?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Strain CG-9, isolated from human saliva and identified as Enterococcus faecalis, produced a component that inhibits the growth of Gram-positive and Gram-negative bacteria. The active peptide from the cell-free supernatant of Enterococcus faecalis CG-9 was purified in three steps: (1) precipitation with 70% saturated ammonium sulfate: (2) cation-exchange chromatography, (3) gel filter chromatography. The purified bacteriocin exhibited a wide range of antimicrobial activity against many food-borne spoilage and Pathogenic bacteria such as Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, as well as some yeast. The bacteriocin was heat stable, pH resistant and protease sensitive. Ethylenediaminetetraacetic acid, sodium dodecyl sulfate, Tween 80 and 6% sodium chloride did not decrease the activity. The characterization, antimicrobial spectrum and molecular mass of this bacteriocin suggested that it was a novel bacteriocin with potential research and application value in food industry.
Introduction
Food contaminated by pathogens and spoilage bacteria or their toxins can lead to disease or even death [Citation1]. They can be widely spread in the globalized world. Therefore, foodborne pathogens pose a great threat to public health [Citation2]. In addition, the abuse of antibiotics also promotes the development of drug-resistant bacteria. There is no new class of antibiotics which has entered clinical practice in over two decades except oxazolidinones and lipopeptides, which only target Gram-positive pathogens [Citation3]. The emergence of antibiotic resistant pathogens aggravates the difficulty of treatment. Controlling food spoilage and foodborne pathogens is a great challenge.
The main methods used to control bacteria include pasteurization, ultrasonic treatment, high pressure and chemical preservatives [Citation4]. In addition to physical and chemical methods, there are biological methods. The biological method is the most promising method. Biological methods mainly include antibacterial natural products (such as antimicrobial peptides, phenols) and phages [Citation5]. Lactic acid bacteria are the producers of antibacterial metabolites, which have attracted extensive attention.
Lactic acid bacteria can inhibit pathogens by competitive exclusion (nutrition and space) [Citation6], and produce antibacterial substances, such as organic acids, hydrogen peroxide, bacteriocin, etc [Citation7]. Bacteriocin has been regarded as a substitute for preservatives and antibiotics [Citation8]. Such as fruit juices [Citation9], canned fruit and vegetable foods [Citation10], canned food and coconut milk [Citation11] and cooked ham [Citation12]. In recent years, a variety of bacteriocins have been found and studied, such as enterocin faecium R.A5 [Citation13], lactocin lactis W2 [Citation14], plantaricin LPL-1 [Citation15], paraplantaricin L-ZB1 [Citation16]. Thus, lactic acid bacteria are the most promising method to control foodborne pathogens and food spoilage bacteria.
There are few studies on the existence and application of lactic acid bacteria in human saliva. This study aimed to screen bacteriocin-producing bacteria with antimicrobial activity against foodborne pathogenic bacteria and putrefactive bacteria. We report the purification and characteristics of a novel bacteriocin.
Materials and methods
Materials
Enterococcus faecalis CG-9 was isolated from saliva of volunteers in the College of Life Science and Technology, Guangxi University. The strain was cultured in MRS liquid medium for bacteriocin-producing bacteria. Escherichia coli (CMCC440102) was grown in LB liquid culture medium and was used as the indicator strain to measure bacteriocin activity.
Identification of strain
Strain CG-9 was characterized and identified on the basis of its morphological, biochemical and genetic properties [Citation17]. The cell morphology and Gram-staining reaction were examined by light microscopy. Then the catalase and oxidase activity was measured. Phenotypic identification was based upon biochemical characteristics, including the ability to grow at 10, 20, 37 and 45 °C, pH 3 to 10, in the presence of 6% (w/v) NaCl and in the presence of 0.5% (w/v) bile salts. The genomic DNA of strain CG-9 was extracted and amplified by using primers (5′-ACAG/LTTTG/LATCCTG/LG/LCTCAG/L-3′ and 5′-TACG/LG/LCTACCTTG/LTTACG/LACTT-3′) to obtain about 1500 bp DNA sequence. Sequences were submitted to the National Center for Biotechnology Information (www.ncbi.nlm.nlh.gov) for similarity searches. After pH, catalase and protease experiments, the antibacterial substance was identified as protein.
Bacteriocin activity assay
Strain CG-9 was grown in MRS broth for 15 h at 37 °C and the culture was centrifuged (10,000 g, 10 min, 4 °C) to obtain the crude cell-free supernatant (CFS). Neutralized cell-free supernatant (NCFS) was prepared by adjusting the pH of CFS to 6.5 using NaOH. The NCFS was tested for antibacterial activity against selected indicator bacteria (Escherichia coli CMCC44102, LB medium) by the agar well diffusion assay. Briefly, 10 mL of soft LB agar (0.8% agar) was seeded with about 1 × 106 CFU/mL of their corresponding indicator bacteria and poured into plates already containing a layer of the same agar medium on top of which were placed 8 mm diameter Oxford cups. After solidification of the soft agar lawn, the Oxford cups were removed, and each well received 50 µL of NCFS. The plates were incubated at the optimum growth conditions of the indicator strain, and the diameter of the clear zone around each well was recorded. The bacteriocin titer was determined and expressed as arbitrary units (AU/mL) using the formula:
where D is the dilution factor, and V is the volume of the NCFS spotted (50 µL).
