Abstract
To assess the current regulatory frameworks of cannabidiol-containing products/preparations on the European market, we analyzed data gathered from 19 European countries using the functionalities of the computer software EPITT (European Pharmacovigilance Issues Tracking Tool). In addition, we investigated pharmacists’ knowledge, awareness and attitudes toward cannabidiol (CBD) in Bulgaria. We used questionnaires covering various domains including availability of CBD products or preparations, some of their general characteristics (dosage forms, CBD and THC content, safety profile, etc.) and use for medical conditions in accordance with the countries’ national regulatory requirements. The results indicate three main approaches for the application of CBD: (1) according to its licensed therapeutic indications (Sativex®, Epidiolex®); (2) for additional specific indications (drugs, magistral or standardized preparations) and (3) as an Investigational New Drug or for supplementation. The results for CBD on the pharmacy market in Bulgaria (2019) showed that it is sold as a food supplement in various formulations (oral drops, oils, sprays, capsules, etc.) but is also used for one or more medical conditions. Top three are: pain, anxiety and cancer. Good tolerance was reported in 96% of the cases. Bulgarian pharmacists showed a good knowledge of the differences between Marijuana and industrial hemp, and are well aware of the THC content in the available hemp-derived products. In conclusion, a compelling rationale exists for further scientific research and legal regulation in order to better use the therapeutic value of the non-psychotropic cannabidiol for the treatment of many medical conditions.
Introduction
The market of medical cannabis has been exuberantly growing to the extent of being outlined as a top number-one trend of 2019 in the pharmaceutical industry [Citation1–3]. The most relevant driver of growth by far appears to be regulatory change, and particularly reductions in restrictions on the use of cannabinoid products. Prominent political and social debates still exist about its use and regulations [Citation4].
The term ‘Industrial hemp’ is often used in order to refer to the cultivar of Cannabis sativa containing low concentration of the main psychoactive cannabinoid Δ9-tetrahydrocannabinol (THC). According to pharmaceutical standards, medicinal cannabis has been standardized in relation to its consistent composition of cannabinoids and terpenes. A variety of hemp legally cultivated in Western Europe has been ‘Fedora 17’ with a consistent cannabinoid profile of CBD around 1.5–3%, and THC less than 0.2%. However, legal criteria for hemp are not defined under European Union law and limitations on THC content may differ from country to country. In contrast, in the United States of America, hemp is legally defined as any part of the Cannabis plant, whether growing or not, which contains THC in concentration of no more than 0.3% on a dry-weight basis, and has CBD exceeding 10% by dry weight [Citation5,Citation6].
The flourishing medical cannabis industry in recent years is thought to be related to the green light that the regulatory bodies of pharmaceutics gave to the first approved pure cannabidiol product Epidyolex® in 2019. This has fuelled optimism for many more cannabis-based therapy approvals yet to come [Citation1]. Cannabidiol is unique as the main non-psychoactive phytocannabinoid in the Cannabis plant. It has been evaluated as a well-tolerated compound with a good safety profile [Citation7]. The interest in its medical applications has been related to its claimed use to treat symptoms of chronic pain, cancer, depression, anxiety, sleep disorders and neurological diseases [Citation8]. Cannabidiol is classified by the US Food and Drug Administration (FDA) as an Investigational New Drug (IND) [Citation9], and has been extensively investigated as such in recent reports of the World Health Organization (WHO) [Citation7,Citation10].
The aim of this study was to analyze the current regulatory frameworks of the CBD-containing products/preparations on the European market. In this context, our survey assessed the use of the available CBD-containing products in Bulgaria and pharmacists’ knowledge of the phytocannabinoids.
Methods
Data analyses
We used the European Medicine Agency’s software EPITT (European Pharmacovigilance Issues Tracking Tool) in order to gather information about the available CBD-containing products and their regulation in the European Union member states (2019). The countries included in our study were chosen on a random principle using EPITT’s functionalities that allow data exchange between up to 20 National Competent Authorities. We analyzed the obtained data according to the products’ regulatory status and medical use, including off-label use. We provide processed detailed country-to-country information about the authorized medicinal products, the licensed products and others that exist in the European countries, as well as evaluation of the main approaches for use of CBD.
