Abstract
The adaptations that halophilic microorganisms have developed due to their extreme habitat, promote the production of active natural compounds with the potential to control microorganisms causing infections associated with health care. The prime purpose of this study was to isolate and identify extremophilic black yeast Hortaea werneckii from intertidal decayed leaves of Avicennia marina located on the Red Sea Coast of Saudi Arabia and to evaluate the antibacterial activity of the crude extract against both gram-positive and gram-negative pathogenic strains. Three new H. werneckii strains with distinguished morphological features, MF135, MF140 and MF141, were isolated and were placed in a monophyletic clade through phylogenetic analysis based on the large subunit ribosomal ribonucleic acid (LSU rRNA). The ethyl acetate crude extract active fractions of these strains possessed significant activities against Methicillin-resistant Staphylococcus aureus (MRSA), Campylobacter jejuni and Salmonella typhimurium. The gas chromatography–mass spectrometry analysis of the active fractions revealed the presence of antimicrobial-related biochemicals, i.e. 4-Acetoxy-2-azetidinone, sec-Butyl nitrite and Fatty Acid Methyl Ester (FAME). This is the first detailed report of black yeast detected with potential antibacterial activity against various pathogenic microorganisms. These results promise potential and interesting biotechnological tools to overcome the staggering problem of emerging antibiotic resistance among pathogenic bacteria.
Introduction
The continuous emergence of pathogenic microorganisms resistant to antibiotics is a worldwide threat that has drawn public attention to the need for the identification and development of new antimicrobial alternatives [Citation1,Citation2]. This threat has extended beyond clinical applications to reach other fields which rendered the current antimicrobial chemicals less effective [Citation3]. Historically, a great number of bioactive compounds have been isolated from natural sources, particularly plants, and have been readily applied for the treatment of various diseases [Citation4]. However, recently attention has been given more to microorganisms that possess great potential for the synthesis of bioactive compounds, which can eventually lead to new drug discovery [Citation5].
Extremophiles are a unique group of microorganisms capable of producing numerous bioactive compounds as a consequence of their adaptation to harsh environmental conditions. A large body of evidence has revealed the role of extremophiles as a promising source of novel antimicrobial agents, although most studies have laid focus only on a few of these diverse biological compounds [Citation6,Citation7]. Halophiles are extremophiles with the ability to live at elevated levels of salt concentrations up to nearly saturation levels, i.e. different hypersaline environments. Among the halophilic microorganisms, fungi such as Black yeasts show a great ability to produce natural bioactive compounds, due to their survival abilities in competing for substrates and in producing a wide range of metabolites allowing their preservation in such extreme environmental conditions [Citation8,Citation9].
The extraordinary features of the unicellular fungi referred to as “Black yeasts”, i.e. a thick cell wall, thermo- and osmo-tolerance due to the ability to grow in a meristematic shape, and the production of extracellular polysaccharides and secondary metabolites, allow them to thrive despite being subjected to numerous stresses. These stresses include fluctuating salt concentrations, high temperature, low water activity, scarce nutrients and high ultraviolet (UV) light intensity [Citation10,Citation11].
Hortaea werneckii is the best known halophilic eukaryotic model species so far for its resilient halophilic nature and the dynamic polymorphic lifespan, which supports its typical prevalence among black yeast species in high saline water at salinity levels over 20% [Citation12,Citation13]. It can develop in a wide optimum growth range of 1.0–3.0 mol/L NaCl, and can grow in a nearly saturated solution of 5.2 mol/L NaCl although at a relatively slow growth rate [Citation11]. This is possible due to its specific adaptive mechanisms to high salt conditions that have not been reported in fungal species showing moderate salt tolerance or salt sensitivity [Citation14]. Its antibacterial activity was studied preliminarily against the gram-positive species Bacillus subtilis [Citation9]. The polymorphism of H. werneckii has led to the assigning of many designations to the same species [Citation14–16]. Therefore, the application of polymerase chain reaction (PCR) in molecular identification and genotyping, particularly based on the sequencing of the internal transcribed spacer (ITS) rDNA regions has been widely described and applied to overcome the aforementioned problem [Citation17–19]. PCR fingerprinting is a flexible method since the suitable selection of primers enables the analyses to be targeted at various taxonomic levels [Citation20]. The aim of the present study was to isolate and identify strains of the extremophilic black yeast H. werneckii from mangrove Avicennia marina located on the Red Sea Coast of Saudi Arabia, then to investigate their antimicrobial potential in detail for the first time, and finally to determine the potential bioactive compounds responsible for this activity.