One AU was defined as the reciprocal of the highest serial two-fold dilution showing a clear zone of growth inhibition of the indicator strain [Citation18].
Purified bacteriocin
Ammonium sulfate precipitation
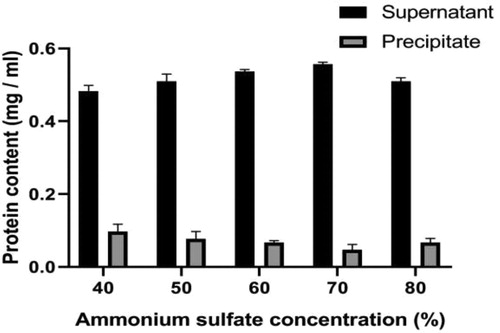
After centrifuging at 4 °C 10,000 g, 50 mL of the fermentation broth was added into 5 small beakers respectively. After coding, it was placed in an ice box and kept at a low temperature. Different amounts of ammonium sulfate were added into five small beakers to make the final saturated concentration of ammonium sulfate reach 40%, 50%, 60%, 70% and 80% respectively. After continuous stirring, ammonium sulfate was fully dissolved. Following centrifugation at 4 °C 10,000 g for 10 min, the protein content in the supernatant was measured after precipitation with different concentrations of ammonium sulfate, and the precipitates were resuspended with deionized water to determine the bacteriostatic effect. Finally, the optimal concentration of antibacterial substances precipitated by ammonium sulfate was 70%. Following centrifugation at 4 °C 10,000 g for 10 min, the precipitate was resuspended in deionized water, and then was put into a 1 kDa dialysis bag for 48 h; the water was changed every 6 h in the middle, and dialysis stopped when the salt ions were completely dialyzed. Finally, 20 mL of crude sample was obtained by vacuum freeze dryer.
Cation exchange chromatography
The column was equilibrated and washed with 50 mmol/L sodium phosphate buffer (pH 5). After the crude sample was added into the cation exchange chromatographic column, it was continuously eluted with 0.1 mol/L NaCl buffer eluent, and the eluent was collected at the same time. The elution rate was about 1 mL/min; one tube was taken for labeling every 5 mL. The protein content and antibacterial activity of each canal were determined. The active eluent was collected together and concentrated by vacuum freeze dryer for later use.
Gel filtration chromatography
Active fractions obtained by cation exchange chromatography were combined and applied onto a Superdex G-75 column, previously equilibrated with 20 mmol/L sodium phosphate buffer, pH 5 at a flow rate of 1 mL/min. The column was washed with the same buffer and the absorbance was recorded at 220 nm [Citation19]. Different peptide fractions were collected, respectively. And activity tests were performed by using the agar well diffusion method as described before. The active fractions were dialyzed, lyophilized and stored at −20 °C.
Protein concentration determination
Protein concentration for each sample at different steps of the purification was determined using the Pierce BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Beijing, China), as recommended by the supplier.
Molecular mass determination
The samples were analyzed by Tricine-SDS-PAGE polyacrylamide gel electrophoresis (15% separating gel, 5% concentrating gel). At the end of electrophoresis, the electrophoretic gel was dyed for 30 min. The dyed electrophoretic gel was put into the decolorizing solution. The decolorizing solution was changed every 1 h until clear electrophoretic bands appeared. Finally, the electrophoretic gel was photographed and analyzed.
Determination of antimicrobial spectrum
In order to investigate the bacteriocin produced by Enterococcus faecalis CG-9, eight strains of indicator bacteria were selected for bacteriostatic spectrum determination. (Bacillus subtilis CGMCC 1.3358, Staphylococcus aureus ATCC 700698, Listeria innocua BNCC128908, Listeria monocytogenes ATCC19111, Bacillus coagulans ATCC7050, Escherichia coli CMCC440102, Salmonella typhimurium ATCC14028, and Saccharomyces cerevisiae ATCC204508). All the strains used in this experiment were maintained at our laboratory.
Physico-chemical characteristics of bacteriocin
Effect of enzymes on bacteriocin activity
Pepsin, trypsin, proteinase K and other enzymes were dissolved in sterile water with a final concentration of 1 mg/mL, and adjusted to the optimum pH of each enzyme (Beijing SolarbioScience & Technology Co., Ltd.) with 2 mol/L HCI and NaOH solution. Pepsin, trypsin, proteinase K and other enzymatic solutions (lipase, catalase and amylase) were added to the purified bacteriocin samples separately. After 1 h in 37 °C water bath, the pH was returned to the original value, and the bacteriostatic activity assay was carried out after the samples were cooled down to room temperature. E. coli was used as the indicator bacterial species, and the diameter of the inhibition zone was used as a reference index to reflect the activity changes of bacteriocin samples treated by different enzymes. Three parallels were set for each group, with bacteriocin that had not been treated with enzymes as the control group.
Effect of temperature on bacteriocin activity
The bacteriocin samples after preliminary purification were placed at 50 °C, 60 °C, 70 °C, 80 °C and 90 °C for 30 min and 121 °C for 10 min, then they were taken out and cooled to room temperature for bacteriostatic test. Escherichia coli was used as the indicator bacteria and the diameter of the inhibition zone was used as a reference index to reflect the activity change of bacteriocin samples after heat treatment. Three parallels were set in each group, and the bacteriocin without high temperature treatment was used as the control group.