Survey
As a next step, we conducted a questionnaire survey among 101 Master of Pharmacy (MPharm) professionals (pharmacy managers) in Bulgaria working in the community setting. The answers were collected over a period of six months (August to September 2019). The targeted pharmacies were situated in the most important regional cities, including the capital Sofia, Plovdiv and Varna. The questionnaires were distributed on a random principle to the pharmacies and afterwards repeated as online questionnaires. No changes in the answers were detected during the review of the online survey. The questionnaires consisted of 13 different domains, associated with market supply and demand of CBD in pharmacies, types of available products, their use for supplementation or medical conditions, safety hazards and drug interactions. Response options were captured by ‘True’ or ‘False’ statements in regards to the medical use for various conditions; by ‘Positive’ or ‘Negative’ to capture pharmacists’ attitudes towards CBD supplementation and by ‘Yes’ or ‘No’ in regards to the other questions. The results were processed with Google Sheets and MS Office for Windows 10. Statistical analyses for accuracy were conducted with the IBM statistical package for Social Sciences SPSS v.17.
Results and discussion
Availability of CBD-containing products on the European market
Despite the skyrocketing of medical cannabis many controversies still exist in regards to its regulations and applications. Only in November 2019, the National Institute for Health and Care Excellence (NICE) in Great Britain issued guidance on the use of the cannabinoids across a range of medical conditions [Citation11,Citation12]. Currently, there is no available classification of the cannabinoids on the basis of their therapeutic use. presents the available CBD-containing products/preparations in 19 European countries, divided by us into four groups on the basis of their different regulatory status, pharmaceutical procedures and applications: approved drugs (Sativex®, Epidiolex®), magistral preparations, standardized preparations and CBD as an investigational new drug. The synthetic cannabinoids dronabinol and nabilone are not included as they are commonly available by a compassionate use permit and usually applied in accordance to their approved indications, thus differing from the phytocannabinoids.
Table 1. Cannabidiol-containing products on the market in European countries.
Sativex®, a medicinal drug containing nabiximols with equal parts of THC (27 mg/mL) and CBD (25 mg/mL), is a formulated extract from Cannabis, approved to treat moderate to severe spasticity due to multiple sclerosis. It is authorized in many European countries as a narcotic drug [Citation13], but has not been authorized in the United States of America. Epidyolex®/Epidiolex®, containing pure cannabidiol, documented as the first non-psychoactive plant-derived cannabis compound, has been authorized in June 2018 by FDA and in September 2019 by EMA. Its approved indication is with the orphan designation for rare and severe forms of epilepsy with an early onset age in children (Dravet syndrome and Lennox–Gastaut syndrome) [Citation14].
Magistral preparations (MPs), namely extemporaneous CBD forms, are prepared by pharmacists, according to a specified medical prescription for an individual patient. In many cases, medical doctors prescribe these medications for ‘off-label use’ and, of course, doctors possess the therapeutic freedom to do so. The standardized preparations (SPs) Bediol® (granulate) and Tilray® (oral solution) have been introduced in some European countries since 2007. Bediol® is obtained by manufacturers from the cultivar C. sativa L. ‘Elida’, developed specifically with a higher CBD content and a balanced ratio of CBD (8%) and THC (6.3%) [Citation15]. Tilray® oil contains equal parts of CBD and THC (10 mg/mL) [Citation16]. Nowadays, Cannabidiol is accepted as a food supplement, and also as an IND with a good safety profile for the treatment of many medical conditions [Citation4,Citation7–9].
The marketing and regulatory status of cannabidiol-containing products in 19 European countries outlined in prompted us to assess the pharmacists’ knowledge of CBD’s applications in Bulgaria.
Analysis of the approaches for medical use of CBD products
Regulatory procedures for authorization of new medicines take into consideration evidence of both clinical effectiveness and safety but many regulatory systems for pharmaceuticals may have schemes (often called ‘compassionate use programs’) that allow doctors to prescribe unauthorized medicines. Thus, on the basis of scientific evidence and substantial clinical investigations, CBD has been available in the list as an IND.