Materials and methods
Samples collection and isolation of H. werneckii
Intertidal decayed leaves of A. marina were collected from two different locations at Yanbu Red Sea Coast (N 24°02.551̛ - E 38° 68.897̛) and placed in sterilized plastic bags. Upon arrival to the laboratory, the surface contaminants of the decayed leaf samples were removed through disinfection with 75% ethanol for 2 min followed by sterilized distilled water for 1 min. The samples were then dried on sterilized filter paper. Each leaf sample was divided into four parts (leaf tip, two middle parts, and leaf petiole) as described by [Citation21]. These parts were placed on Corn Meal Agar (CMA) (17 g CMA in 1 L of seawater + 0.5 g chloramphenicol) on Petri plates and incubated under dark conditions at 25 °C. Plates were examined periodically for 3 weeks for the presence of germinating hyphae of H. werneckii using a dissecting microscope (WILD HEERBRUGG, Switzerland). The germinated hyphae were subcultured onto new CMA plates and incubated in darkness at 25 °C. Pure cultures of each H. werneckii strain were preserved in glycerol solution (10%) at −80 °C and deposited with ID numbers MF135, MF140 and MF141 at Molecular Microbiology Laboratory MycoBank, Department of Botany and Microbiology, College of Science, King Saud University. Photography and measurements of the isolated fungi were taken using a compound microscope (Nikon ECLIPSE 80i, Japan).
DNA extraction, sequencing and phylogenetic analysis
The DNA extraction procedure was carried out using a MOBIO DNA extraction kit (Mo Bio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. Single-spore isolates were grown in Yeast, Maltose and Glucose (YMG) broth (4 g yeast extract, 10 g glucose, 10 g malt extract in 1 L seawater) until sufficient mycelium was formed for DNA extraction.
For molecular identification, the large subunit (LSU) rDNA was amplified using forward primer LROR (5′-ACC CGC TGA ACT TAA GC-3′) and LR7 (5′-TAC TAC CAC CAA GAT CT-3′) as the reverse [Citation22]. Sequencing was performed by Macrogen Inc., Korea, using MGTM Taq-HF DNA Polymerase; with the following cycling profile: initial denaturation at 96 °C for 3 min, 96 °C for 15 s, 52 °C for 45 s, 72 °C for 1 min 30 s, and a final extension at 72 °C for 7 min. The molecular phylogenetic tree was analyzed using phylogenetic analysis using parsimony program (PAUP). Posterior probabilities were obtained by Bayesian phylogenetic inference using the program MrBayes 3.1.2 [Citation23,Citation24].
Crude extract preparation
The crude extract was prepared using a previously described protocol [Citation25] with slight modification, in which three strains of H. werneckii were inoculated into Potato Dextrose Broth and incubated for 9 days at 25 °C. Crude fermentation broths were centrifuged (15,000 g; Mikro 200 R; Hettich, Salford, U.K.) for 10 min and the supernatants were passed through a filtration membrane. The supernatant was mixed with ethyl acetate 1:1 and left for 15 min, then the upper phase was collected. This step was repeated three times. Then, the collected crude extracts were concentrated by rotary evaporation at 77 °C. Column chromatography was applied for the separation and fractionation of active compounds from the crude extract. Around 150 mg of the extract was dissolved in 12 mL of HPLC grade ethyl acetate and the solutions were applied to a chromatographic column (glass column: 10 mm diameter × 90 mm height contained 6 g silica gel). Different volumes of petroleum ethyl acetate were used as an elution solvent, and the fraction eluted ethyl acetate was collected and evaporated to dryness under reduced pressure at 60 °C. Then the crude extract fractions were collected at regular intervals of 15 min. Eight active fractions were evaporated via a rotary evaporator and their weights were determined, which were used for subsequent testing against the test microorganisms.
