Abstract
The Asian citrus psyllid, Diaphorina citri Kuwayama, is one of the most destructive pests of citrus and the primary vector of Candidatus Liberibacter spp., which cause citrus Huanglongbing (HLB). Microbial insecticides based on entomopathogenic fungi are safer alternatives to chemical pesticides. They can mitigate the spread of HLB by suppressing D. citri populations in citrus groves. Purpureocillium lilacinum strain ZJPL08, previously isolated from D. citri cadavers, is highly pathogenic to D. citri. We determined the virulence of strain ZJPL08 conidial suspensions and the efficacy of a wettable powder formulation. The LC50 of strain ZJPL08 against D. citri at 7 d post-inoculation was 2.11 × 104 conidia/mL, and the LT50 of a 1.0 × 108 conidia/mL suspension was 3.14 d in the laboratory. Under the same conditions, the LC50 of the wettable powder was 1.39 × 104 conidia/mL, and the LT50 was 2.64 d. The corrected mortality caused by the 1500-times diluted solutions of strain ZJPL08 wettable powder in D. citri reached 86.67% at 7 d post-treatment and 100% at 14 d post-treatment under field conditions. Our study revealed that strain ZJPL08 infected D. citri by hyphae penetrating directly through the cuticle and into the body cavity and tissues. These findings show that P. lilacinum strain ZJPL08 has potential for the effective control of D. citri.
Introduction
The Asian citrus psyllid, Diaphorina citri Kuwayana (Hemiptera: Psyllidae), is the major vector of Candidatus Liberibacter asiaticus and Candidatus Liberibacter americanus, which are the bacteria responsible for spreading Huanglongbing (HLB). HLB is the most destructive disease of citrus in the world and results in loss of yield, reduced fruit quality and a shortened plant life [Citation1,Citation2]. Between 2005 and 2016, the estimated losses to the Florida citrus industry caused by HLB have exceeded $8 billion [Citation3].
HLB can spread rapidly via D. citri in a citrus grove. The psyllids can acquire the causative agents after feeding on HLB-infected plants in 15–30 min and then transmit them to other citrus plants in less than 1 h after a latent period of 8–12 days [Citation4]. D. citri also has a substantial flight capability, and it can traverse potential geographic barriers and disperse at least 2 km within 12 d in the absence of severe weather events [Citation5]. HLB and D. citri have spread to nearly all citrus-producing areas worldwide [Citation1,Citation6]. In addition, D. citri is the main pest of the new shoot stage of citrus; it can directly feed on the young leaves of new shoots and produce wax and honeydew to encourage sooty mold disease [Citation7,Citation8]. Therefore, it is important to increase the control level of D. citri populations, both to reduce HLB prevalence and to lessen the direct harm caused by D. citri feeding.
Chemical pesticides are commonly used for D. citri control and reduction of HLB [Citation6,Citation9]. However, long-term and frequent application of chemical insecticides may lead to resistance in D. citri populations, harm natural enemies, pollute the environment, and have negative effects on food safety [Citation10]. Biological control agents are eco-friendly alternatives to chemical insecticides. These agents include entomopathogenic fungi, which are insect-specific pathogens and not hazardous to mammals.
Several species of entomopathogenic fungi can infect D. citri, including Isaria fumosorosea Wize [Citation11,Citation12], Capnodium citri Berk. et Desm. [Citation13], Paecilomyces javanicus (Friederichs & Bally) Brown and Smith [Citation14], Hirsutella citriformis Speare [Citation15], Lecanicillium spp. [Citation16,Citation17] and so forth. The efficacy of different entomopathogens against D. citri may vary under different conditions, so the addition of new entomopathogens will provide more possibilities for screening out strains suitable for certain field conditions. It is also necessary to clarify the infection mechanism of entomopathogens and the efficacy of their formulations. This is valuable information that can be used to improve entomopathogens to become potential biological pesticides.
Purpureocillium lilacinum (Thom) Luangsa-ard, Hywel-Jones, Houbraken & Samson strain ZJPL08 is an entomopathogenic fungus isolated from D. citri cadavers found in Zhejiang province, China [Citation18]. Strain ZJPL08 is highly pathogenic to D. citri and has the characteristics of rapid mycelial growth, production of many conidia and high germination rate of the conidia [Citation19]. The ZJPL08 strain is attractive for development and practical application. In this study, we further determined the virulence and observed the infection process of strain ZJPL08 against D. citri. We prepared a wettable powder formulation of strain ZJPL08 and determined its efficacy on D. citri in laboratory and field conditions.
