Abstract
Schisandra chinensis, classed as a climbing woody vine, is representative of the Schisandraceae family regarded as a significant plant in Chinese herbal medicine. However, the application of molecular breeding is constrained by the fact that the number of genetic markers is small for this species. In this study, transcriptome sequencing of S. chinensis was performed using the Illumina HiSeq platform to construct a library of expressed sequence tag-simple sequence repeat (EST-SSR) markers. A total of 59,786 unigenes were obtained on the basis of 8.57 Gbp clean data. A total of 3460 putative SSR sites were detected with a frequency of 5.79%. The predominant type of repeat markers was dinucleotide (64.54%), followed by trinucleotide (23.87%), hexanucleotide (0.90%), tetranucleotide (0.12%) and pentanucleotide (0.40%). Besides, 50 pairs of EST-SSR primers were randomly selected for verification, among which 14 pairs (28%) showed polymorphism in 42 different types of S. chinensis accessions. The mean values of Na, Ne, PIC, Ho, He and SI were 2.71, 1.7642, 0.4295, 0.6016, 0.3934 and 0.6078, respectively. All of the 42 accessions were successfully identified and formed four major clusters, indicating that the EST-SSR markers are applicable for genetic diversity analysis and identification in S. chinensis. According to the result, the transcriptome data are an effective resource for developing SSR markers. These markers can provide a basis for the identification of S. chinensis accessions, genetic diversity analysis, the conservation and construction of genetic linkage maps as part of selective breeding and the conservation efforts for this valuable plant.
Introduction
Schisandra chinensis (Turcz.) Baill. (Schisandraceae) is regarded as an essential plant in respect of Chinese herbal medicine [Citation1,Citation2]. The fruit of S. chinensis possesses multiple beneficial therapeutic and physiological properties, including adaptogenic, hepatoprotective, anticancer, antioxidant and anti-inflammatory effects. The abundance of Schisandra species has declined significantly in recent years due to habitat loss and excessive economic exploitation. S. chinensis has been listed as a ‘Category III’ protected species in the Chinese National Key Protected Wild Plants [Citation3,Citation4]. Most of the studies on S. chinensis centered on its pharmacological properties, while there is still little attention brought to its mechanism on the molecular level.
DNA molecular markers can be used to analyze the genetic diversity of germplasm resources. However, previous studies have applied only a few types of markers in Schisandra, including inter simple sequence repeats (ISSRs) [Citation5,Citation6], simple sequence repeats (SSRs) [Citation7,Citation8], amplified fragment length polymorphisms (AFLPs) [Citation9,Citation10] and randomly amplified polymorphic DNA (RAPD) [Citation11]. Adopting the FIASCO method, Yan et al. [Citation8] achieved a success in developing and screening 9 SSR polymorphic primers, with the expected heterozygosity (He) reaching 0.528 on average. Based on Bayes cluster analysis, ten natural populations of S. sphenanthera were divided into two groups [Citation8]. By analyzing the genetic structure of S. sphenanthera and S. chinensis, Sun et al. [Citation6] inidcated that the He value of S. sphenanthera was 0.2782, Shannon’s Information (SI) index was 0.4280, the He value of S. chinensis was 0.2894 and the SI index was 0.4396. In comparison with the traditional method applied for the development of SSR markers, transcriptome sequencing can provide a large number of putative SSR loci with significantly less efforts and costs required. Herein, to increase the collection of available markers, through transcriptome sequencing, we report a large number of identified ESR-SSRs, which provide ample genome resource for further studies about the species.
Materials and methods
Sample collection and total RNA extraction
The plant materials were obtained from the germplasm resource garden of Jilin Agriculture University (125°24′15″E, 43°48′5″N; Changchun, Jilin Province, China) in July, 2019. The information on 42 accessions is shown in . ‘Yanhong’ and ‘Jinwuwei’ were examined and approved by Jilin Provincial Variety Examination and Approval Committee in 2012 and 2016, respectively. Young leaves (0.5–1.0 g) were collected and ground into powder after freeze drying in liquid nitrogen. Total RNA was extracted using a modified version of the cetyltrimethylammonium bromide (CTAB) method [Citation12]. RNA was visualized by 1.5% agarose gel electrophoresis.
Table 1. Schisandra chinensis accessions analyzed in this work.