Effect of pH on bacteriocin activity
After the initial purification of bacteriocin samples, the pH was adjusted to 3, 4, 5, 6, 7, 8, 9, 10 [Citation19] with 2 mol/L HCl and NaOH solution, and then the pH was adjusted back to the original value for 1 h for antibacterial test. Escherichia coli was used as the indicator, and the diameter of the inhibition zone was used as the reference index to reflect the activity changes of bacteriocin samples treated with different acidity/alkalinity. There were three parallel measurements in each group, and the bacteriocin without acid treatment was used as the control group.
Effect of salt concentration on bacteriocin activity
Aliquots of 200 µL of purified bacteriocin sample were added to Eppendorf tubes (EP)tubes; 200 µL of salt solution (2% to 10% NaCl) was added to each tube; 200 µL of sterile water was added into one EP tube as a control sample. The bacteriostatic test was conducted after 1 h. E. coli was used as indicator and the diameter of the inhibition zone was used as a reference index to reflect the activity changes of bacteriocin samples treated with different concentrations of salt solution.
Effect of surfactants on bacteriocin activity
Three surfactants, sodium dodecyl sulfate (SDS), Tween-80 and ethylenediaminetetraacetic acid (EDTA), were selected to determine the effect on bacteriocin activity. SDS and Tween-80 were prepared with deionized water as 2%, 6% and 10% respectively, and EDTA was prepared as 2,6,10 mmol/L solution. Equal volumes of purified bacteriocin and surfactant solutions were mixed in EP tubes, in which the final concentration of the above surfactant was 1%, 3%, 5% or 1, 3, 5 mmol/L. The last group of bacteriocin samples were mixed with the same amount of sterile water to serve as the control group. After 1 h, the bacteriostatic experiment was carried out. Escherichia coli was used as the indicator, and the diameter of the inhibition zone was used as the reference index to reflect the activity changes of bacteriocin samples treated with different surfactants.
Results and discussion
Identification of strain CG-9
Eighteen strains were screened from human saliva; the basic characteristics showed that all strains belonged to Enterococcus and Lactobacillus. Strain CG-9 formed round and smooth colonies. It was identified as gram-positive, catalase negative and oxidase negative. It can survive at 10 °C to 45 °C, pH 9, 6% sodium chloride and 0.5% bile salt. The DNA sequence of strain CG-9 is 1500 bp. The similarity searches with sequences in the National Center for Biotechnology Information database revealed that the identity with Enterococcus faecalis MT516172.1 was the highest(99.9%). These results showed that strain CG-9 was Enterococcus faecalis and the bacteriocin produced by the strain was enterococcin.
In recent years, the research into bacteriocins has attracted much attention. Some bacteriocin-producing strains have been isolated from cheese, fermented sausage and other foods [Citation20,Citation21]. The bacteriocins produced by these strains showed strong antibacterial activity. In this study, we obtained a bacteriocin-producing Enterococcus faecalis CG-9, isolated from human saliva, whose bacteriocin showed strong bacteriostatic activity. When protease was added, it lost its activity.
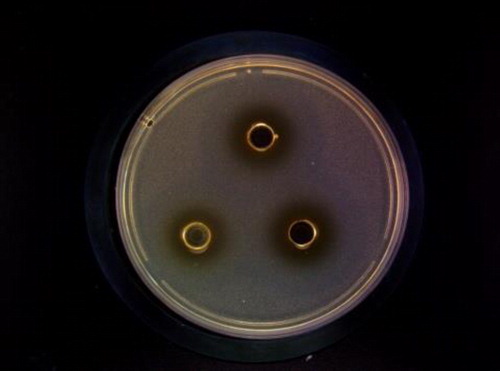
After eliminating the interference of acids and hydrogen peroxide, using the agar diffusion method and Staphylococcus aureus as the indicator bacteria, the bacteriocin produced by CG-9 formed an inhibition zone shown in . Strain CG-9 was the most effective strain; therefore, it was selected for further research. It was identified as Enterococcus faecalis.
Bacteriocin production
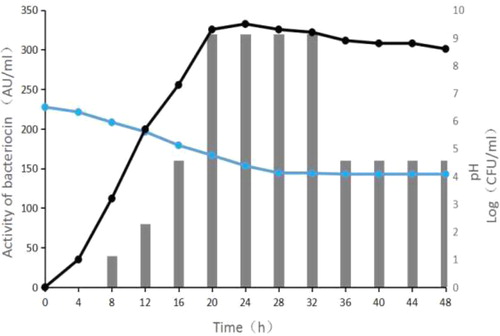
The growth curve of Enterococcus faecalis CG-9 at 37 °C without pH control is shown in . E. faecalis CG-9 began to produce bacteriocin at 8 h. The bacteriocin production increased during the exponential growth phase, reached a maximum of 320 AU/mL at 18 h, remained stable at 18–32 h during the stationary phase, and began to decrease after 32 h. This suggested that the production of bacteriocin depends on the cell density. At the same time, the pH of the medium decreased from 6.5 to 4.08. Bacteriocins are secondary metabolites and their production depends on the cell density [Citation22]. Similar results have also been observed in some bacteriocins produced by Lactic acid bacteria, such as bacteriocin FGC-12 [Citation23]. At the end of growth, the bacteriocin activity decreased due to the decrease in the number of viable cells, the decrease in pH or the secretion of protease [Citation24].