Material and methods
Entomopathogen and cultivation
A pure culture of the entomopathogenic fungus P. lilacinum strain ZJPL08, previously isolated from D. citri cadavers in Zhejiang province in July 2012, was maintained in potato dextrose agar (PDA) slants and stored at 4 °C in our laboratory for 2 mouths. One mycelial plug of strain ZJPL08 was inoculated in the middle of the PDA medium and cultured at 28 °C for 5–7 d. A conidial suspension was obtained by eluting the conidia of the fungus cultured on PDA plates with sterile water.
Virulence of P. lilacinum strain ZJPL08
Healthy D. citri were reared on Murraya paniculata (L.) Jack. seedlings in a greenhouse at 28 °C and a 14:10 h (light:dark) photoperiod. Conidial suspensions were prepared by mixing conidia of strain ZJPL08 into 0.1% (v/v) Tween-80 to give 1.0 × 108, 1.0 × 107, 1.0 × 106, 1.0 × 105 and 1.0 × 104 conidia/mL.
D. citri adults were picked up using a fine brush and immersed in conidial suspensions for 30 s, and then, placed on the detached leaves of M. paniculata in 15 cm diameter petri dishes. The petri dishes were covered with 60-mesh nylon screen and incubated in an artificial climate chamber maintained at 28 °C, > 90% relative humility and a 14:10 h (light:dark) photoperiod. Healthy D. citri treated only with sterile water containing 0.1% Tween-80 were used as the control. Three replicates were performed for each treatment with 30 D. citri adults per replicate. Mortality was assessed by counting the number of dead insects.
Examination of infection process
D. citri adults inoculated with a conidial suspension of 1.0 × 108 conidia/mL were incubated as above in a climate chamber. External characteristics of the treated D. citri were observed at 12, 24, 36, 48 and 72 h post-inoculation using a SMZ1000 stereo microscope (Nikon Co. Ltd., Tokyo, Japan).
At 1, 6, 12, 36 and 48 h post-inoculation, the inoculated D. citri were sampled, fixed overnight with a 2.5% glutaraldehyde solution at 4 °C, and then, treated using the previous method [Citation20]. Prepared specimens were observed with a JSM-6390LV scanning electron microscope (JEOL Co. Ltd., Tokyo, Japan).
The D. citri sampled at 6 h, 12 h, 24 h, 36 h and 60 h post-inoculation were first fixed overnight with 4% paraformaldehyde solution at 4 °C, and then, treated using the previous method [Citation21] to prepare sections of 5 μm thickness. Finally, hematoxylin–eosin or Grocott’s methenamine silver were used to stain the sections to highlight fungal tissues, and the stained sections were examined with an Eclipse E200 light microscope (Nikon Co. Ltd., Tokyo, Japan).
Preparation of wettable powder
Conidia of strain ZJPL08, cultured on PDA plates, were inoculated into potato sucrose broth (PSB) medium with a sterile inoculation ring, and incubated at 28 °C on a shaking incubator at 120 r/min for 3 d to prepare the seed solution. Then, the seed solution was inoculated onto sterilized solid rice in a petri dish at a ratio of 10% (v/w), and incubated at 28 °C in darkness for 7 d until the dark purple conidia had completed growth. Then, the culture was dried in a blast drying oven at 35 °C for 36 h, and sieved using 325-mesh sieve to prepare conidia powder.
The properties of the additives and their effects on mycelial growth and conidial germination of strain ZJPL08 were determined according to previously described methods [Citation22,Citation23]. Calcium carbonate, kaolin, talcum powder, diatomaceous earth, attapulgite and wheat flour were studied to select the best carrier. Sodium dodecyl sulfate, sodium dodecyl benzene sulfonate and nekal BX were evaluated for selection of the best wetting agent. Sodium lignin sulfonate, calcium lignosulfonate and sucrose fatty acid ester were evaluated to select the best dispersant. Humic acid, fluorescein sodium, sodium alginate and ascorbic acid were evaluated to select the best UV protectant. Boric acid, zinc sulfate, calcium chloride and potassium chloride were evaluated to select the best conidial germination promoter. Xanthan gum, sucrose and soluble starch were studied to select the best adhesive.
The wettable powder of strain ZJPL08 was prepared by mixing conidia powder and additives in a certain proportion and screening through 325-mesh sieve. The quality index of the prepared wettable powder, including conidia content, germination rate, moisture content, wetting time, suspension rate, fineness and pH, was determined by the previous method [Citation24,Citation25].
Efficacy of wettable powder
The lethal effect of strain ZJPL08 wettable powder was determined in a manner similar to that described above. A conidial suspension of 1.0 × 108 conidia/mL was prepared by mixing 1 g wettable powder with 298 mL sterile water, and then, diluted with sterile water to concentrations of 1.0 × 107, 1.0 × 106, 1.0 × 105 and 1.0 × 104 conidia/mL before spraying on adult D. citri. Healthy D. citri were treated with sterile water only containing additives as the control. There were three replicates per treatment with 30 D. citri per replicate. Mortality was assessed by counting the number of dead insects.