Sequencing, assembly and functional annotation
RNA was extracted using the CTAB method before the reverse-transcription into cDNA, which was sequenced by Illumina (San Diego, CA, USA) high-throughput deep sequencing platform in 2018. The Trinity program [Citation12] was involved to assemble the high quality reads. The unigenes annotation was performed on the basis of COG, GO, KEGG, KOG, Pfam, Swissprot, NR and eggNOG databases.
SSR site identification and primer design based on transcriptome data
SSR loci of unigenes were analyzed using MISA software (https://omictools.com/misa-tool). The minimum number of di-, tri-, tetra-, penta- and hexanucleotide repeats was 6, 5, 5, 5 and 5, respectively. Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/) was adopted to design primers with the following characteristics: (1) a length varying between 18 and 23 bp; (2) an annealing temperature ranging from 55 °C to 65 °C, with a difference of less than 2 °C between forward and reverse primers; (3) an amplification product sized between 80 and 300 bp; and (4) a GC concentration that varies from 40% to 60%. In total, 50 EST-SSR primers were randomly selected and synthesized by Shenggong Biological Engineering (Shanghai, China).
Polymerase chain reaction (PCR) amplification and data analysis
The 50 EST-SSR primers were tested to identify those with both stability and capability to detect polymorphisms. The PCR reaction, with a total volume of 16 µL, contained 8 µL of 2× Ex Taq Master Mix, 0.8 µL of each of primer, 5.4 µL of ultrapure water, and approximately 20 ng of DNA template. PCR amplification was conducted as previously described [Citation6] with a T100TM thermal cycler (Bio-Rad, USA). The products were separated using a 5% polyacrylamide gel. The bands were visualized by means of silver staining. A clear band was assigned a value of 1 while a weak or no band at the same position was given a value of 0.
Data analysis
Trinity version r20131110 [Citation12] software was used to assemble the transcriptome data. The parameters of S. chinensis genetic diversity (Na, Ne, Ho, He, PIC and SI) were analyzed using Popgene version 1.32 software [Citation13]. Final dendrograms were constructed by NTSYS-PC software [Citation14] based on the unweighted pair group method with arithmetic mean (UPGMA).
Results and discussion
RNA-Seq and functional annotation
A total of 8.57 Gbp clean data were obtained with 92.51% Q30 bases. The raw data of RNA-seq were deposited in Sequence Read Archives Database (http://www.ncbi.nlm.nih.gov/bioproject/609148) under the accession number PRJNA609148.
A total of 59,786 unigenes were assembled using the Trinity method and were treated as the background data required for the development of EST-SSR markers. The functional annotation of unigenes is presented in .
Table 2. Functional annotation of S. chinensis unigenes.
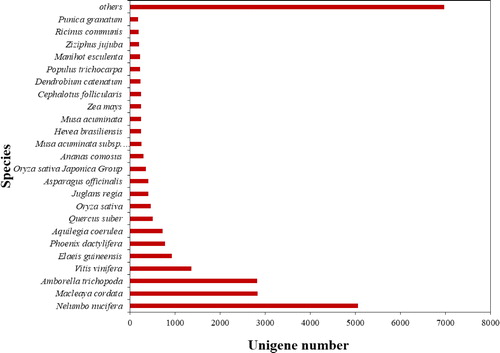
The number of hits against Nelumbo nucifera at 5059 hits (19.2%) was the highest, followed by Macleaya cordata at 2835 hits (10.76%), Amborella trichopoda at 2818 hits (10.69%), Vitis vinifera at 1358 hits (5.15%) and Elaeis guineensis at 932 hits (3.54%) ().
