Abstract
The aim of this study was to evaluate the effect of ascorbic acid (Vitamin C) and 2-mercaptoethanol (ME) added to buffalo semen samples after thawing for 1 h and 2 h incubation. Ejaculates (n = 20) from eight Bulgarian Murrah buffaloes were frozen in pellets and straws at a final sperm concentration of 80 × 106 mL. We investigated the effect of supplementations on the motility, morphology of spermatozoa at 1 h and 2 h after thawing, sperm plasma membrane status and mitochondrial trans-membrane potential. The comparative CASA analysis of the motility and velocity parameters of spermatozoa showed significant differences in the progressive motility and in the VCL values in favour of control vs. samples with additives (p < 0.05). The molecular changes in PM phospholipid asymmetry were higher after thawing (p < 0.05). The semen treated with Vit C showed significant (p < 0.05) lower number of apoptotic spermatozoa (6CF+/Ann V Cy3+) in comparison with samples incubated with ME. The numbers of dead spermatozoa (6CF-/Ann V Cy3+) in samples with ME were significantly higher than other groups. The percentage of spermatozoa with well preserved and functioning mitochondria in control semen was higher (p < 0.05) than that in the experimental group with ME. The results obtained revealed that addition of Vit C (5 µmol/L) and ME (50 µmol/L) has no positive effect on post-thaw semen quality of buffalo sperm, because of the impaired integrity of PM and mitochondrial trans-membrane potential.
Introduction
Artificial insemination (AI) is one of the important reproductive biotechnologies, which helps the widespread dissemination of semen, limiting of sexually transmitted diseases and primarily facilitating genetic improvement programs. As highlighted by Mittal et al. [Citation1], the fertilizing capacity of the diluted or cryopreserved semen is essential to the success of AI. This fertilizing capacity depends upon the quality of sperm cells and the semen extender to maintain semen quality for a maximum period at refrigerator temperature (4–5 °C) as well as at −196 °C in liquid nitrogen. However, the cryo-damage that occurs during the freeze-thawing process is well documented. Some authors report that cryopreservation causes sublethal damage to sperm cells due to osmotic, thermal, and mechanical stress, potentially leading to destruction of the acrosomal plasma membrane [Citation2], inactivation of acrosin and hyaluronidase, prostaglandin depletion, reduction of adenosine triphosphate (ATP) and adenosine di-phosphate synthesis, decrease in acrosomal proteolytic activity [Citation3] and DNA integrity [Citation4]. Majzoub and Agarwal [Citation5] reported that the production of reactive oxygen species (ROS) increases, and the concentration of natural antioxidants decreases during sperm cryopreservation and thawing. ROS cause membrane lipid peroxidation in spermatozoa as a result of reduced antioxidant potential during cryopreservation [Citation1,Citation6]. All this amounts to spermatozoon lesions such as oxidative damage or apoptosis, which can lead to negative results during the AI process [Citation7]. Research has shown poor quality of keeping and freezability of buffalo semen [Citation8,Citation9]. Negative changes in buffalo spermatozoa are due to their unique physiology and higher polyunsaturated phospholipid levels in the plasma membrane [Citation1], probably affecting the sperm motility, viability, acrosomal and DNA integrity [Citation10,Citation11]. The poor post-thaw characteristics of buffalo semen could be attributed to higher generation of ROS leading to sperm damage [Citation4].
The motility and fertilization ability of spermatozoa can improve after addition of various motility enhancing agents or antioxidants [Citation9,Citation12]. The antioxidants check the chemical breakdown of the substrate resulting from oxidation, neutralize the free radicals, and reduce the risk of damage to spermatozoa during cryopreservation [Citation13]. The major antioxidants naturally present in mammalian semen that regulate ROS are Vitamin C and E. These antioxidants protect the sperm from lipid peroxidation and ensure the integrity of the plasma membrane and mitochondria as well as better kinematics parameters for post-cryopreservation sperm [Citation1,Citation14,Citation15]. Ascorbic acid in the seminal plasma accounts for about 65% of the antioxidant capacity of semen [Citation9]. Tariq et al. [Citation16] have shown that the concentration of ascorbic acid in buffalo seminal plasma is 364 μmol/L, while that in blood plasma is 40 μmol/L. The ascorbic acid content of bull semen is significantly higher as compared to buffalo bull semen [Citation17], which could explain the differences in the quality preservability of semen. The addition of antioxidants to the semen extender may lead to mitigation of oxidative damage in buffalo spermatozoa during the post-thawing process [Citation9,Citation18]. In view of these facts, the present investigation aimed to evaluate the effect of antioxidant additives such ascorbic acid (Vit C) and 2-mercaptoethanol (ME) added to buffalo semen samples after thawing for 1 h and 2 h incubation.