Efficacy determination was performed under natural conditions (average temperature 25 °C, average relative humidity 71%). In this test, healthy D. citri adults were released onto a potted M. paniculata plant enclosed in an insect-proof cage. Then, the D. citri were sprayed with the diluted 1500 times solutions of the prepared wettable powder and a conidial suspension of 1.99 × 107 conidia/mL, respectively. Positive controls were sprayed with 10% imidacloprid wettable powder (diluted 2000 times), 30% nitenpyram soluble liquid (diluted 4500 times) and 24.5% abamectin mineral oil emulsifiable concentrate (diluted 1500 times), respectively. These concentrations were chosen based on the conventional dilution ratios currently used for the control of citrus psyllids in the field. Negative controls were treated with water only containing additives. Five replicates were performed for each treatment with 60 D. citri per replicate, and the experiment was conducted three times. Mortality was evaluated at 3, 7 and 14 d after treatment.
Statistical analysis
Mortality data of D. citri in the conidial suspension and wettable powder of strain ZJPL08 treatments were analyzed by using the SAS v9.2 software. Linear regression equations derived from the regression analysis were used to calculate the median lethal concentration (LC50) and median lethal time (LT50), respectively. The mortality of D. citri caused by wettable powder and other treatment under natural conditions were analyzed by analysis of variance (ANOVA) using SAS v9.2. Multiple comparisons of the means were performed using the Duncan’s new multiple-range test at a significance level of p < .05.
Results and discussion
Virulence of P. lilacinum strain ZJPL08
P. lilacinum strain ZJPL08 is an entomopathogenic fungus isolated from D. citri in Zhejiang province, China. The pathogenicity of strain ZJPL08 was determined by its virulence to D. citri. At 3 d post-inoculation, the LC50 was 6.06 × 109 conidia/mL, while it was 2.11 × 104 conidia/mL at 7 d post-inoculation (). The LT50 of the conidial suspension of 1.0 × 104 conidia/mL was 6.89 d, while the LT50 was 3.14 d at the concentration of 1.0 × 108 conidia/mL (). Generally, the higher the concentration of conidial suspension, the more the number of conidia adsorbed, germinated and infected on the insect surface, and the stronger the pathogenicity to the host. The results demonstrated that a high concentration of conidial suspension of strain ZJPL08 had a higher fatality rate to D. citri. Suitable concentrations can be chosen according to the population quantity of D. citri in field application.
Table 1. LC50 of conidial suspension of Purpureocillium lilacinum strain ZJPL08 in Diaphorina citri.
Table 2. LT50 of conidial suspensions of Purpureocillium lilacinum strain ZJPL08 in Diaphorina citri.
Examination of infection process
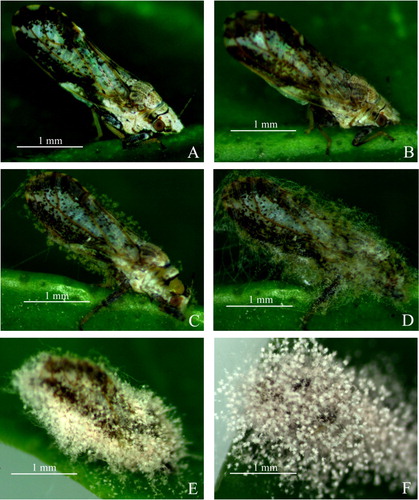
The changes in external symptoms of the treated D. citri body as a function of time post-inoculation were visible under a stereo microscope (). shows the control D. citri treated only with sterile water containing 0.1% Tween-80. At 12 h post-inoculation, white short hyphae were found growing mainly on the pronotum, wing margin and femora of D. citri (). However, the infected insects appeared to be normal in their behavior and had no significant difference compared to the control. After that, white hyphae grew progressively longer, and were also clearly visualized in the head, chest, abdomen, legs, anus and genitalia at 24 h post-inoculation (). At this time, the infected insects showed dull responses, sluggish movements and loss of balance. At 36 h post-inoculation, fungal hyphae were visible all over the body of D. citri, and pale purple conidia began to grow on the hyphae, causing the death of the infected insect (). Even after death, the hyphae continued to develop in the insect body. A layer of dense hyphae and purple conidia covered the whole surface of the insect body at the end of the infection ().