SSR site distribution in S. chinensis
We searched 59,786 unigene sequences in the transcriptome data and detected 3460 SSR sites in 3062 sequences. There were 352 unigene sequences containing two or more EST-SSR sites. The SSR frequency was 5.79%. This value was affected by not only the SSR search threshold but also a large number of single nucleotide repeats. In order to facilitate comparisons with other species, we analyzed the results in two groups: one including and the other excluding mononucleotide repeats. In the former context, the SSR frequency in S. chinensis was 10.46%, which is higher than Allium cepa (5.57%) [Citation15] and Phoebe nanmu (9.90%) [Citation16] but lower than Elaeis guineensis (11.26%) [Citation17], Miscanthus (14.44%) [Citation18] and Solanum melongena (18.32%) [Citation19]. Without considering mononucleotide repeats, the SSR distribution frequency was 5.79%, which is higher than corn (Zea mays) (1.5%), barley (Hordeum vulgare) (3.4%) and rice (Oryza sativa) (4.7%) [Citation20], cucumber (Cucumis sativus) (4.03%) [Citation21], Chinese yew (Taxus chinensis) (2.24%) [Citation22] and Korean pine (Pinus koraiensis)(4. 24%) [Citation23]; similar to peanut (Arachis hypogaea) (6.63%) [Citation24], pepper (Capsicum annuum) (7.83%) [Citation25] and dal (Cajanus cajan) (7.6%) [Citation26]; and lower than thorn pear (Rosa roxburghii) (20.37%) [Citation27], oak (Quercus austrocochinchinensis) (27.61%) [Citation28] and precocious trifoliate orange (Citrus reticulata) (26.88%) [Citation29]. It is speculated that these differences indicate the actual variations in SSR abundance across various species if the impact of the amount and source of EST data was ignored [Citation27]. Thus, transcriptome SSRs are abundant in Schisandra, making these an ideal marker for genetic mapping.
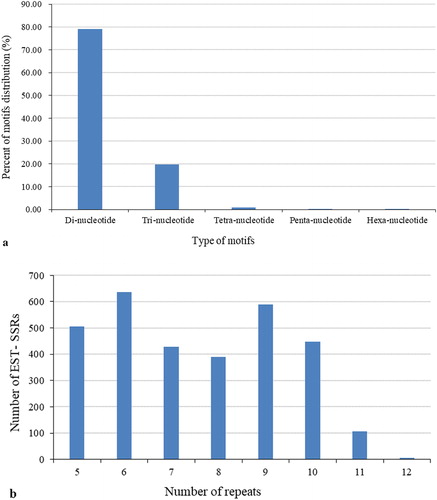
The types of EST-SSR detected in the transcriptome varied and their frequencies differed significantly (). Di-, and trinucleotides were the most common, accounting for 64.54% and 23.87%, respectively. Tetra-, penta- and hexanucleotides were rare, only accounting for 1.42%.
Table 3. Type, number and frequency of expressed sequence tag-simple sequence repeat markers in Schisandra chinensis.
More than two times higher frequency of the dinucleotides compared to the trinucleotides conformed to the trend as observed in peanut (Arachis hypogaea) [Citation24], precocious trifoliate orange (Citrus reticulata) [Citation29], Korean pine (Picea neoveitchii) [Citation30] and litchi (Litchi chinensis) [Citation31], despite some evidence suggesting that trinucleotides are the most common SSR type [Citation17,Citation19,Citation22,Citation32,Citation33]. This difference is potentially attributed to the characteristics and quantity of EST-SSR and the EST resources in plants.
Characteristics of EST-SSRs
An analysis was conducted of di-, tri-, tetra-, penta- and hexanucleotides. Due to the potential of poor sequencing quality caused by homopolymerization, mononucleotides were excluded [Citation34]. In total, 82 types of motif were identified, with 8, 30, 25, 5 and 14 di-, tri-, tetra-, penta- and hexanucleotide repeats, respectively (complementary sequences were considered one type of motif) (). The most highly represented EST-SSR type was dinucleotide (64.54%); TC/GA was the most common motif (48.46%) followed by CT/AG (39.52%), with other motif types comprising merely 12.02% of all EST-SSR dincleotides. TC/GA was revealed as the most common dinucleotide repeat unit in S. chinensis, which is identical to pigeonpea (Cajanus cajan) and tree rhododendron (Rhododendron arboreum) [Citation26,Citation35] but distinct from barley (Hordeum vulgare), wheat (Triticum aestivum), corn (Zea mays), sorghum (Sorghum bicolor), rice (Oryza sativa) [Citation20], eggplant (Solanum melongena) [Citation19], peanut (Arachis hypogaea) [Citation24], sugarcane (Saccharum officinarum) [Citation36], grass (Miscanthus) [Citation18], daylily (Hemerocallis citrina) [Citation37], Chinese gansui (Euphorbia kansui) [Citation38], thorn pear (Rosa roxburghii) [Citation27], and Chinese yew (Taxus chinensis) [Citation22]. There were 30 types of trinucleotide repeat motifs among S. chinensis ESTs. More specifically, the most frequent ones include GAA/TTC and AGA/TCT, accounting for 16.59% (846) and 13.49% (688) of the total, respectively. GAA/TTC was the most abundant trinucleotide repeat motif, as reported for pansy [Citation39].