Materials and methods
Animals and sampling
The study was done with eight sexually mature breeding buffalo bulls of Bulgarian Murrah breed, aged 4–6 years. All bulls were in good health and under veterinary care. The semen was collected by an artificial vagina. Each ejaculate was divided into two equal parts that were frozen in pellets and straws at sperm concentration of 80 × 106/mL. The pellets (0.5 mL) were frozen using the cryoprotective diluent GH22L [Citation19] on dry ice (Nagaze-Niva method). The straws (0.25 mL) were frozen using the Triladyl (Minitube) diluent by programmable freezer Digitcool (IMV Technologies; Cassou method). All semen samples (pellets and straws) were stored in liquid nitrogen (–196 °C) for a minimum of 72 h. After that, pellets were thawed at 37 °C for 5 s using 0.5 mL of 2.8% sodium citrate; the straws, at 37 °C for 30 s. All thawed samples were incubated at 37 °C in air-humidified incubator (Binder GmbH, Tuttlingen) for 10 min before CASA analysis. Vitamin C (Vit C, 5 µmol/L) and 2-mercaptoethanol (ME, 50 µmol/L) as pharmaceuticals, were added to semen samples immediately after thawing. Control semen samples without supplements were diluted with the same amount of semen extender.
Computer-assisted sperm analysis (CASA)
The motility, progression, velocity and kinematic characteristics of frozen-thawed spermatozoa at 0 h, 1 h and 2 h after thawing were analyzed using Leja 20 slides on Sperm Class Analyzer (Microptic). The studies were performed by ‘Motility & Concentration’ software on: static cells, progressive and rapid motility spermatozoa; curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), linearity (LIN); straightness (STR); wobble (WOB), ALH (lateral head displacement) and BCF (beat frequency of sperm cells).
Morphological analysis of spermatozoa
The analysis was performed by the triple-staining method. Smears from each semen sample were made at 0 h, 1 h, and 2 h after thawing. After fixation in ethanol for 10 min, the slides were stained with method for differential staining of the acrosome through consistent use of the following dyes: 0.5% aqueous solution of eosin for 5 min, saturated aqueous solution of Congo red for 5 min and 0.5% aqueous solution of gentian violet for 5 s. After three washings and drying, morphology analysis of 200 spermatozoa was performed on each slide.
Sperm plasma membrane (PM) status evaluation
The sperm PM status was analyzed using Annexin V-Cy3™ Apoptosis Detection Kit (Sigma-Aldrich). Double staining was done on frozen-thawed spermatozoa from all groups (control, Vit C, ME in granules and straws) by a protocol adapted for use with spermatozoa. Sperm cells were washed two times with binding buffer (1 × BB, Sigma-Aldrich) by centrifugation at 300g for 3 min in order to remove the seminal plasma and extenders. The spermatozoa were resuspended in 1 × BB up to a final concentration of 0.5–1 × 106 mL. Then, 50 μL of the cell suspension was added on Poly-L-Lysine treated slides. The staining procedure followed the Annexin V-Cy3™ Apoptosis Detection Kit protocol (Sigma-Aldrich). The stained spermatozoa were evaluated using a fluorescence microscope Olympus BX51 at 40-fold and 100-fold magnification. The protein Annexin V in the Annexin V Cy3 (λ = 532 nm) binds specifically to the phosphatidylserine which may be externalized on the outer monolayer of the PM. This binding was visualized as red fluorescence. The 6-carboxyfluorescein diacetate (6CFDA) dye was used to evaluate sperm viability. When this non-fluorescent component enters living cells, the esterase causes its hydrolysis, producing the fluorescent 6-carboxyfluorescein (6CF; λ = 492nm), visualized as green fluorescence. Each sperm cell was evaluated by using filters for 6CF (λ = 492nm) and for Cy3 (λ = 532 nm) successively. The results were reported as live spermatozoa with preserved PM integrity (6CF+/Ann V Cy3–), apoptotic spermatozoa with initial molecular changes in PM (6CF+/Ann V Cy3+), dead spermatozoa (6CF-/Ann V Cy3+).
Mitochondrial trans-membrane potential (MTP) evaluation
Mitochondrial specific fluorescent dye Rh123 (Sigma-Aldrich) was used to determine the mitochondrial bioenergetics of frozen-thawed spermatozoa from all the groups (control, Vit C, ME in granules and straws) after 30 min of incubation at 37 °C. The stained spermatozoa were evaluated using fluorescent microscopy (λ = 534nm). The classification of the spermatozoa was based on the intensity of Rh123 absorption as follows: spermatozoa with non-functional mitochondria (Rh123–; no fluorescence); live spermatozoa with some MTP disturbances (Rh123+; low fluorescence); normal spermatozoa with functionally preserved mitochondria (Rh123 ++; bright fluorescence).