Antibacterial activity
The antibacterial activity of the whole crude extracts and subsequently their fractions was determined using the Agar Disc Diffusion method [Citation26] against six gram-negative and four gram-positive pathogenic bacteria () of clinical importance. Pathogenic bacterial strains were grown for 24 h on Muller-Hinton Agar plates and a cell suspension was prepared in physiological saline (phosphate buffer saline). An inoculum of the prepared cell suspension of each bacterial strain was mixed with freshly prepared Muller-Hinton agar medium and poured on Petri plates. After media solidification, sterilized filter paper discs (0.5 cm) were placed on the plates in an aseptic condition with an aliquot of 50 µL of 200 µg/mL concentration of the crude extract [Citation27]. Plates were incubated at 37 °C for 24 h, then the bacterial growth and inhibition zone diameter around the discs were measured and recorded. The same procedure was applied using crude extract fractions in which an aliquot of 50 µL of 100 µg/mL concentration was used to determine the antibacterial activity. The solvent acetone was used as the negative control, while the standard antibiotic ciprofloxacin at a concentration of 3 μg/mL served as the positive control for evaluation of the antibacterial activity.
Table 1. Pathogenic bacterial strains used in antibacterial activity assay.
Minimum inhibitory concentration (MIC) determination
Two-fold micro-dilution method was used to determine MIC via a 96-well plate (FALCON, France); 100 μg/mL was the starting concentration for the selected fractions, whereas 0.7 μg/mL was the endpoint concentration reached by serial micro-dilutions. Optical density measurement of the plates was done using an ELISA reader (ELX 800 microplate reader; BioTek, USA).
Gas chromatography-mass spectrometry (GC-MS)
The active compounds in the fractions acquired using ethyl acetate were identified using GC-MS (Konikrom Plus - HRGC 5000B) equipped with a 100% dimethylpolysiloxane column, using helium as the mobile phase with the pressure of 60 psi; 1 μL of the active fraction was injected by a Hamilton needle with a split ratio of 25:1. The temperature profile was run with a start of 80–240 °C at 8 deg/min; then 240–300 °C at 12 deg/min and finally a 20-min hold at 300 °C [Citation28]. The fractions chemical components were identified via surveying the mass spectral libraries from Central Laboratory College Science (CLCS), King Saud University (KSU).
Results and discussion
Current research has highlighted the urgent need to identify alternative natural sources of compounds with biological activity, which is extremely crucial to overcome the ever-emerging clinical threats. Many extremophilic fungi, especially those which grow in a unique habitat (i.e. marine environments), have proven to be a valuable source for unusual bioactive compounds [Citation29,Citation30]. It is believed that these secondary metabolites are being expressed as a chemical defense mechanism in competing for substrates and with other microorganisms as well, thus acting as a sort of adaptation of fungi to their environment [Citation8, Citation31]. The black yeast H. werneckii favors environments with hypersaline waters at salinities above 3.0 mol/L NaCl [Citation11].