In , we present data analysis of the CBD-containing products/preparations in 19 European countries. As shown, the observed regulatory status of these products is not connected to the geographic regions. However, we may define three main approaches for medical use of the CBD-containing products/preparations. The first one is traditional: CBD products used according to their licensed therapeutic indications. Both Sativex® and Epidiolex® have been authorized in a number of countries and began to be used according to their licensed indications shortly after they had received approval. Sativex® has been regulated as a narcotic drug and has received authorization in 14 out of the 19 European countries in this study. In total, in nine countries it is currently not being marketed. However, in some of these countries (Czechia, Ireland, Netherlands, Slovakia and Slovenia) there is still a possibility for it to be made available for an individual patient through a special permit. On the other hand, Epidiolex®, because of its orphan designation, has been available to patients EU-wide, including in Iceland, Liechtenstein and Norway.
Table 2. Data analysis of the approaches for medical use of CBD-containing products in European countries.a
The second approach consists of licensed products being applied not only according to their approved indications, but also for additional indications. Such is the current use of Sativex® and Epidiolex® for neuropathic pain and intractable epilepsy, respectively.
The third approach, which we named specific, regards other CBD-containing products/preparations, and their medical use. These are applied as oral pharmaceutical forms by special schemes. The reason for that is the accumulation of scientific data for their pharmacological activity and awaited results from clinical trials. For example, doctors may prescribe unapproved CBD formulations for various medical purposes such as control of chronic/persistent pain or palliative/supportive care [Citation17]. Generally, these schemes mimic the regulatory approach for medicinal CBD products: they must be in oral formulations and must have a standardized CBD and THC content. In particular, according to European standards, the level of THC content must be no more than 0.2% (although it may slightly vary), or no more than 0.3% according to FDA. Today, various medical applications and models for oral or inhalation CBD are observed, as presented in .
Our comparative country-to-country analysis shows that access to non-psychotropic CBD for medical purposes is extensive. In Germany, the 2017 Act allowed Cannabis formulations to be prescribed for any life-threatening illness and for improvement of the quality of life of patients with severe diseases. Today, the governmental policy advocates for domestic and standardized production. The cannabis policy in Italy is associated with the application of pharmacologically active plant-origin Cannabis compounds based on doctors’ prescriptions for medical use: pain or cachexia due to cancer or HIV. Croatia provides greater access to medical cannabinoids than Czechia but lesser than Germany or the Netherlands [Citation4].
In regard to the limitations of THC content, in Hungary only products with content of THC < 10 mg/kg are accepted as licensed CBD; CBD supplements and novel foods derived from Cannabis seeds must be with a maximum THC content of less than 0.2 mg/kg of dry weight. In Norway, all drugs that contain any amount of THC used to be accepted as narcotics. But since 2019, there have been changes regarding the common use of CBD oils with less than 0.3% content of THC. According to the legislation in Sweden and the United Kingdom, CBD products may be divided into products without pharmacological effects used for supplementation, and such with pharmacological activity used for medical purposes. Thus, the use of CBD as an IND is reasonable on the basis of extensive scientific data. In Austria, CBD was introduced in 2019 as IND only. Some countries (Slovakia, Portugal) have restricted CBD use, or limited it only to magistral preparations (Finland, Slovenia).
The analysis presented above shows that considerable variations between countries exist, but policies for the medical use of cannabinoids remain in place based on the established rules and restrictions.
Study: CBD in Bulgaria
We investigated CBD as a supplement in three aspects: availability of CBD products on the pharmacy market, their use for medical conditions and pharmacists’ knowledge, awareness and attitudes towards CBD.
CBD on the pharmacy market
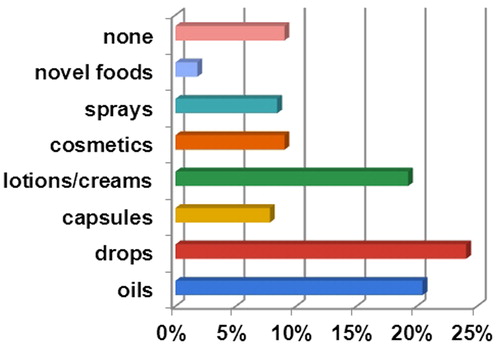
CBD supplements and novel foods became available freely in the community setting over a short period in 2019, identical to the period of conducting of our study. The portfolio of CBD products included various formulations, the most common one being oral drops (24%), followed by oils, lotions or creams (both near 20%) and sprays (approx. 10%) (). CBD products were not available in less than 10% of the pharmacies included in this survey. In Bulgaria, magistral preparations of Cannabis are not permitted and a clear strategy does not exist for the use of medicinal cannabis, and respectively for the use of CBD as an IND for medical conditions.