Figure 1. Adult D. citri infected with Purpureocillium lilacinum strain ZJPL08 observed under a stereo microscope. (A) Control D. citri. (B − F) Infected D. citri at 12, 24, 36, 48 and 72 h post-inoculation.
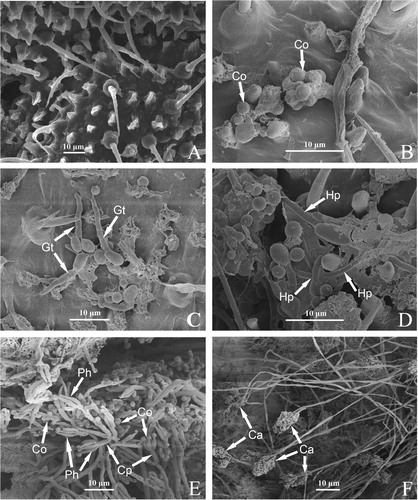
The invasion process of strain ZJPL08 on the cuticle of D. citri was observed using a scanning electron microscopy (). shows the control D. citri inoculated only with sterile water containing 0.1% Tween-80. In the treatment, the conidia of strain ZJPL08 were observed adhering to the surface of the insect body at 1 h post-inoculation, especially in the intersegmental fold, setal alveolus, anus, genitalia and other folds or sunken regions such as the suture and sulcus (). At 6 h post-inoculation, conidia had germinated to form germ tubes, some of which appeared to be penetrating the epicuticle directly, while others grew horizontally along the insect body surface (). At 12 h post-inoculation, the hyphae had become branched, developed a variety of hyphal formations, and anastomosed to form groupings surrounded by extracellular mucilage (). At 36 h post-inoculation, the hyphae continued to grow, and some had differentiated to form conidiophores with conidia produced at the apex. The cuticle of the insects became fractured (). At 48 h post-inoculation, a mass of conidiophores and conidia were produced ().
Figure 2. Invasion of Purpureocillium lilacinum strain ZJPL08 in D. citri observed under a scanning electron microscopy. (A) Control D. citri. (B − F) Fungal development on the surface of D. citri body at 1, 6, 12, 36 and 48 h post-inoculation. Co: conidia, Gt: germ tubes, Hp: hyphae, Ph: phialides, Cp: conidiophore, Ca: Conidiogenous apparatus.
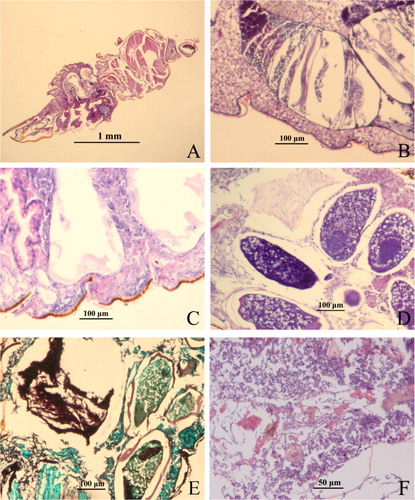
Light microscopy revealed the internal progress of strain ZJPL08 infection in D. citri (). The observations were similar to those made with scanning electron microscopy. shows the control D. citri inoculated only with sterile water containing 0.1% Tween-80. In the treatment at 6 h post-inoculation, the conidia adhering to the insect body surface were visible and some had germinated. The germ tubes penetrated the epicuticle or grew along the insect body surface (). At 12 h post-inoculation, the hyphae had entered the body cavity through the cuticle (). The hyphae proliferated massively and occupied the whole haemocoel at 24 h post-inoculation (). At 36 h, the internal tissues and organs, such as the fat body, muscle tissue, digestive tract and ovary, were infected and began to undergo pathological changes (). At 60 h, a mass of intertwined hyphae developed, and most organs of D. citri were totally destroyed ().
Figure 3. Invasion of Purpureocillium lilacinum strain ZJPL08 in D. citri observed under a light microscopy. (A) Control D. citri. (B − F) Fungal invasion at 6, 12, 24, 36 and 60 h post-inoculation.
The pathogenic process of entomopathogenic fungi usually includes the following stages: conidia attaching to the host surface, conidia germinating on the insect cuticle, penetrating the epidermis, mycelia growing in the haemocoel, toxin production, host death, mycelia invading all the organs of the host, mycelia penetrating the epidermis, producing and spreading conidia [Citation26,Citation27]. P. lilacinum can infect plant parasitic nematodes, such as root-knot nematodes, in many ways. It can parasitize the eggs and cysts of nematodes by physical means, combine mechanical pressure and secreted extracellular enzymes in the infection process, and produce toxic metabolites that paralyze the nematode to promote its colonization on the nematode body and obtain nutrients [Citation28–31].