Purification of bacteriocin
The best concentration for salting out bacteriocin was 70% ammonium sulfate (). Ammonium sulfate with a concentration of 70% was used for salting out precipitation. After being desalted by a 1 kDa dialysis bag, it was concentrated in a vacuum freeze dryer. Finally, the activity of the concentrated antibacterial substance increased from 320 AU/mL to 5120 AU/mL, the yield was 32%, and the purification was 3.48 times. Compared with the ethyl acetate extraction method, the ammonium sulfate precipitation method can maintain the bacteriocin activity better and gives a higher yield [Citation25]. Therefore, in this study, we chose the ammonium sulfate precipitation method to extract bacteriocin.
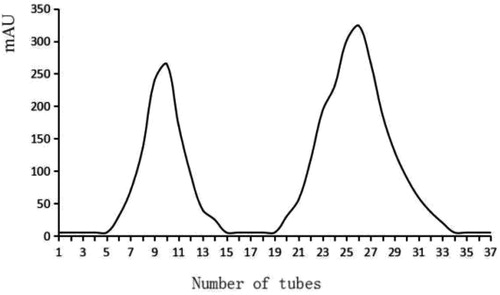
After 70% ammonium sulfate precipitation and 1 kDa dialysis bag dialysis desalination, the antibacterial substances were extracted and further purified by cation exchange chromatography. There were two absorption peaks in the ion exchange chromatography (). The samples with two absorption peaks were collected and the antibacterial activities of the two substances were detected respectively. The results showed that the first absorption peak had no antibacterial activity, which could be identified as a heteroprotein. The substance in the second absorption peak had antibacterial activity, indicating it to be a cationic peptide in nature [Citation26]. After concentration, the bacteriocin titer was 5120 AU/mL, the yield was 16%, and the purification was 16.68 times.
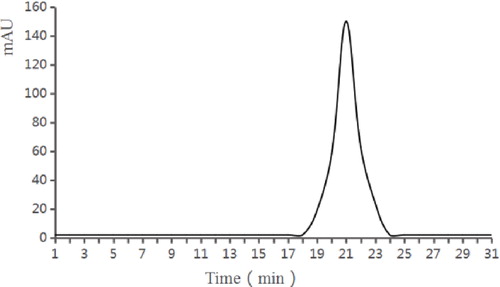
The purified crude samples were then purified by Superdex G-75 gel filtration chromatography. There was only 1 absorption peak in the gel chromatogram (). There was only 1 absorption peak in the gel chromatogram. The active substance of the peak was collected to detect the bacteriostatic activity, and it was identified as the bacteriocin sample. After concentration, the antibacterial activity was 10,240 AU/mL, the yield was 16%, and the purification was 34.37 times. The traditional methods for the isolation of bacteriocin from complex mixtures frequently involve complicated chromatographic separations [Citation27]. Although the process is complex, it is very effective [Citation28].
Determination of protein content
The fermentation broth of 1 L Enterococcus faecalis CG-9 was separated and purified by70% ammonium sulfate precipitation, cation exchange chromatography and Superdex G-75 gel filtration chromatography. The antibacterial activity and protein content were determined respectively, and the total activity, total protein, specific activity, yield and purification times were calculated. The results () showed that the yield was only 32% after ammonium sulfate precipitation. During ammonium sulfate precipitation, the bacteriocin produced by E. faecalis CG-9 lost a lot of its activity. The reason may be that the activity of some bacteriocin was lost after ammonium sulfate precipitation, or some bacteriocin residues were not completely collected in the beaker during salting out. After cation exchange chromatography, the specific activity of the sample was 8366.01 AU/mg, the purification was 16.68 times and the yield was 16%. The purity of the bacteriocin sample was improved and the yield was satisfactory. Finally, after Superdex G-75 gel filtration chromatography, the specific activity of bacteriocin reached 17239.06 AU/mg, the final yield was 16% and the purification was 34.37 times. After purification, the purity of the sample was greatly improved, which laid a foundation for the subsequent steps in this study. Although high purification magnitude was obtained, the final yield of bacteriocin was relatively low. Similar results have also been reported by other researchers during purification of plantaricin K25 [Citation29] and lactocin MM4 [Citation30]. The issue could be resolved by optimization of culture medium and growth conditions of the producer strain. Moreover, genetic engineering of bacteriocin-producing strains may result in the enhanced expression of the bacteriocin [Citation31].
Table 1. Bacteriocin purification results.
Following purification of the bacteriocin produced by E. faecalis CG-9 by the three-step method [Citation32], precipitation with 70% ammonium sulfate, ion exchange chromatography and Superdex G-75 gel filtration chromatography, the fractions were collected and checked by the agar diffusion assay. The obtained purification and the final yield were higher than others [Citation33].