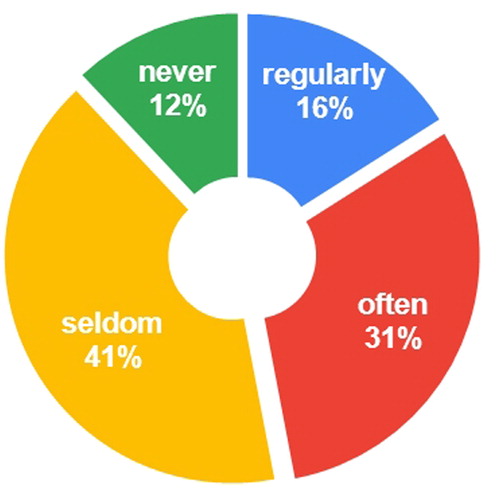
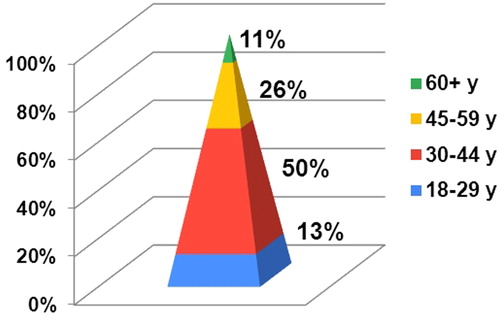
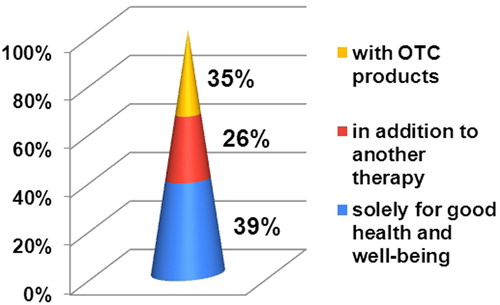
In our survey, 88% of the surveyed MPharms reported that patients were looking for CBD products regularly, often or seldom, while only 12% of the pharmacists had never encountered such a demand (). This observation suggests that there is a demand for CBD products in the pharmacies in Bulgaria. Half of the adults looking for CBD products were between 30 and 44 years old, followed by adults between the ages of 45 and 59 (26%) (). Cannabidiol was used solely for good health and well-being in 39% of the cases (). In the rest of the cases, patients with one or more underlying medical conditions used oral CBD simultaneously with OTC products (35%) or in addition to another drug therapy (26%). The observed disproportion illustrates the key role of pharmacists in having knowledge about pharmacologically active CBD and advising patients accordingly.
Analysis of the medical use of CBD
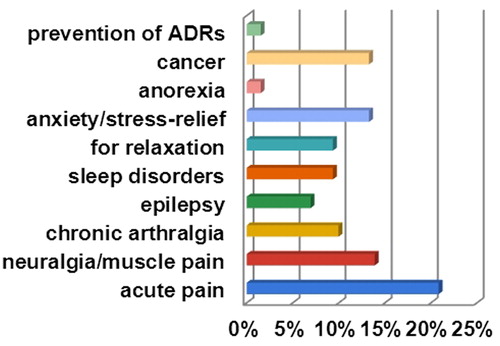
The results for the medical use of CBD in Bulgaria show that it is used most commonly for pain, cancer and anxiety (10–21%) (). Patients also use it for sleep disorders, for relaxation and epilepsy (7–9%). Only 2% reported use of CBD for anorexia or prevention of unwanted effects related to another therapy. These results correspond to the medical use of CBD in other European countries, although in some of these countries the emphasis is on pharmacologically active products/preparations and their prescription by a licensed medical doctor. In the USA, various formulations of CBD supplementation are widely used by patients for different medical conditions [Citation18].
The medical use of CBD for the above-mentioned conditions is relevant on the basis of data from many preclinical and clinical studies. CBD-based analgesia is potent on the basis of CBD proven modulatory effect and possibility to influence the opioid receptors in higher doses [Citation19,Citation20]. CBD affects central neural activities, including serotonin, acetylcholine and GABA receptors, and thus has a potential to influence anxiety, depression and other neurological disorders. Anti-inflammatory and antioxidant activity is observed in some investigations [Citation21,Citation22], and also in our current research (unpublished data). In addition, experimental studies of CBD show anti-proliferative, anti-invasive and anti-metastatic activity in many tumour models [Citation23,Citation24].