Statistical analysis
All statistical analyses were carried out using the Statistical Package for Social Sciences program (SPSS), version 16.00 for Windows. Statistical significance was set at the 0.05 probability level. The results are expressed as mean values with standard error of the mean (±SEM).
Results and discussion
Computer-assisted sperm analysis (CASA)
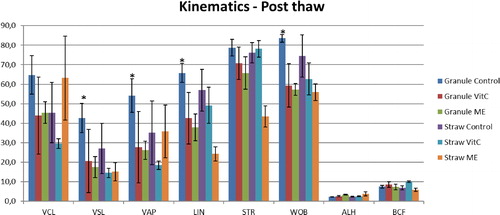
The motility characteristics of sperm cells assessed by CASA in post-thawed semen of all samples revealed that the percentage of static cells frozen in granules and straws was lowest in the control samples (). In the samples with added Vit C and ME, the levels of static cells were significantly higher in granules and in straws (p < 0.05), but a lower percentage of static spermatozoa was noticed in the samples frozen in granules. Addition of Vit C and ME did not decrease the percentage of static cells immediately post thaw. The results in progressive motility and rapid spermatozoa were similar, where a statistically highest percentage cells has been recorded in control samples in comparison with the experimental group (p < 0.05).
Table 1. Motility characteristics post thaw of buffalo spermatozoa frozen in granules and straws with added Vit C (5 µmol/L) and ME (50 µmol/L).
The results from the thermal resistance test at 1 h and 2 h post thaw at 37 °C showed a significantly lower percentage of static spermatozoa in control samples, both in granules and in straws, than in the experimental groups after 1 h incubation (p < 0.05). Among the treatments, better results were visible in the samples with Vit C, especially the straw samples unaffected by the time of incubation ().
Table 2. Motility characteristics after post thaw incubation for 1 h and for 2 h at 37 °C of buffalo spermatozoa frozen in granules and straws with supplementation of Vit C ( 5 µmol/L) and ME (50 µmol/L).
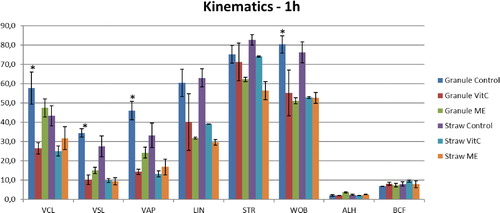
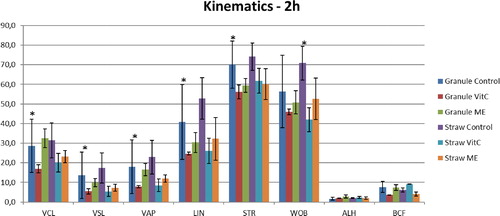
The addition of Vit C and ME significantly decreased the number of progressive and rapidly motile spermatozoa (p < 0.05) after the thermal resistance test at 1 h and 2 h at 37 °C, except that the granules treated with ME had a better result than the other two (p > 0.05). The kinematic parameters VSL (straight-line velocity), VAP (average path velocity), LIN (linearity), STR (straightness), and WOB (wobble) after post thaw showed best results (p < 0.05) in the control sample granules (). The samples with Vit C and ME had generally lower values, except for VCL (curvilinear velocity), which showed better values in the sample with ME in straws (p > 0.05) compared to the control sample. The values of ALH (lateral head displacement) and BCF (beat frequency of sperm cells) were very low in all samples, but non-significantly higher in the straws treated with vit C.
Figure 1. Kinematic parameters of buffalo spermatozoa frozen in granules and straws with addition of Vitamin C (Vit C) and 2-mercaptoethanol (ME). *p < 0.05.
The effect of semen extender supplementation with Vit C and ME on the kinematic parameters of post-thaw buffalo semen with different incubation times is presented in and , respectively. In an effect undifferentiated by incubation time −1 h or 2 h, VCL, VSL, VAP, LIN, STR, and WOB were significantly higher in the control samples (p < 0.05) compared with the two experimental treatments. The granules treated with ME showed higher VCL, VSL, and VAP values than the samples with Vit C, although these differences were not significant.