Morphological identification
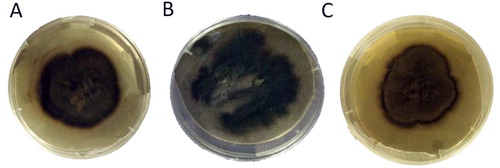
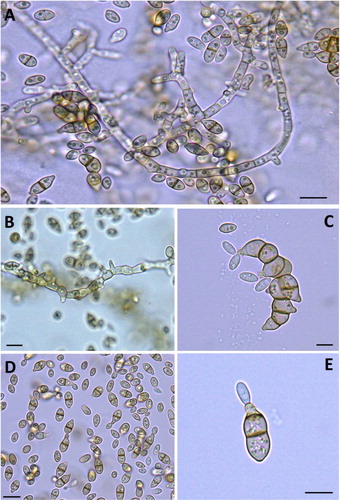
The colonies of isolated pure cultures of H. werneckii showed three distinct morphologies indicating the presence of three candidate isolates MF135, MF140 and MF141 (). Under the light microscope, the three isolates appeared identical. They were composed of brown septate hyphal elements and numerous two-celled, cylindrical to spindle-shaped yeast-like pale brown cells that form an annellide due to the tapered ends. Additionally, most yeast-like cells have noticeable darkly pigmented septa. Annellides may likewise emerge from the hyphae. Conidia are cylindrical to spindle-shaped, one- or two-celled, hyaline to pale brown, and more often appear in aggregated masses ().
Figure 2. H. werneckii in 3-week-old culture. Different shapes of budding conidia and pseudomycelium (A–E).
Note: Bright-field light micrographs (mounted in water). Scale bars: 0.5 µm (A–D), 0.1 µm (E).
Decayed wood and leaves from mangroves are the main habitats of H. werneckii [Citation32]. In this study, three H. werneckii isolates were obtained from 54 intertidal samples of decayed leaves examined from two mangrove stands near Yanbu City, Saudi Arabia. The three isolates of H. werneckii were confirmed through a combination of morphological and molecular tools (i.e. phylogenetic analysis of LSU ribosomal gene) [Citation33] and were recognized as three new strains, namely H. werneckii MF135, 140 and 141. Despite being grown on the same media and under the same conditions, these strains showed distinct culture features () but revealed similar microscopic characteristics (). Similar observations have been witnessed in Saccharomyces cerevisiae, in which different strains isolated from different plant sources, including dates, sugarcane and figs, possessed the same microscopic features and needed further genetic identification via PCR based markers to differentiate between them [Citation34].
Strain MF135 was a dark brown color with transparent newly formed mycelia all around the colony, which provided an additional zone that can be clearly seen in the culture plate. The MF140 colony was black to dark greenish with a high rate of growth and non-uniform distribution. The MF141 colony was light brown; new mycelial growth was darker in color and protruded around the culture.
Phylogenetic analysis
Phylogenetic analysis based on LSU rDNA proved the existence of genetic variation between the three isolates MF135, MF140 and MF141 and placed them in a monophyletic clade with H. werneckii (Teratosphaeriaceae, Capnodiales, Ascomycetes). Moreover, the sequences were placed in NCBI under accession numbers (KY746723, KY746724 and KY746725, respectively).
The LSU dataset included three query isolates and 19 taxa, of which 17 belong to the Capnodiales, whereas two belong to the order Dothideales used as the outgroup. The large subunit dataset consisted of 919 total characters, of which 709 are constant, 65 are variable and parsimony-uninformative and 145 are parsimony-informative characters. The maximum parsimony (MP) phylogenetic tree was produced using a heuristic search with a length of 416 steps, a consistency index of 0.6370, a retention index of 0.6696 and a rescaled consistency index of 0.4254 ().
Figure 3. Bayesian phylogenetic tree of Hortaea spp. and related taxa, based on the nucleotide sequences of the large subunit (LSU) rDNA sequence. Numbers are bootstrap support, Maximum parsimony (MP). The H. werneckii isolates are highlighted.
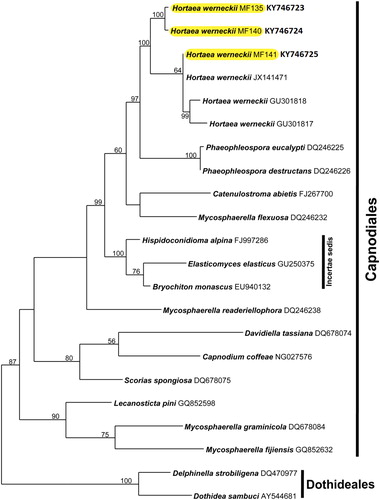
Molecular identification proved to be a valuable tool in differentiating the three strains. H. werneckii phylogenetic analysis placed the new strains in the order Capnodiales (). Both MF135 and MF140 showed remarkable genetic variation through forming a sister taxon to H. werneckii (Horta) Nishim. & Miyaji. by a well-supported clade (100 for MP). MF141 was joined to the selected BLAST sequences with a moderate-support clade (64 for MP).