Referring to CBD’s pharmacological activity, it is well-known that it possesses a wide range of protein-dependent and protein-independent mechanisms of action. CBD shows different biological activities depending on the doses. Experimentally, it is shown that the concentration range of 0.1–5.0 μmol/L influences cell signalling through CB1 receptors [Citation25]; at 3–30 μmol/L, it acts on the TRPV1 ion channels [Citation26] and at 1–30 μmol/L, it affects the activity of GPR55 [Citation27]. At 100 μmol/L, CBD acts on the opioid μ receptors [Citation19]. On the basis of these data, we suppose that many efforts will be needed to differentiate between the dosage regimens for different medical conditions.
Pharmacists’ knowledge
We assessed pharmacists’ knowledge provided anonymously in three aspects: content of CBD products, associated unwanted effects and awareness of CBD supplementation.
As shown in , three-fourths of the pharmacists have a good knowledge about industrial hemp, and more than half of them are well aware of the established limitations of THC content for CBD products in Europe and the USA.
Figure 6. Pharmacists’ knowledge, awareness and attitudes towards CBD. Note: In red (dark): No/Negative; in blue (light): Yes/Positive.
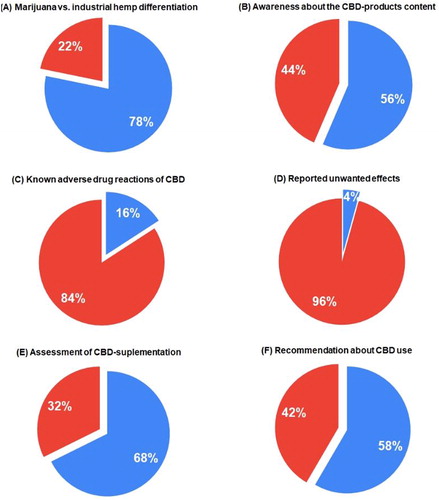
In relation to unwanted effects, 84% of the pharmacists identified adequately the possible side effects of pharmacologically active cannabidiol. The number of unwanted effects associated with CBD use reported by patients to MPharms was as low as 4% (). These data confirm that generally cannabidiol is a well-tolerated substance with a good safety profile, as shown by other authors [Citation28]. According to a report of the Expert Committee on Drug Dependence, adverse effects in many cases may be due to drug–drug interactions between cannabidiol and concomitant medications [Citation7]. There are limited studies of CBD interactions with other drugs. In the field of experimental oncology, antagonistic interactions have been proved between BCNU and cisplatin when applied simultaneously with CBD [Citation29].
The recommendations of the majority of the surveyed pharmacists (67%) in favour of cannabidiol were principally correct, even though they concern only oral supplementation (). In the survey, 58% of the pharmacists stated they would give a positive recommendation for the use of non-prescribed cannabidiol to their patients ().
The results show that Bulgarian MPharms have a good general knowledge for cannabidiol but are not confident in its adequacy because of the lack of supporting data from clinical trials for many conditions. In this aspect, we believe that the topic of medical cannabis should be covered in the pharmacy curricula, as suggested by Corroon and Phillips [Citation18].
Conclusions
In general, in Europe access to medicinal cannabis products including magistral preparations is often restricted to the individual patient by a strict case-to-case evaluation, and use is limited only to the few approved indications. The observed trend is for specific control exerted by each country’s National Competent Authority, especially regarding the use of non-psychotropic CBD as an investigational new drug and/or supplement. In Bulgaria, the medical cannabis market has been associated only with CBD as supplementation. Despite that, Bulgarian pharmacists show good general knowledge about the cannabinoids. We believe that the presented real-world evidence associated with the cannabidiol-containing products provides a compelling rationale for a better clinical and regulatory decision-making for the use of these products.