Molecular mass determination
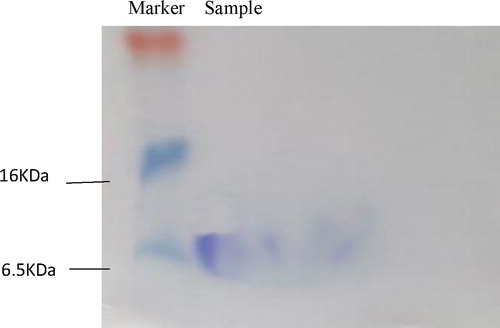
The purified bacteriocin samples collected from different steps of purification were analyzed by Tricine-SDS-PAGE. A single target band appeared (), indicating that the resultant bacteriocin is a single active peptide in nature. The molecular weight of the bacteriocin was about 6.5 kDa. It is consistent with many reports that the molecular weight of enterococcin is in the range of 3.5–6.5 kDa. This is within the range of molecular weights of some bacteriocins such as crude bacteriocin produced by Enterococcus faecalis RJ-11 isolated from rice bran [Citation34] and partially purified bacteriocins produced by Enterococcus faecalis isolated from mangrove forests in southern Thailand [Citation35]. Within this range of molecular weight, some bacteriocins such as Enterococcus faecium TJUQ1 with high bacteriocin-producing ability was isolated from pickled Chinese celery [Citation36]. A novel bacteriocin, PE-ZYB1, was produced by Pediococcus pentosaceus zy-B that was isolated from the intestine of Mimachlamys nobilis and collected from the South China Sea [Citation37]. They are all small molecule proteins in nature. To the best of our knowledge, this is the first report to screen for bacteriocin produced by Enterococcus faecalis from human saliva, suggesting that this bacteriocin is also novel.
Characteristics of bacteriocin
Enzyme stability
The changes of bacteriocin activity after treatment with pepsin, trypsin, protease K, lipase, catalase and amylase are shown in .
Table 2. Effect of enzyme on bacteriocin activity against E. coli.
It can be seen from that the bacteriocin produced by E. faecalis CG-9 lost its activity after being treated with pepsin, trypsin and protease K, while lipase, catalase and amylase did not affect the bacteriocin activity. This is consistent with the characteristics of bacteriocins reported by other scholars [Citation38,Citation39].
Temperature stability
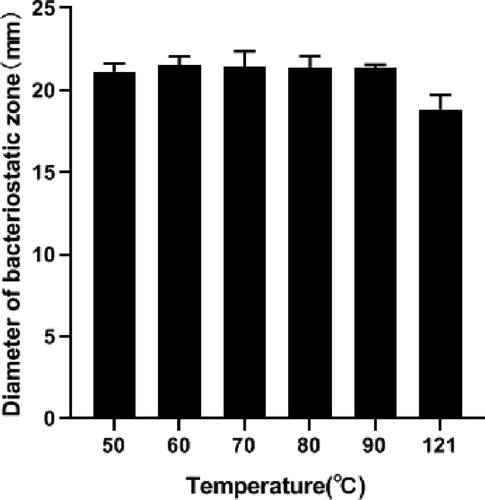
The activity of bacteriocin was not affected in the range of 50 °C–90 °C (). The activity of the bacteriocin was not completely lost after high temperature treatment at 121 °C for 10 min, and the bacteriocin still retained high antibacterial activity. Its antibacterial activity was not affected by temperature, which may be related to the disulfide bond of low molecular weight polypeptide. Some scholars pointed out that bacteriocins produced by Enterococcus have high temperature tolerance and high temperature resistance. For example, the good thermal stability of the bacteriocin produced by E. faecalis CG-9 is in agreement with the bacteriocins from E. faecium JCM 5804 T [Citation40] and E. faecium LM-2 [Citation41]. The effect of temperature on bacteriocin activity showed that enterococcin was heat-resistant. To our knowledge, enterolysin A retains 50% of its activity after treatment at 75 °C and is inactivated after treatment at 100 °C, helveticin J is inactivated after treatment at 100 °C for 30 min, and the activity of zoocin A is abolished in 20 min at temperature of 60 °C [Citation42]. The small amount of data limits the possibility of comprehensive comparison of our bacteriocin with other bacteriocins. However, our study revealed that it retains practically 100% activity after treatment at 90 °C. High activity was also maintained after heat treatment at 121 °C.
pH stability
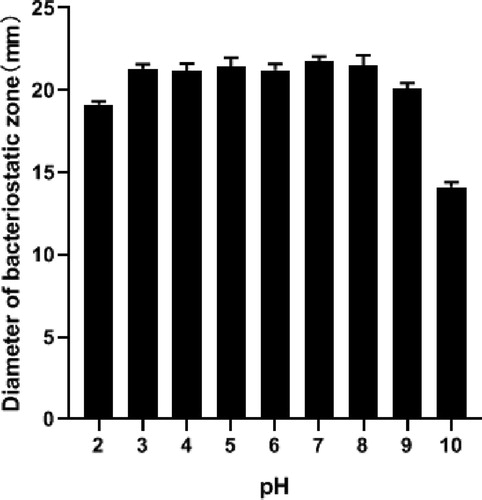
The bacteriostatic activity of the bacteriocin was not affected by pH in the range of pH 3 to 9 (). At pH 2, the bacteriocin activity decreased, i.e. it did not inhibit the indicator bacteria. The results showed that the bacteriocin produced by Enterococcus had good acid and alkali resistance. This bacteriocin remained relatively stable at pH 3–9. However, plantaricin GZ1-27 lost most of its activity at pH 7.0 and was fully inactive at pH values > 8.0 [Citation43]. This result was similar to previous findings; neutral and alkaline conditions readily inactivate most bacteriocins, including nisin, plantaricin JLA-9 [Citation44], and bacteriocin R1333 [Citation45]. The effect of pH on bacteriocin activity showed that enterococcin was found active over a wide range of pH from 3 to 9. This will lay a foundation for its application in acidic environment and weak alkaline environment.