Table 4. Distribution of EST-SSR motifs in the transcriptome of S. chinensis.
The frequency of different motifs in the EST datasets varied, with 20572 (79.39%) dinucleotides, 5100 (19.68%) trinucleotides, 206 (0.81%) tetranucleotides, 27 (0.1%) pentanucleotides and 6 (0.02%) hexanucleotides (). The number of motif repeats ranged from 5 to 12, with six as the most-seen number of repeats, as shown in .
Development of S. chinensis EST-SSR primer pairs and detection of polymorphisms
To obtain high quality SSRs that could detect polymorphisms, we randomly selected 50 primer pairs to evaluate polymorphisms among four accessions of S. chinensis (Yanhong, Zaohong, Jinwuwei and 12-(-2)-1). Among the 50 EST-SSR primer pairs tested, 40 were successfully amplified, whereas 14 produced stable and polymorphic bands of the expected size (), with a polymorphic amplification efficiency of 28%, which is comparable to Chinese bajitian (Morinda officinalis) (24%) [Citation40], higher than pigeonpea (Cajanus cajan) (12.9%) [Citation26] but lower than onion (Allium cepa) (60%) [Citation15], thorn pear (Rosa roxburghii) (54.76%) [Citation27] and Chinese yew (Taxus chinensis) (53.23%) [Citation22]. A total of 38 alleles were detected by 7 pairs of primers, with an average of 2.71. The polymorphism information content (PIC) ranged from 0.2604 to 0.5817, with an average of 0.4295. The He value for diversity ranged from 0.0235 to 0.4997, and the highest SI index was found for SSC0035 with 1.0842, the lowest for SSC0062 ().
Table 5. Information of 14 SSRs used for assessment of genetic diversity among 42 S. chinensis accessions.
Table 6. Estimation of genetic diversity based on 14 SSRs among 42 S. chinensis.
Discrimination between different S. chinensis genotypes using EST-SSR primer pairs
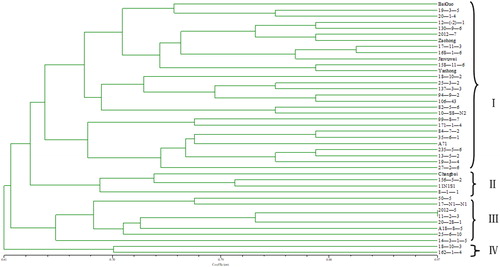
In the genetic diversity analysis, the 14 EST-SSR primer pairs identified as described above could be used to differentiate between the 42 S. chinensis accessions. Using NTSYS-pc software to analyze genotype data, the accessions were classified into four groups at a similarity index of 0.63 (). A dendrogram revealed clear distinctions between the accessions, reflecting a high genetic diversity that can be exploited for S. chinensis identification based on a DNA fingerprint. The relatedness of the 42 accessions was supported by similarity coefficients ranging between 0.61 and 0.97. Group I, which comprised 28 varieties mostly originating in Jilin, was the largest group with four subgroups and a similarity coefficient of 0.682. Group II included four varieties; most were from Heilongjiang, with one accession from Jilin. ‘18-10-3’and ‘162-1-4’ did not cluster with any of the groups and were designated as group IV, and the remaining accessions constituted group III. All 42 accessions were distinguishable based on the 14 EST-SSR markers and their clustering pattern was concordant with their distribution, indicating that EST-SSR data obtained by transcriptome analysis can reveal the genetic relatedness of S. chinensis germplasm resources.
Figure 3. Dendrogram of S. chinensis germplasms generated using the unweighted pair group method with arithmetic mean.
To date, there are as few as nine EST-SSR markers identified for S. chinensis. In this study, the large number of SSR loci and polymorphic markers identified on the basis of transcriptome data are effective in facilitating the identification of accessions and the construction of genetic linkage maps in S. chinensis as part of broader efforts for the selective breeding and the conservation of this valuable plant.