Antibacterial activity
Preliminary tests of the three crude extracts against the selected pathogenic bacteria showed inhibitory activity () against C. jejuni, S. typhimurium and Methicillin-Resistant S. aureus (MRSA), whereas crude extract MF135 exhibited the highest activity ().
Figure 4. Agar disc diffusion method using MF135 crude extract against MRSA (A), C. jejuni (B) and S. typhimurium (C).

Table 2. Preliminary bioactivity of crude extracts from H. werneckii strains against tested pathogenic bacteria (disc 5 mm).
The results from the preliminary test of the fractionated crude extract demonstrated that fraction 6 of MF135 exhibited the highest inhibitory potential against MRSA, C. jejuni and S. typhimurium with an inhibition zone of 23, 20 and 17 mm, respectively. Both MF140 fraction 3 and MF141 fraction 6 showed moderate inhibitory activity with no significant difference between them (), which was further confirmed using the MIC test.
Table 3. Preliminary bioactivity of H. werneckii extract fractions against tested pathogenic bacteria.
Two-fold microdilution method revealed the MIC of the selected ethyl acetate extract fractions () against the tested pathogenic strains. The active fraction 6 in MF135 showed the lowest MIC against MRSA and C. jejuni at 6.2 μg/mL, while slightly higher MIC against S. typhimurium with 12.5 μg/mL. Both fraction 3 of MF140 and 6 in MF141 showed moderate MIC values against MRSA, S. typhimurium and C. jejuni which ranged between 20 and 50 μg/mL ().
Figure 5. Illustration of two-fold micro-dilution plate to determine the MIC of the selected three active fractions of H. werneckii MF135, MF140 and MF141. Positive control, ciprofloxacin (3 µg/mL); Negative control, acetone.
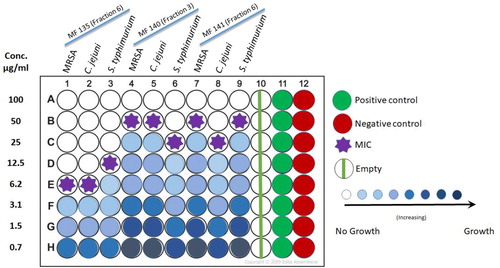
The preliminary test proved that the active fractions of Black Yeast strains demonstrated significant antibacterial activity against well-known human pathogens, including MRSA. The preliminary tests were carried out in two steps: the first aimed to determine whether the crude extracts of the strains show any sign of activity against the selected pathogenic bacteria. The second step applied the fractions of the crude extract to determine which fraction contains the bioactive compounds responsible for the inhibitory effect. Ethyl acetate was used in the crude extract fractionation, as it possesses medium polarity allowing the separation of as many of the bioactive compounds as possible [Citation35]. The results of this study revealed the antibacterial activities of the three strains against both gram-negative and gram-positive bacteria, in which the crude extract fraction 6 of the MF135 strain showed the best inhibitory activity against MRSA, C. jejuni and S. typhimurium with an inhibition zone of 23, 20 and 17 mm, respectively. The MIC test was performed using a microtiter two-fold microdilution method, which proved herein to be a reliable quantitative method for antibacterial testing, as it allows for estimation of the minimal concentration of the tested antimicrobial agent that exerts an inhibitory effect in the used broth medium [Citation27]. The low MIC value of 6.2 μg/mL against MRSA, C. jejuni and 12.5 μg/mL against S. typhimurium obtained from the MF135 active fraction 6 reflects its efficacy as an antibacterial agent, as it requires a small amount of the agent to achieve high inhibitory activity in comparison to other reports of MIC values, which ranged from 6.5 to more than 125 μg/mL against different pathogenic bacteria [Citation36–38]. Hence, the antibacterial activity of the H. werneckii strains assayed in this study suggests that strain MF135 could be a strong candidate for bioactive compounds through its promising potential as an alternative biological source that needs to be explored further in future investigations against some pathogenic bacteria.