Our results showed that similar infection mechanisms as above were utilized by strain ZJPL08 to infect D. citri. The attachment site of conidia helps us understand the relationship between entomopathogenic fungi and insects, and provides a basis for improving the infection efficiency of strain ZJPL08 and expanding its range of application. We found that D. citri began to die before the hyphae had completely destroyed the internal tissues and organs. Strain ZJPL08 may produce toxins in the host haemocoel which accelerated the speed of death. The hyphae on the body of D. citri produce many new conidia, which will disperse and contribute to the initiation of new infection cycles.
Preparation of wettable powder
Based on the properties of various additives and their biocompatibility with strain ZJPL08 in our preliminary experiments (data not shown), diatomaceous earth was selected as the best carrier of the wettable powder. Sodium lauryl sulfate was the best wetting agent, and sodium lignosulfonate was the best dispersant. Humic acid was the best UV protectant, calcium chloride was the best conidial germination promoter, and xanthan gum was the best adhesive.
The conidia powder of strain ZJPL08 was mixed with the screened additives to prepare the wettable powder. The formula of the wettable powder is: 10% conidia powder of strain ZJPL08, 3% sodium lauryl sulfate, 5% sodium lignosulfonate, 0.25% humic acid, 0.1% calcium chloride, 0.05% xanthan gum and 81.6% diatomaceous earth. The quality index of wettable powder was further determined. The results showed that the prepared wettable powder was of high quality, with a conidia content of 2.98 × 1010 conidia/g, germination rate of 91.32%, moisture content of 2.59% and a wetting time of 90 s ().
Table 3. Quality index of a wettable powder formulation of Purpureocillium lilacinum strain ZJPL08.
Environmental factors such as relative humidity, temperature, wind, sunlight and photoperiod can influence the effect of entomopathogens. In most cases, high humidity is required during all of the developmental stages and infection processes of entomopathogens [Citation32–35]. The optimum temperature for Hyphomycetes is generally in the range of 20–30 °C, whereas for Entomophthorales, it is considered 15–25 °C. Wind is important for conidia dispersal of entomopathogens, but it can also reduce humidity by removing free water in some cases [Citation36]. Sunlight and ultraviolet (UV) rays can reduce the conidial viability and impair the infection persistence of entomopathogenic fungi [Citation36–40]. To maximize the efficacy of strain ZJPL08 wettable powder formulation in D. citri management, humic acid was added to help protect against UV irradiation, calcium chloride was added to promote conidia germination, and xanthan gum was added to enhance adhesion to D. citri.
Efficacy of wettable powder
As with the conidial suspension, the efficacy of strain ZJPL08 wettable powder against D. citri increased with the increase of conidial concentration and time. Under the same conditions, the wettable powder had a better lethal effect on D. citri than the conidial suspension. At the same length of culture post-inoculation, the higher conidial concentration of wettable powder, the higher the mortality of D. citri. At 3 d post-inoculation, the LC50 was 9.67 × 107 conidia/mL, while it was 1.39 × 104 conidia/mL at 7 d post-inoculation (). The LT50 of the wettable powder with a concentration of 1.0 × 104 conidia/mL was 6.17 d, whereas the LT50 of the wettable powder with a concentration of 1.0 × 108 conidia/mL was only 2.64 d ().
Table 4. LC50 of a wettable powder formulation of Purpureocillium lilacinum strain ZJPL08 in Diaphorina citri.
Table 5. LT50 of a wettable powder formulation of Purpureocillium lilacinum strain ZJPL08 in Diaphorina citri.
The field trials conducted to determine the efficacy of strain ZJPL08 wettable powder against D. citri showed that the wettable powder was effective at D. citri control (). At 3 d post-treatment, the corrected mortality of D. citri treated with ZJPL08 wettable powder was 42.83%. This was less than that of 24.5% abamectin mineral oil emulsifiable concentrate and 10% imidacloprid wettable powder, but comparable with the 30% nitenpyram soluble liquid treatment and higher than the mortality rates produced by strain ZJPL08 conidial suspension. The control of D. citri produced by the wettable powder increased with time. The corrected mortality of D. citri produced by the wettable powder was 86.67% at 7 d post-treatment and 100% at 14 d post-treatment. These values were significantly different from those of the 30% nitenpyram soluble liquid.
Table 6. Efficacy of a wettable powder formulation of Purpureocillium lilacinum strain ZJPL08 on Diaphorina citri.
The wettable powder was more effective for D. citri control than the conidial suspension, indicating that the additives enhanced the efficacy of strain ZJPL08 on D. citri. Compared with chemical pesticides, such as imidacloprid, strain ZJPL08 wettable powder acts more slowly but has better persistence. It may be more effective in citrus growing areas with a low or moderate population density of D. citri because the infected D. citri can carry strain ZJPL08 for a longer time and aid in its transmission. Strain ZJPL08 can also be mixed or alternated with chemical pesticides when the population density of D. citri is high.