Salt concentration
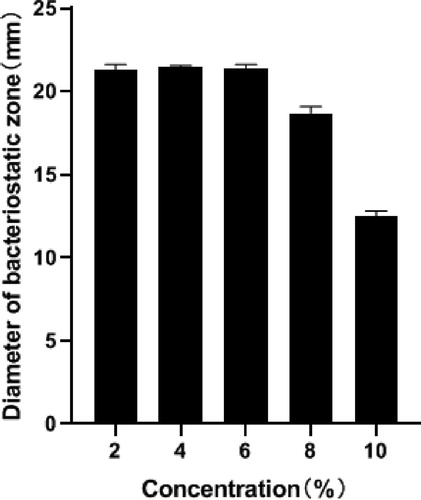
According to the results in , compared with the control group, the activity of bacteriocin treated with 2%, 4% and 6% sodium chloride solution did not change, but the bacteriostatic activity of bacteriocin after treatment with 8% and 10% sodium chloride solution decreased to varying degrees. It could tolerate 6% sodium chloride. In the environment of 8% sodium chloride, the activity was still high. The experimental results showed that the bacteriocin has a certain tolerance to salt concentration, which can be utilized in high-salt foods in the future. This is also one of the advantages of bacteriocins.
Surface active agents
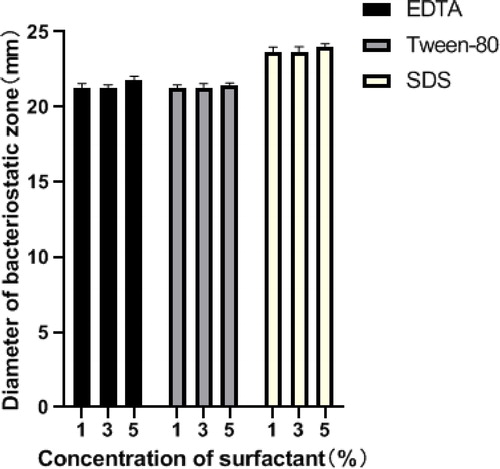
EDTA and Tween-80 at different concentrations did not affect the antibacterial activity of the bacteriocin Produced by E. faecalis CG-9 (). Another report indicated that some solvents did not change the activity of bacteriocin CV7, but Tween-20, Tween-80 and urea decreased its activity [Citation46]. Liu et al. [Citation22] reported that Tween-80, Tween-20, EDTA and urea did not reduce the activity of bifidocin A; however, with SDS-36%, the activity of bifidocin A was lost. This means that solvents and detergents could cause structural changes in bacteriocin. Studies on bacteriocins have shown that they have the advantage of being degradable by proteases [Citation24]. However, the antibacterial activity of the bacteriocins treated with SDS was significantly higher, and increased with the increase of SDS concentration. This could most likely be attributed to certain antibacterial activity that SDS itself has, resulting in synergistic effect, rather than to SDS improving the bacteriocin’s antibacterial activity [Citation46].
Detection of antimicrobial agents
Bacteriocins with broad antibacterial activity can be useful and valuable in the food industry. However, nisin, the only bacteriocin currently approved for use with food, only inhibits Gram-positive bacteria. The antimicrobial activity of the purified bacteriocin is shown in . This bacteriocin inhibited both gram-positive and gram-negative bacteria (E. coli), and even some fungi (Saccharomyces cerevisiae). This activity may be due to the fact that the bacteriocin has a different mode of action from that of antibiotics. It is also worth noting that this bacteriocin was also active against the gram-negative bacteria E. coli, because most bacteriocins were have been reported to be active only against gram-positive bacteria [Citation43]. Most of the new bacteriocins have narrow antimicrobial spectrum and only inhibit gram-positive bacteria [Citation27]. Firstly, bacteriocins bind to bacterial outer membrane receptors and then are introduced into the cell forming pores and causing leakage of intracellular materials [Citation47]. On the other hand, multiple studies have suggested that some bacteriocins have the ability to interact with intracellular enzyme systems and nucleic acids, besides damaging the integrity of the bacterial cell membrane [Citation48–50]. These bacteriocins may be active against gram-negative bacteria and even against some fungi [Citation28,Citation43]. The bacteriocin studied here has a wide range of antibacterial activity, and has inhibitory effect on Gram-positive bacteria and Gram-negative bacteria. Therefore, compared with antibiotics, this bacteriocin has great potential for application in the food industry. Bacteriocins have long been used in many aspects of life [Citation51,Citation52]. According to the molecular weight, properties and antibacterial spectrum of the bacteriocin produced by E. faecalis CG-9, enterococcin may be a new bacteriocin with research and development potential. Limited by the experimental conditions, the bacteriocin peptide sequence was not determined here. This is one of the directions that we should expand our investigations in the future.
Table 3. Antimicrobial spectrum of Enterococcus faecalis CG-9 bacteriocin.