Identification of active fractions
The active fractions of the three H. werneckii strains were identified via GC-MS (). MF135 active fraction 6 showed three active compounds at retention time ranging between 5.35 and 11.48 min, while its molar mass was between 103 to 154 g/mol. Fraction 3 of MF140 revealed a wide range of compounds (11) between retention times of 5.39 to 41.5 min and molar mass between 56 and 290 g/mol. MF141 fraction 6 showed the least number of compounds, comprising only two, between 6.6 and 64.2 min retention times and molar mass of 56 to 210 g/mol ().
Figure 6. GC-MS chromatogram for active fractions of H. werneckii MF135 (A), MF140 (B) and MF141 (C).
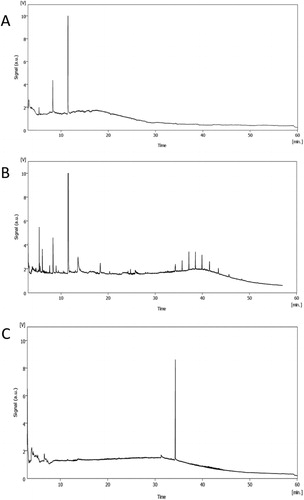
Table 4. GC-MS analysis of active fractions from H. werneckii ethyl acetate.
Melanin pigment of black yeasts was recognized as the main bioactive compound responsible for antibacterial activity [Citation39]. However, the GC-MS results of this study revealed the potential of the three strains in producing diverse compounds with antibacterial activity in the absence of melanin. Bioactive compounds with inhibitory activity, i.e. 4-acetoxy-2-azetidinone, sec-butyl-nitrite, octadecadiynoic-acid-methyl-ester and aspidofractinin-3-ylmethanol were identified. The saturated form of nitrogen 4-acetoxy-2-azetidinone in the MF135 extract has been previously reported to be produced by Penicillium species and to act by inhibiting cell wall biosynthesis [Citation40]. The nitrite compound based sec-butyl nitrite was identified in both MF135 and MF140 extracts at high levels, and proved to be of a bacteriostatic nature as it has been witnessed to effectively inhibit Clostridium perfringens growth [Citation41]. Octadecadiynoic-acid-methyl ester expressed by MF140 is among the Fatty Acid Methyl Ester (FAME) compounds produced by the fungi order Glomales and many halophylic plant species such as A. marina and members of the family Chenopodiaceae [Citation42,Citation43]. FAME have been reported to be with antibacterial effect against Bacillus subtilis, Streptococcus pyogenes and Escherichia coli [Citation44]. Moreover, an elevated level of the alkaloid compound aspidofractinin-3-ylmethanol was identified in the active fraction 6 of strain MF141. This compound has been used in the development of many antibacterial drugs such as quinolones, which play a major role in the inhibition of bacterial nucleic acid synthesis [Citation45]. Other compounds of unknown function have been detected in active fractions such as 2,5-methano-2H-furo[3,2-b]pyran-8-one, hexahydro-,1-propanamine, N,2-dimethyl-N-nitroso-, N-carbobenzyloxy-cysteinylcysteine, 9-oxabicyclo[6.1.0]nonan-4-one, undefined long alkyl chain, 2,4,6,8-tetramethyl-1-undecene and 3-methyl-1,2-diazirine; all of which need further separation and investigation. Since different strains of the same species may produce different bioactive metabolites in order to adjust to their competitive environment, future studies of the three Hortaea werneckii strains isolated from Red Sea mangrove may identify novel compounds with useful applications in different fields of biotechnology.