P. lilacinum (formerly Paecilomyces lilacinus) is an important bio-control fungus that can control many species of nematodes, such as Meloidogyne spp. and Heterodera spp [Citation41–45]. Many formulations of P. lilacinum have been commercialized for the control of agricultural pest nematodes. For example, P. lilacinus strain 251, marketed by Intrachem Bio. Italia under the trade-name ‘BioAct’ and by Certis USA as ‘MeloCon’, was introduced as an agricultural bionematicide in the United States after several years of commercial use in southern Europe [Citation46–49]. In addition, P. lilacinum can parasitize a variety of insects, such as thrips, cotton bollworm, litchi stink bug, leafhoppers and the brown planthopper [Citation50–54]. It has antagonistic effects on Sclerotinia sclerotiorum (Lib.) de Bary, Botrytis cinerea Pers., Colletotrichum lagenarium (Pass.) Ell.& Halst, Fusarium graminearum Schw. and other plant pathogens [Citation55–57]. In addition, the metabolites of P. lilacinum can promote seed germination and plant growth, and its various functional enzymes can help degrade certain pesticides [Citation58–61]. Strain ZJPL08 is not only pathogenic to D. citri, but also to citrus red mite and aphids, and is compatible with imidacloprid and other insecticides when used as a tank mix (our unpublished data). Therefore, strain ZJPL08 is expected to be used in commercial citrus orchards and nurseries for the control of D. citri and other pests. Owing to its uncomplicated production process, we believe that a large-scale production of the wettable powder is economically feasible.
Conclusions
In this study, we observed the infection process of P. lilacinum strain ZJPL08 on D. citri, prepared a P. lilacinum wettable powder formulation, and determined its efficacy. Currently, there are no microbial pesticides registered for the control of D. citri in China. We studied the possibility of using P. lilacinum strain ZJPL08 in a sustainable control for D. citri.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bové JM. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol. 2006;88:7–37.
- Keppanan R, Krutmuang P, Sivaperumal S, et al. Synthesis of mycotoxin protein IF8 by the entomopathogenic fungus Isaria fumosorosea and its toxic effect against adult Diaphorina citri. Int J Biol Macromol. 2019;125:1203–1211.
- Singerman A, Burani-Arouca M, Futch S. The profitability of new citrus plantings in Florida in the era of HLB. HortScience. 2018;53(11):1655–1663.
- Li W, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus Huanglongbing. J Microbiol Methods. 2006;66(1):104–115.
- Lewis-Rosenblum H, Martini X, Tiwari S, et al. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J Econ Entomol. 2015;108(1):3–10.
- Hall DG, Hentz MG, Meyer JM, et al. Observations on the entomopathogenic fungus Hirsutella citriformis attacking adult Diaphorina citri (Hemiptera: Psyllidae) in a managed citrus grove. BioControl. 2012;57(5):663–675.
- Tsai JH, Wang JJ, Liu YH. Seasonal abundance of the Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) in southern Florida. Fla Entomol. 2002;85(3):446–451.2.0.CO;2]
- Warnert JE. Asian citrus psyllid and Huanglongbing disease threaten California citrus. Calif Agr. 2012;66(4):127–130.
- Stansly P, Qureshi J. Controlling Asian citrus psyllids, sparing biological control. Citrus Ind. 2008;89:20–24.
- Meyer JM, Hoy MA, Boucias DG. Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. J Invertebr Pathol. 2008;99(1):96–102.
- Samson RA. Paecilomyces and some allied Hyphomycetes. Stud Mycol. 1974;6:1–119.
- Subandiyah S, Nikoh N, Sato H, et al. Isolation and characterization of two entomopathogenic fungi attacking Diaphorina citri (Homoptera, Psylloidea) in Indonesia. Mycoscience. 2000;41(5):509–513.
- Aubert B, Quilici S. Monitoring adult psyllas on yellow traps in Reunion Island. International Organization of Citrus Virologists Conference Proceedings (1957-2010). 1988;10(10):249–254. https://escholarship.org/uc/item/4mn0b3tj.
- Yang Y, Huang M, Beattie GAC, et al. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: a status report for China. Int J Pest Manage. 2006;52(4):343–352.
- Meyer JM, Hoy MA, Boucias DG. Morphological and molecular characterization of a Hirsutella species infecting the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), in Florida. J Invertebr Pathol. 2007;95(2):101–109.