Conclusions
In this study, specific SSR markers were developed based on the transcriptome sequencing data of S. chinensis, and the distribution of SSR markers was analyzed. Using these markers, we successfully analyzed the genetic diversity and identified different accessions of S. chinensis. The results demonstrated that transcriptome sequencing is an effective method to identify molecular markers. Our work may lay a foundation for genetic diversity, genetic mapping and marker-assisted selection in S. chinensis. It may facilitate S. chinensis breeding, as well as studies with other Schisandra plants with economic and medicinal value.
Disclosure statement
The authors declare that they have no conflict of interest.
Availability of data and materials
The raw data of RNA-seq are deposited in Sequence Read Archives Database (http://www.ncbi.nlm.nih.gov/bioproject/609148) under accession number PRJNA609148. Other datasets supporting the conclusions of this article are included within the article (and its additional file).
Additional information
Funding
References
- Szopa A, Ekiert H. In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese Magnolia vine) - a potential biotechnological rich source of therapeutically important phenolic acids. Appl Biochem Biotechnol. 2012;166(8):1941–1948.
- Yan BQ, Wang J, Chen GP, et al. Isolation and characterization of polymorphic microsatellite loci in a traditional Chinese medicinal plant, Schisandra sphenanthera. Conserv Genet. 2009;10(3):615–617.
- Yu LH, Sun QL, Dou SS, et al. A studies on the investigation and evaluation germplasm of Schisandra chinensis. Asia Pac Tradit Med. 2005;3:84–87.
- The Ministry of Agriculture and the State Administration of Forestry. National key protected wild plants (first batch). Beijing (China): The State Council of the People’s Republic of China; 1999.
- Luo C. Population genetic structure and genetic diversity of Schisandra sphensanthera in Qinling of China [dissertation]. Shanxi (China): Shanxi Normal University; 2012.
- Sun Y, Wen XY, Huang HW. Population genetic differentiation of Schisandra chinensis and Schisandra sphenanthera as revealed by ISSR analysis. Biochem Syst Ecol. 2010;38(3):257–263.
- Xu M, Wu S, Liu XX. Genetic diversity analysis of Schisandra sphenanthera in Qinling mountains based on SSR markers. J Chin Med Mater. 2013;36:1215–1218.
- Yan B, Wang T, Hu L. Population genetic diversity and structure of Schisandra sphensanthera, a medicinal plant in China. Chin J Ecol. 2009;28:811–819.
- Gu W. Studies on genetic diversity of germplasm in Schisandra sphenanthera Rehd. et Wils [PhD dissertation]. Shanxi (China): Shanxi Normal University; 2010.
- Valente M. Clonality and patterns of genetic diversity in Schisandra glabra (Brickell) Rehder (Schisandraceae), a threatened woody vine [MSc dissertation]. Knoxville (TN): University of Tennessee; 2007.
- Gao JP. Comparative studies on Schisandra chinensis and Schisandra sphenanthera [PhD dissertation]. Shanghai (China): Fudan University; 2003.
- Grabherr MG, Haas BJ, Yassour M, et al. Full length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. https://doi.org/10.1038/nbt.18.
- Yeh F, Boyle T. Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg J Bot. 1997;129:157.
- Rolf JF. NTSYS-PC, numerical taxonomy and multivariate analysis system version 2.11T Exeter Software. New York: Setauket; 2000.
- Li MT, Zhang SL, Deng P, et al. Analysis on SSR information in transcriptome of onion and the polymorphism. Acta Hort Sinica. 2015;42:1103–1111.
- Shi XD, Zhu XH, Sheng YZ, et al. Development of SSR markers based on transcriptome sequence of Phoebe zhennan. Sci Silvae Sin. 2016;52:71–78.
- Xiao Y, Zhou L, Xia W, et al. Exploiting transcriptome data for the development and characterization of gene-based SSR markers related to cold tolerance in oil palm (Elaeis guineensis). BMC Plant Biol. 2014;14:384.
- Nie G, Tang L, Zhang Y, et al. Development of SSR markers based on transcriptome sequencing and association analysis with drought tolerance in perennial grass Miscanthus from China. Front Plant Sci. 2017;8:801.
- Wei MM. Development and application of SSR markers in eggplant based on transcriptome sequencing [MSc dissertation]. Beijing (China): Chinese Academy of Agricultural Sciences; 2016.