Morphological analysis of spermatozoa
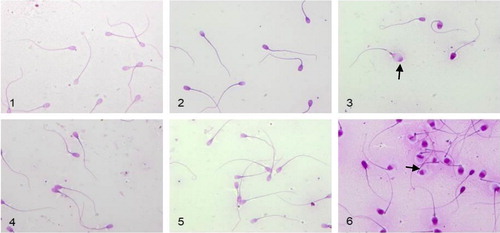
The samples treated with Vit C manifested no changes in morphology. The samples treated with ME revealed impaired morphology with swollen heads and acrosomes. Some sperm cells even were with lysed heads (, arrows).
MTP evaluation
Independent of the treated or non-treated groups, the use of Annexin V/6CF test registered the following cell types in the ejaculates: 6CF+/Ann V Cy3– (live), 6CF+/Ann V Cy3+ (apoptotic), 6CF–/Ann V Cy3+ (dead). Although the preliminary sperm motility was preserved, the Annexin V binding test differentiated some spermatozoa with an initialized process of cell destruction ().
Table 3. Comparative analysis of the integrity of PM by double staining Annexin V Cy3 apoptosis detection kit of buffalo spermatozoa.
It was noteworthy that the semen treated with Vit C after thawing showed a significantly (p < 0.05) lower number of apoptotic spermatozoa with commencing changes in the PM (6CF+/Ann V Cy3+) in comparison with the samples incubated with ME. In the samples with ME, the numbers of dead spermatozoa (6CF–/Ann V Cy3+) were significantly higher than those in the other groups.
The percentage of spermatozoa with well preserved and functioning mitochondria in control semen was higher (p < 0.05) in comparison with the experimental group with ME, but the differences were not statistically significant in comparison with the sample incubated with Vit C ().
Table 4. Comparative analysis of mitochondrial trans-membrane potential measured by Rh123 of buffalo spermatozoa.
Potential post-thawing effect of vit C and ME
This study evaluated the potential post-thawing antioxidant effect of Vit C and ME on the buffalo semen quality, as a follow-up to another study that demonstrated the protective role of medium GH22L® on sperm motility, membrane integrity and mitochondrial potential [Citation20]. It is well known that the freeze–thawing process has a negative impact on semen parameters due to the deleterious effect of ROS [Citation21,Citation22]. The addition of different antioxidants in semen extenders has some positive effect [Citation23,Citation24]. In the present study we observed essential differences, unlike other authors [Citation23,Citation25–28]. Addition of Vit C and ME had no positive influence on the motility and velocity parameters; there was even deterioration of semen parameters. In the samples incubated for 1 h at 37 °C, semen with added Vit C revealed the highest percentage of static cells, lowest percentage of progressive motility spermatozoa and fast movement unlike other studies [Citation1,Citation29,Citation30]. The results from the present study are in agreement with a previous report [Citation31] that higher concentrations of ascorbic acid were detrimental to sperm motility of frozen–thawed bull semen.
The effect of antioxidants is related to differences in the susceptibility to antioxidants among species and breeds, as Fernandez-Santos et al. [Citation32] reported no positive effect on post-thaw sperm parameters in deer with ascorbic supplementation. In our study, it is noteworthy that the samples with added ME had better motility results compared with the Vit C samples, despite that both showed lower values than the control samples, both in the form of granules and straws. Maybe these differences could be attributed to differences in the mode of action of both antioxidants: ME increases the glutathione synthesis [Citation27,Citation33] and inhibits lipid peroxidation [Citation30], while Vit C is a direct scavenger of ROS [Citation34]. Probably, this could explain the clearly visible differences in the morphological analysis. In the samples with ME there was a clearly visible swallowing of spermatozoa heads and acrosome region. In contrast, some studies report that ME did not influence the post thaw quality and oxidative activity of ram spermatozoa [Citation35], but ME has been added in semen extender before the freezing process.
Contrasting results may be also due to different procedures in the cryopreservation process [Citation36]. Temperature-related alterations in cooling and freezing procedures have profound effects on the organization of structural components of the plasma membrane and may cause changes in the permeability and viability of the spermatozoa [Citation37]. Although it is not yet clear, probably during freezing and post thawing, there is a lipid–protein rearrangement resulting in the loss of the selective permeability characteristic of the biological membranes of viable cells [Citation38]. Some more pronounced molecular changes of the PM registered after incubation with antioxidants compared to the control semen indicate that probably the antioxidants were not utilized after thawing, due to changes in the sperm membrane induced by disturbances in the functions of transporter proteins.