- Lu L, Cheng B, Du D, et al. Morphological, molecular and virulence characterization of three Lencanicillium species infecting Asian citrus psyllids in Huangyan citrus groves. J Invertebr Pathol. 2015;125:45–55.
- Lu L, Cheng B, Du D, et al. Genetic diversity of Lecanicillium lecanii and its pathogenicity against Diaphorina citri. J Zhejiang Univ. 2015;41:34–43.
- Du DC, Lu LM, Hu XR, et al. Isolation and identification of Purpureocillium lilacinum and its pathogenicity against Diaphorina citri. Acta Agric Zhejiangensis. 2015;27:393–399.
- Du DC, You MQ, Hu XR, et al. Study on the biological characteristics of the Purpureocillium lilacinum strain ZJPL08 infecting Asian citrus psyllids. Zhejiang Citrus. 2014;31:17–21.
- Leemon DM, Jonsson NN. Comparative studies on the invasion of cattle ticks (Rhipicephalus (Boophilus) microplus) and sheep blowflies (Lucilia cuprina) by Metarhizium anisopliae (Sorokin). J Invertebr Pathol. 2012;109(2):248–259.
- Liu WM, Xie YP, Xue JL, et al. Histopathological changes of Ceroplastes japonicus infected by Lecanicillium lecanii. J Invertebr Pathol. 2009;101(2):96–105.
- Zhu M. Research on fungal biocontrol agents of the Hyphomycetes against green peach aphid Myzus persicae (Sulzer). Jinan (China): Shandong Polytechnic University; 2012. p. 13–19.
- Zhang L. Studies on the wettable powder processing and bioassay of Acremonium hansfordii. Lanzhou (China): Gansu Agricultural University; 2008. p. 25–29.
- Lu L, Yang M, Qin X, et al. Study on biological characteristics virulence and wettable powder of Verticillium lecanii from weevil on Dendrobium nobil. J Anhui Agric Sci. 2009;37:8042–8043, 8069.
- Zhu M, Tian L, Liu X. Screening of the formula for wettable powder of Beauveria bassiana with conidia. J Anhui Agric Sci. 2012;40:4560–4563.
- Pu ZL, Li ZZ. Insect mycology. Hefei (China): Anhui Science and Technology Press; 1996. p. 93–95.
- Keppanan R, Sivaperumal S, Hussain M, et al. Molecular characterization of pathogenesis involving the GAS 1 gene from entomopathogenic fungus Lecanicillium lecanii and its virulence against the insect host Diaphorina citri. Pestic Biochem Physiol. 2019;157:99–107.
- Bonants PJM, Fitters PFL, Thijs H, et al. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiology. 1995;141(4):775–784.
- Khan A, Williams K, Molloy MP, et al. Purification and characterization of a serine protease and chitinases from Paecilomyces lilacinus and detection of chitinase activity on 2d gels. Protein Expr Purif. 2003;32(2):210–220.
- Park JO, Hargreaves JR, Mcconville EJ, et al. Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Lett Appl Microbiol. 2004;38(4):271–276.
- Xie J, Li S, Mo C, et al. Genome and transcriptome sequences reveal the specific parasitism of the nematophagous Purpureocillium lilacinum 36-1. Front Microbiol. 2016;7:1–17.
- Sharififar M, Mossadegh MS, Vazirianza B. Effects of temperature and humidity on the pathogenicity of the entomopathogenic fungi in control of the house fly, Musca domestica L. (Diptera: Muscidae) under laboratory conditions. J Entomol. 2012;9(5):282–288.
- Meyling NV, Pell JK. Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol Entomol. 2006;31(2):162–171.
- Acheampong MA, Coombes CA, Moore SD, et al. Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J Invertebr Pathol. 2020;174:107436.
- Bahadur AB. Entomopathogens: role of insect pest management in crops. Trend Hortic. 2018;1(3):1–9.
- Gul HT, Saeed S, Khan FZA. Entomopathogenic fungi as effective insect pest management tactic: a review. Appl Sci Bus Econ. 2014;1:10–18.
- Cagáň L, Švercel M. The influence of ultraviolet light on pathogenicity of entomopathogenic fungus Beauveria bassiana (Balsamo) vuillemin to the European corn borer, Ostrinia nubilalis HBN. (Lepidoptera: Crambidae). J Cent Eur Agric. 2001;2:227–233.
- Fernandes ÉKK, Rangel DEN, Braga GUL, et al. Tolerance of entomopathogenic fungi to ultraviolet radiation: a review on screening of strains and their formulation. Curr Genet. 2015;61(3):427–440.
- Kaiser D, Bacher S, Mène-Saffrané L, et al. Efficiency of natural substances to protect Beauveria bassiana conidia from UV radiation. Pest Manag Sci. 2019;75(2):556–563.