Conclusions
In this paper we first reported a novel bacteriocin produced by Enterococcus faecalis CG-9 from human saliva. The bacteriocin could inhibit Gram-positive bacteria and Gram-negative bacteria. It is stable at a wide range of temperature and pH, and can be degraded by protease. The bacteriocin has a broad application prospect in the control of pathogenic bacteria and spoilage bacteria in food. The mechanisms of action of the bacteriocin produced by E. faecalis CG-9 against foodborne pathogens will be investigated in our further study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Heredia N, Garcia S. Animals as sources of food-borne pathogens: a review. Anim Nutr. 2018;4(3):250–255.
- Zhang B, Li HH, Pan WX, et al. Dual-color upconversion nanoparticles (UCNPs)-based fluorescent immunoassay probes for sensitive sensing foodborne pathogens. Food Anal Methods. 2017;10(6):2036–2045.
- Poli JP, Guinoiseau E, Luciani A, et al. Investigating the antibacterial action of Corsican honeys on nosocomial and food borne pathogens. J Apic Res. 2018;57(1):186–194.
- Franz C, den Besten HMW, Bohnlein C, et al. Microbial food safety in the 21st century: emerging challenges and foodborne pathogenic bacteria. Trends Food Sci Technol. 2018;81:155–158.
- McLay JC, Kennedy MJ, Orourke AL, et al. Inhibition of bacterial foodborne pathogens by the lactoperoxidase system in combination with monolaurin. Int J Food Microbiol. 2002;73(1):1–9.
- Ayala DI, Cook PW, Franco JG, et al. A systematic approach to identify and characterize the effectiveness and safety of novel probiotic strains to control foodborne pathogens. Front Microbiol. 2019;10:1108.
- Galvez A, Abriouel H, Lopez RL, et al. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120(1-2):51–70.
- Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11(2):95–105.
- Grande MJ, Lucas R, Abriouel H, et al. Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int J Food Microbiol. 2005;104(3):289–297.
- Lucas R, Grande MJ, Abriouel H, et al. Application of the broad-spectrum bacteriocin enterocin AS-48 to inhibit Bacillus coagulans in canned fruit and vegetable foods. Food Chem Toxicol. 2006;44(10):1774–1781.
- Viedma PM, Abriouel H, Ben Omar N, et al. Inactivation of Geobacillus stearothermophilus in canned food and coconut milk samples by addition of enterocin AS-48. Food Microbiol. 2009;26(3):289–293.
- Ananou S, Banos A, Maqueda M, et al. Effect of combined physico-chemical treatments based on enterocin AS-48 on the control of Listeria monocytogenes and Staphylococcus aureus in a model cooked ham. Food Control. 2010;21(4):478–486.
- Rim EJ, Monia EB, Pilar CM, et al. In vitro probiotic profiling of novel Enterococcus faecium and Leuconostoc mesenteroides from Tunisian freshwater fishes. Can J Microbiol. 2016;62(1):60–71.
- Feliatra F, Muchlisin ZA, Teruna HY, et al. Potential of bacteriocins produced by probiotic bacteria isolated from tiger shrimp and prawns as antibacterial to Vibrio, Pseudomonas, and Aeromonas species on fish. F1000Res. 2018;7:415.
- Wang Y, Qin Y, Xie Q, et al. Purification and characterization of plantaricin LPL-1, a novel class IIa bacteriocin produced by Lactobacillus plantarum LPL-1 isolated from fermented fish. Front Microbiol. 2018;9:2276.
- Zhao X, Liu Z, Li W, et al. In Vitro synergy of nisin and coenzyme Q0 against Staphylococcus aureus. Food Control. 2014;46:368–373.
- Toure R, Kheadr E, Lacroix C, et al. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J Appl Microbiol. 2003;95(5):1058–1069.
- Van Reenen CA, Dicks LMT, Chikindas ML. Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J Appl Microbiol. 1998;84(6):1131–1137.
- Hu Y, Liu X, Shan C, et al. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: purification, identification and antimicrobial characteristics. Food Control. 2017;77:290–297.
- Cavicchioli VQ, Camargo AC, Todorov SD, et al. Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J Dairy Sci. 2017;100(4):2526–2526.
- Lv X, Ma H, Sun M, et al. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as biopreservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fifillets. Food Control. 2018;89:22–31.
- Liu G, Ren L, Song Z, et al. Purifification and characteristics of bififidocin A, a novel bacteriocin produced by Bififidobacterium animals BB04 from centenarians' intestine. Food Control. 2015;50:889–895.
- Lv X, Du J, Jie Y, et al. Purification and antibacterial mechanism of fish-borne bacteriocin and its application in shrimp (Penaeus vannamei) for inhibiting Vibrio parahaemolyticus. World J Microbiol Biotechnol. 2017;33(8):156
- Rasheed HA, Tuoheti T, Zhang Y, et al. Purification and partial characterization of a novel bacteriocin produced by bacteriocinogenic Lactobacillus fermentum BZ532 isolated from Chinese fermented cereal beverage (Bozai). LWT. 2020;124:109113.
- Chen C, Chen X, Jiang M, et al. A newly discovered bacteriocin from Weissella hellenica D1501 associated with Chinese Dong fermented meat (Nanx Wudl). Food Control. 2014;42:116e124.