Acknowledgements
This study was initiated by the Bulgarian Drug Agency. The authors thank the National Competent Authorities of the European Member States for the received data about the availability and use of cannabidiol-containing products in these countries.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Top 5 trends in the pharmaceutical industry in 2019. MasterControl Inc.; [cited 2019 Nov]. Available from: https://www.mastercontrol.com/learning-center/pharmaceutical/top-5-trends-in-the-pharmaceutical-industry-in-2019
- Medical marijuana market worth over $55 billion by 2024: Global Market Insights, Inc. GlobeNewswire; [cited 2018 Aug 22]. Available from: https://globenewswire.com/news-release/2018/08/22/1555007/0/en/Medical-Marijuana-Market-worth-over-55-billion-by-2024-Global-Market-Insights-Inc.html
- The global cannabis market continues to grow as medical cannabis legalization spreads. Cision, PR Newswire; [cited 2018 Nov 13]. Available from: https://www.prnewswire.com/news-releases/the-global-cannabis-market-continues-to-grow-as-medical-cannabis-legalization-spreads-816722673.html
- Medical use of cannabis and cannabinoids: Questions and answers for policymaking. EMCDDA, Lisbon; [cited 2018 Dec]. Available from: https://www.emcdda.europa.eu/system/files/publications/10171/20185584_TD0618186ENN_PDF.pdf
- Mead A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front Plant Sci. 2019;10:697–614.
- Marinotti O, Sarill M. Differentiating full-spectrum hemp extracts from CBD isolates: implications for policy, safety and science. J Diet Suppl. 2020;17(5):517–526.
- CANNABIDIOL (CBD) Critical Review Report Expert Committee on Drug Dependence Fortieth Meeting Geneva, 4–7 June 2018. Available from: https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf
- The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. National Academies Press for the National Academies of Sciences Engineering and Medicine, Washington, DC; 2017.
- Office of the Commissioner. Public Health Focus—FDA and Marijuana: Questions and Answers. 2018. Available from: https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm421168.htm
- CANNABIDIOL (CBD) Pre-Review Report Agenda Item 5.2 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6–10 November 2017. Available from: https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf
- Wiffen P. The cannabis conundrum. Eur J Hosp Pharm. 2020;27(1):1. [32064080]
- NICE. NICE guidance on cannabis based medicinal products. Available from: https://www.nice.org.uk/guidance/ng144
- Medicinal cannabis and derivatives: a legal analysis of the options, their limitations, and current practice in the EU. EMCDDA, ELDD Comparative Study, May 2002. Available from: http://eldd.emcdda.org/databases/eldd_comparative_analyses.cfm
- Monograph Epidyolex® [cannabidiol] for seizures associated with Lennox-Gastaut syndrome [adjunctive treatment with clobazam] (specialist use only), and seizures associated with Dravet syndrome [adjunctive treatment with clobazam] (specialist use only). Joint Formulary Committee. British National Formulary (online), London. BMJ Group and Pharmaceutical Press; 2020. Available from: https://about.medicinescomplete.com/publication/british-national-formulary/
- Specification sheet (version: January 2019. Product: Cannabis flos, ssp. sativa, variety Bediol (hemp flowers). Office of Medicinal Cannabis. CIBG Ministerie van Volksgezondheid, Welzijn en Sport. Available from: https://english.cannabisbureau.nl/
- Tilray Medical Cannabis Products Card. Tilray; 2020. Available from: https://www.tilray.ca/en/drops
- Krcevski-Skvarc N, Wells C, Häuser W. Availability and approval of cannabis-based medicines for chronic pain management and palliative/supportive care in Europe: a survey of the status in the chapters of the European Pain Federation. Eur J Pain. 2018;22(3):440–454.
- Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3(1):152–161.
- Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(5):354–361.
- Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018; 19(3):833.
- Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16(1):30–42.
- Do Val-da Silva RA, Peixoto-Santos JE, Kandratavicius L, et al. Protective effects of cannabidiol against seizures and neuronal death in a rat model of mesial temporal lobe epilepsy. Front Pharmacol. 2017;8:131. [28367124]
- Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75(2):303–312.
- Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–1172.
- Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–4805.
- Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131–1141.
- Piñeiro R, Maffucci T, Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30(2):142–152.
- Fasinu PS, Phillips S, ElSohly MA, et al. Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy. 2016;36(7):781–796.
- Deng L, Ng L, Ozawa T, et al. Quantitative analyses of synergistic responses between cannabidiol and DNA-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J Pharmacol Exp Ther. 2017;360(1):215–224.