Conclusions
The results from this work demonstrate that fungi from marine habitats could be considered a prospective source of natural bioactive compounds, including ones with pharmacological potential. To our knowledge, this work is the first detailed study reporting the active antibacterial fractions of H. werneckii extracts. The isolated strains showed relatively strong antibacterial activity due to the presence of highly effective bioactive compounds with unique nature and known antimicrobial and antifungal activity, such as fatty acids methyl ester, diazirine and azetidinone compounds. These compounds have a great potential for further research and can be explored for various biological activities. In addition, they should be purified and clinically tested in the near future as a step toward potential approval for incorporation in drug manufacturing to tackle the problem of emerging antibiotic resistance among pathogenic bacteria. In depth studies on these fungal strains to produce adequate amounts of the antibacterial compounds, i.e. large-scale growth and modifying culture conditions for better production, are needed. Furthermore, in vivo tests are required to understand the cellular targets of the active compounds responsible for the bioactivities.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Prakash D, Saxena R. Antimicrobial susceptibility pattern of human pathogenic bacteria related to Enterobacteriaceae family causing urinary tract infection. Adv Appl Sci Res. 2013;4:98–104.
- Wiyakrutta S, Sriubolmas N, Panphut W, et al. Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plants. World J Microbiol Biotechnol. 2004;20(3):265–272.
- Amiri A, Dugas R, Pichot AL, et al. In vitro and in vivo [corrected] activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int J Food Microbiol. 2008;126(1–2):13–19.
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303–336.
- Gunatilaka AL. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod. 2006;69(3):509–526.
- Ravot G, Masson J-M, Lefèvre F. 34 applications of extremophiles: the industrial screening of extremophiles for valuable biomolecules. Methods Microbiol. 2006;35:785–813.
- Sánchez LA, Gómez FF, Delgado OD. Cold-adapted microorganisms as a source of new antimicrobials. Extremophiles. 2009;13(1):111–120.
- Bhadury P, Mohammad BT, Wright PC. The current status of natural products from marine fungi and their potential as anti-infective agents. J Ind Microbiol Biotechnol. 2006;33(5):325–337.
- Sepcic K, Zalar P, Gunde-Cimerman N. Low water activity induces the production of bioactive metabolites in halophilic and halotolerant fungi. Marine Drugs. 2010;9(1):43–58.
- Gostinčar C, Lenassi M, Gunde-Cimerman N, et al. Fungal adaptation to extremely high salt concentrations. Adv Appl Microbiol. 2011;77:71–96.
- Gunde-Cimerman N, Zalar P, De Hoog S, et al. Hypersaline waters in salterns–natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol. 2000;32(3):235–240.
- Butinar L, Santos S, Spencer-Martins I, et al. Yeast diversity in hypersaline habitats. FEMS Microbiol Lett. 2005;244(2):229–234.
- Zalar P, Kocuvan MA, Plemenitaš A, et al. Halophilic black yeasts colonize wood immersed in hypersaline water. Botanica Marina. 2005;48(4):323–326.
- Plemenitaš A, Gunde-Cimerman N. Cellular responses in the halophilic black yeast Hortaea werneckii to high environmental salinity. In Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Dordrecht: Springer; 2005. p. 453–470.
- Gunde-Cimerman N, Plemenitaš A. Ecology and molecular adaptations of the halophilic black yeast Hortaea werneckii. In: Life in extreme environments. Springer; 2006. p. 177–185.
- Plemenitaš A, Vaupotic T, Lenassi M, et al. Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: a molecular perspective at a glance. Stud Mycol. 2008;61:67–75.
- De Hoog G, Zalar P, Urzi C, et al. Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8 S and ITS2 rDNA sequence comparison. Stud Mycol. 1999;43:31–37.
- Mayr BM, Kobold U, Moczko M, et al. Identification of bacteria by polymerase chain reaction followed by liquid chromatography − mass spectrometry. Anal Chem. 2005;77(14):4563–4570.