- Kantety RV, Rota ML, Matthews DE, et al. Data mining for simple sequence repeats in expressed sequence tags from barley, maize, rice, sorghum and wheat. Plant Mol Biol. 2002;48(5–6):501–510.
- Guo S, Zheng Y, Joung JG, et al. Transcriptome sequencing and comparative analysis of cucumber flowers with different sex types. BMC Genomics. 2010;11:384.
- Li YL, Yang XX, Zhang JY, et al. Studies on SSR molecular markers based on transcriptome of Taxus chinensis var. mairei. Acta Hort Sinica. 2014;41:735–745.
- Zhang Z, Zhang HG, Mo C, et al. Transcriptome sequencing analysis and development of EST-SSR markers for Pinus koraiensis. Sci Silvae Sin. 2015;51:114–120.
- Zhang J, Liang S, Duan J, et al. De novo assembly and characterisation of the transcriptome during seed development, and generation of genic-SSR markers in peanut (Arachis hypogaea L.). BMC Genomics. 2012;13:90.
- Liu F, Wang YS, Tian XL, et al. SSR mining in pepper (Capsicum annuum L.) transcriptome and the polymorphism analysis. Acta Hort Sinica. 2012;39:168–174.
- Dutta S, Kumawat G, Singh BP, et al. Development of genic-SSR markers by deep transcriptome sequencing in pigeonpea [Cajanus cajan (L.) Millspaugh]. BMC Plant Biol. 2011;11:17.
- Yan XQ, Lu M, An HM. Analysis on SSR information in transcriptome and development of molecular markers in Rosa roxburghii. Acta Hort Sinica. 2015;42:341–349.
- An M, Deng M, Zheng SS, et al. De novo transcriptome assembly and development of SSR markers of oaks Quercus austrocochinchinensis, and Q. kerrii (Fagaceae). Tree Genet Genomes. 2016;12:103.
- Long D. Large scale development of SSR markers based on transcriptome sequencing of precocious trifoliate orange [MSc dissertation]. Wuhan (China): Huazhong Agriculture University; 2014.
- Chang P, Zhu L, Zhao M, et al. The first transcriptome sequencing and analysis of the endangered plant species Picea neoveitchii Mast. and potential EST-SSR markers development. Biotechnol Biotechnol Equip. 2019;33(1):967–973.
- Sun QM, Ma WC, Ma SP, et al. Characteristics of SSRs derived from ESTs and development of EST-SSR markers in litchi (Litchi chinensis Sonn). Sci Agric Sin. 2011;44:4037–4049.
- Saunders RMK. Monograph of Schisandra (Schisandraceae). Syst Bot Monogr. 2000;58:1–146.
- Sun Y, Liu YF, Huang HW. Isolation and characterization of polymorphic microsatellite markers in Schisandra chinensis (Turcz.) Baill. (Schisandraceae). Conserv Genet Resour. 2009;1(1):119–121.
- Gilles A, Meglécz E, Pech N, et al. Accuracy and quality assessment of 454 GS-FLX titanium pyrosequencing. BMC Genom. 2011;12:245.
- Sharma H, Kumar P, Singh A, et al. Development of polymorphic EST-SSR markers and their applicability in genetic diversity evaluation in Rhododendron arboreum. Mol Biol Rep. 2020;47(4):2447–2457.
- Xiao N, Wang H, Yao W, et al. Development and evaluation of SSR markers based on large scale full-length transcriptome sequencing in sugarcane. Trop Plant Biol. 2020;10:1–10.
- Li S, Ji F, Hou F, et al. Characterization of Hemerocallis citrina transcriptome and development of EST-SSR markers for evaluation of genetic diversity and population structure of Hemerocallis collection. Front Plant Sci. 2020;11:686.
- Zhao X, Wang M, Chai J, et al. De novo assembly and characterization of the transcriptome and development of microsatellite markers in a Chinese endemic Euphorbia kansui. Biotechnol Biotechnol Equip. 2020;34(1):562–574.
- Du XH, Yang YP, Zhu XP, et al. Development of genic-SSR markers by transcriptome sequencing in Viola × wittrockiana. Acta Hort Sinica. 2018;46:797–806.
- Liao BY, Lee SY, Meng KK, et al. Characterization and novel EST-SSR marker development of an important Chinese medicinal plant, Morinda officinalis How (Rubiaceae). Biotechnol Biotechnol Equip. 2019;33(1):1311–1318.