- Acheampong MA, Hill MP, Moore SD, et al. UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biol. 2020;124(5):304–310.
- Xiao S, Zhang SS, Liu GK. Control effect of Paecilomyces lilacinus on Meloidogyne spp. J Fujian Agric For Univ. 2006;35:463–465.
- Sun MH, Liu XZ, Jin ZB. Effects of Paecilomyces lilacinus on egg hatching and juvenile mortality of Heterodera glycines. Acta Phytophy Sin. 2002;29:57–61.
- Wang J, Huang JS, Deng GP, et al. Stress resistance characteristics of the PL04 strain of Paecilomyces lilacinus and parasitism effect of it on Meloidogyne spp. Acta Agric Boreali-Occidentalis Sin. 2009;18:263–267.
- Moosavi MR, Ghani M. The optimal concentrations of Purpureocillium lilacinum and jasmonic acid in controlling Meloidogyn javanica on tomato. Arch Phytopath Plant Protect. 2019;52(7–8):582–600.
- Giannakou IO, Tasoula V, Tsafara P, et al. Efficacy of Purpureocillium lilacinum in combination with chitosan for the control of Meloidogyne javanica. Biocontrol Sci Technol. 2020;30(7):671–684.
- Dimock MB. Commercial introduction of bionematicides based on Paecilomyces lilacinus. J Nematol. 2009;41(4):325.
- Tranier M-S, Pognant-Gros J, Quiroz RDLC, et al. Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Braz Arch Biol Technol. 2014;57(6):831–841.
- Giné A, Sorribas FJ. Effect of plant resistance and BioAct WG (Purpureocillium lilacinum strain 251) on Meloidogyne incognita in a tomato-cucumber rotation in a greenhouse. Pest Manag Sci. 2017;73(5):880–887.
- Dahlin P, Eder R, Consoli E, et al. Integrated control of Meloidogyne incognita in tomatoes using fluopyram and Purpureocillium lilacinum strain 251. Crop Prot. 2019;124:1–7.
- Hotaka D, Amnuaykanjanasin A, Maketon C, et al. Efficacy of Purpureocillium lilacinum CKPL-053 in controlling Thrips palmi (Thysanoptera: Thripidae) in orchid farms in Thailand. Appl Entomol Zool. 2015;50(3):317–329.
- Lopez DC, Sword GA. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol Control. 2015;89:53–60.
- Xie Q, Liang G, Lu Y. Field efficiency of Paecilomyces lilacinus against litchi stinkbug Tessaratoma papillosa Drury. Wuyi Sci. 2002;18:143–145.
- Rombach MC, Aguda RM, Shepard BM, et al. Infection of rice brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae), by field application of entomopathogenic Hyphomycetes (Deuteromycotina). Environ Entomol. 1986;15(5):1070–1073.
- Debnath S. Occurrence of native entomogenous fungus Paecilomyces lilacinus (Thom) Samson on eggs and larvae of bunch caterpillar (Andraca bipunctata). Two Leaves A Bud. 1998;45:24–25.
- Elsherbiny EA, Taher MA, Elsebai MF. Activity of Purpureocillium lilacinum filtrates on biochemical characteristics of Sclerotinia sclerotiorum and induction of defense responses in common bean. Eur J Plant Pathol. 2019;155(1):39–50.
- Wang M, Zhou H, Fu Y, et al. The antifungal activities of the fungus 36-1 to several plant pathogens. Chin J Biol Control. 1996;12:20–23.
- Liu R, Khan RAA, Yue Q, et al. Discovery of a new antifungal lipopeptaibol from Purpureocillium lilacinum using MALDI-TOF-IMS. Biochem Biophys Res Commun. 2020;527(3):689–695.
- Xia H, Liao M, Zhou J, et al. Research on an analogous plant auxin production from Paecilomyces lilacinus PL-HN-16. J Northwest A&F Univ. 2011;39:97–102.
- Li F, Huang S, Liu B. Degradation of Phoxmi by Paecilomyces lilaciuns. Chin J Appl Environ Biol. 2006;12:104–107.
- Sarven MS, Aminuzzaman FM, Huq ME. Dose-response relations between Purpureocillium lilacinum PLSAU-1 and Meloidogyne incognita infecting brinjal plant on plant growth and nematode management: a greenhouse study. Egypt J Biol Pest Co. 2019;29(26):1–9.
- Moreno-Salazar R, Sánchez-García I, Chan-Cupul W, et al. Plant growth, foliar nutritional content and fruit yield of Capsicum chinense biofertilized with Purpureocillium lilacinum under greenhouse conditions. Sci Hortic. 2020;261:108950.