- Yıldırım İH, Yıldırım SC, Koçak N. Molecular methods for bacterial genotyping and analyzed gene regions. J Microbiol Infect Dis. 2011;1:42–46.
- Uijthof J, De Cock A, De Hoog G, et al. Polymerase chain reaction-mediated genotyping of Hortaea werneckii, causative agent of tinea nigra. Mycoses. 1994;37(9–10):307–312.
- Hodhod M. Molecular systematics of Marine Fungi isolated from Yanbu Mangroves in the Kingdom of Saudi Arabia [MS Thesis]. Riyadh, Saudi Arabia: King Saud University; 2013. p. 143.
- Hong J-H, Jang S, Heo YM, et al. Investigation of marine-derived fungal diversity and their exploitable biological activities. Marine Drugs. 2015;13(7):4137–4155.
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755.
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574.
- Li H, Qing C, Zhang Y, et al. Screening for endophytic fungi with antitumour and antifungal activities from Chinese medicinal plants. World J Microbiol Biotechnol. 2005;21(8–9):1515–1519.
- Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49(11):1749–1755.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79.
- Devi N, Prabakaran J. Bioactive metabolites from an endophytic fungus Penicillium sp. isolated from Centella asiatica. CREAM. 2014;4(1):34–43.
- Giddings L-A, Newman DJ. Bioactive compounds from extremophilic marine fungi. In: Fungi in extreme environments: ecological role and biotechnological significance. Cham: Springer; 2019. p. 349–382.
- Ibrar M, Ullah M, Manan S, et al. Fungi from the extremes of life: an untapped treasure for bioactive compounds. Appl Microbiol Biotechnol. 2020;104(7):2777–2801.
- Gallo M, Amonette R, Lauber C, et al. Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol. 2004;48(2):218–229.
- Abdel-Wahab M. 2000. Diversity of fungi in subtropical mangroves [PhD thesis]. Sohag, Egypt: Faculty of Science at Sohag, South Valley University.
- Jones EBG, Suetrong S, Sakayaroj J, et al. Classification of marine ascomycota, basidiomycota, blastocladiomycota and chytridiomycota. Fungal Diversity. 2015;73(1):1–72.
- Kechkar M, Sayed W, Cabrol A, et al. Isolation and identification of yeast strains from sugarcane molasses, dates and figs for ethanol production under conditions simulating algal hydrolysate. Braz J Chem Eng. 2019;36(1):157–169.
- Hossain F, Follett P, Salmieri S, et al. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int J Food Microbiol. 2019;295:33–40.
- Cardozo VF, Oliveira AG, Nishio EK, et al. Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann Clin Microbiol Antimicrob. 2013;12:12–18.
- Elshikh M, Ahmed S, Funston S, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38(6):1015–1019.
- Yang Z, Hao X, Chen S, et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J Colloid Interface Sci. 2019;533:13–23.
- Rani MHS, Ramesh T, Subramanian J, et al. Production and characterization of melanin pigment from halophilic black yeast Hortaea werneckii. Int J Pharma Res Rev. 2013;2:9–17.
- Nikalje APG, Ghodke MS, Mirza SB. 2-azetidinone-a brief review. World J Pharm Pharm Sci. 2013;3:2589–2625.
- Ngutter C, Donnelly C. Nitrite-induced injury of Listeria monocytogenes and the effect of selective versus nonselective recovery procedures on its isolation from frankfurters. J Food Prot. 2003;66(12):2252–2257.
- Chandrasekaran M, Kannathasan K, Venkatesalu V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Zeitschrift Für Naturforschung C. 2008;63(5–6):331–336.
- Graham JH, Hodge NC, Morton JB. Fatty Acid methyl ester profiles for characterization of glomalean fungi and their endomycorrhizae. Appl Environ Microbiol. 1995;61(1):58–64.
- Chandrasekaran M, Senthilkumar A, Venkatesalu V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur Rev Med Pharmacol Sci. 2011;15(7):775–780.
- Cushnie TT, Cushnie B, Lamb AJ. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014;44(5):377–386.