- Liu H, Zhang L, Yi H, et al. Identifification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. LWT - Food Sci Technol. 2016;71:386–390.
- Pei J, Feng Z, Ren T, et al. Purification, characterization and application of a novel antimicrobial peptide from Andrias davidianus blood. Lett Appl Microbiol. 2018;66(1):38–43.
- Hwanhlem N, Ivanova T, Biscola V, et al. Bacteriocin producing Enterococcus faecalis isolated from chicken gastrointestinal tract originating from Phitsanulok, Thailand: isolation, screening, safety evaluation and probiotic properties. Food Control. 2017;78:187–195.
- Wen LS, Philip K, Ajam N. Purifification, characterization and mode of action of plantaricin K25 produced by Lactobacillus plantarum. Food Control. 2016;60:430–439.
- An Y, Wang Y, Liang X, et al. Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control. 2017;81:211–217.
- Garsa AK, Kumariya R, Sood SK, et al. Bacteriocin production and different strategies for their recovery and purification. Probiotics Antimicrob Proteins. 2014;6(1):47–58.
- Pei J, Jin W, El-Aty AMA, et al. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control. 2019;110(106923).
- Baranzan WS, Koshy P. Purification, characterization, mode of action and enhanced production of Salivaricin mmaye1, a novel bacteriocin from Lactobacillus salivarius SPW1 of human gut origin. Electron J Biotechnol. 2018;35:39–47.
- Yamamoto Y, Togawa Y, Shimosaka M, et al. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl Environ Microbiol. 2003;69(10):5746–5753.
- H-Kittikun A, Biscola V, El-Ghaish S, et al. Bacteriocin-producing Enterococcus faecalis KT2W2G isolated from mangrove forests in southern Thailand: purification, characterization and safety evaluation. Food Control. 2015;54:126–134.
- Qiao X, Du R, Wang Y, et al. Purification, characterization and mode of action of enterocin, a novel bacteriocin produced by Enterococcus faecium TJUQ1. Int J Biol Macromol. 2020;144:151–159.
- Zhang Y, Yang J, Liu Y, et al. A novel bacteriocin PE-ZYB1 produced by Pediococcus pentosaceus zy-B isolated from intestine of Mimachlamys nobilis: purification, identification and its anti-listerial action. LWT. 2020;118:108760.
- Ahmadova A, Todorov S, Choiset Y, et al. Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani Motal cheese. Food Control. 2013;30(2):631–641.
- Du Toit M, Franz CM, Dicks LM, et al. Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J Appl Microbiol. 2000;88(3):482–494.
- Park SH, Itoh K, Fujisawa T. Characteristics and identifification of enterocins produced by Enterococcus faecium JCM 5804(T). J Appl Microbiol. 2003;95(2):294–300.
- Liu G, Griffiths MW, Wu P, et al. Enterococcus faecium LM-2, a multi-bacteriocinogenic strain naturally occurring in “Byaslag”, a traditional cheese of Inner Mongolia in China. Food Control. 2011;22(2):283–289.
- Manta V, Marija G, Mindaugas V, et al. Geobacillin 26 - high molecular weight bacteriocin from a thermophilic bacterium. Int J Biol Macromol. 2019;141:333–344.
- Du H, Yang J, Lu X, et al. Purification, characterization, and mode of action of Plantaricin GZ1-27, a Novel Bacteriocin against Bacillus cereus. J Agric Food Chem. 2018;66(18):4716–4724.
- Zhao S, Han J, Bie X, et al. Purification and characterization of plantaricin jla-9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from suan-tsai, a traditional Chinese fermented cabbage. J Agric Food Chem. 2016;64(13):2754–2764.
- Todorov SD, Rachman C, Fourrier A, et al. Characterization of a bacteriocin produced by Lactobacillus sakei R1333 isolated from smoked salmon. Anaerobe. 2011;17(1):23–31.
- Perumal V, Venkatesan A. Antimicrobial, cytotoxic effect and purification of bacteriocin from vancomycin susceptible Enterococcus faecalis and its safety evaluation for probiotization. LWT- Food Sci Technol. 2017;78:303–310.
- Tiwari SK, Noll SK, Cavera VL, et al. Improved antimicrobial activities of synthetic-hybrid bacteriocins designed from enterocin E50-52 and pediocin PA-1. Appl Environ Microbiol. 2015;81(5):1661–1667.
- Zhu Z, Sun H, Zhou Q, et al. Characterization and antibacterial action mode of bacteriocin BMP32r and its application as antimicrobial agent for the therapy of multidrug-resistant bacterial infection. Int J Biol Macromol. 2020;164:845–854.
- Cao S, Zhao F, Du R, et al. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2. Food Control. 2019;96:470–478.
- Goh HF, Philip K. Isolation and mode of action of bacteriocin bacc1 produced by nonpathogenic Enterococcus faecium c1. J Dairy Sci. 2015;98(8):5080–5090.
- Mbandlwa P, Doyle N, Hill C, et al. 2020. Bacteriocins: novel applications in food, and human and animal health. Reference module in food science. Elsevier.
- Wang J, Zhang S, Ouyang Y, et al. Current developments of bacteriocins, screening methods and their application in aquaculture and aquatic products. Biocatal Agric Biotechnol. 2019;22:101395.