Although supplementation with antioxidants can exert some protective effects against lipid peroxidation, there have been conflicting reports about the benefits of such supplementation for the post-thawing viability of the sperm of bulls [Citation39], rams [Citation40], and bucks [Citation41]. Spermatozoa from control samples exhibit high MMP, therefore generally have intact acrosome function and high fertilizing capacity [Citation42] corresponding with the observed normal motility and morphology. The decrease in the motility of the spermatozoa and the changes in PM corresponded with alterations in the mitochondrial status. The results suggest that the increase in apoptotic cells probably occurred at the expense of decreased motile spermatozoa in experimental samples. It is possible that spermatozoa are incapable of metabolizing some antioxidants as somatic cells do [Citation35,Citation43]. Similar to the present study, other authors have observed an increase in the apoptotic and early necrotic cells and a decrease in the viable counts in the buffalo sperm population after cryopreservation [Citation44]. The membrane disturbance is an indicator of initiated cell destruction [Citation45,Citation46]. Functional assays of plasma membrane integrity can potentially characterize the quality of spermatozoa via monitoring early phases of membrane dysfunction or initial phases of apoptosis [Citation47].
Conclusions
The present study demonstrated that that addition of Vit C (5 µmol/L) and ME (50 µmol/L) influenced the post-thawing semen quality of buffalo sperm. The tested antioxidants were associated with poorer integrity of PM and lower mitochondrial trans-membrane potential. Future studies may test the effect for inclusion of antioxidants in freezing extenders, as well as the effect of prolonged time for incubation with antioxidants.
Acknowledgments
This research was support by NNP Reprobiotech, MES-Bulgaria.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mittal PK, Anand M, Madan AK, et al. Antioxidative capacity of vitamin E, vitamin C and their combination in cryopreserved Bhadavari bull semen. Vet. World. 2014;7(12):1127–1131. e-ISSN: 2231–0916.
- Bucak MN, Ateşşahin A, Varışlı Ö, et al. The influence of trehalose, taurine, cysteamine and hyaluronanon ram semen, microscopic and oxidative stress parameters after freeze–thawing process. Theriogenology. 2007;67(5):1060–1067.
- Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009;10(1):49–62.
- Yu D, Simon L, Lewis SEM. The impact of sperm processing and cryopreservation on sperm DNA integrity. Sperm chromatin. New York: Springer; 2011. p. 397–409.
- Majzoub A, Agarwal A. Antioxidants in sperm cryopreservation. In: Parekattil S, Esteves S, Agarwal A, editors. Male infertility. Cham: Springer; 2020. p. 671–678.
- Kumar R, Jagan G, Mohanarao A. Freeze-thaw induced genotoxicity in buffalo (Bubalus bubalis) spermatozoa in relation to total antioxidant status. Mol. Biol. Rep. 2011;38(3):1499–1506.
- Peña FJ, García BM, Samper JC, et al. Dissecting the molecular damage to stallion spermatozoa: The way to improve current cryopreservation protocols? Theriogenology. 2011;76(7):1177–1186.
- Chaudhari DV, Dhami AJ, Hadiya KK, et al. Relative efficacy of egg yolk and soya based extenders for cryopreservation (–196 °C) of buffalo semen. Vet World. 2015;8(2):239–244.
- Patel HA, Siddiquee GM, Chaudhari DV, et al. Effect of different antioxidant additives in semen diluent on cryopreservability (–196 °C) of buffalo semen. Vet World. 2016;9(3):299–303.
- Andrabi SMH. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim. 2009;44(3):552–569.
- Nair SJ, Brar AS, S, Ahuja C, et al. A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim Reprod Sci. 2006;96(1–2):21–29.
- Husna A, Ansari MS, Rakha BA, et al. Melatonin supplementation in extender enhances the post thaw quality of buffalo bull spermatozoa. Pakistan J Zool. 2017;49(1):171–175.
- Strzezek J. Secretary activity of boar seminal vesicle glands. Reprod Biol. 2002;2:243–266.
- Akhter S, Rakha BA, Ansari MS, et al. Storage of Nili-Ravi buffalo (Bubalus bubalis) semen in skim milk extender supplemented with ascorbic acid and α tocopherol. Pak J Zool. 2011;43:273–277.
- Silva SV, Soares AT, Batista AM, et al. Vitamin E (Trolox) addition to Tris-egg yolk extender preserves ram spermatozoon structure and kinematics after cryopreservation. Anim Reprod Sci. 2013;137(1–2):37–44.
- Tariq M, Khan MS, Shah MG, et al. Exogenous antioxidants inclusion during semen cryopreservation of farm animals. J Chem Pharm Res. 2015;7(3):2273–2280.
- Reddy NM, Raja CKV. Seasonal variation in fructose, citric acid and ascorbic acid content of buffalo semen. Indian Vet J. 1979;56(8):660–662.
- Ansari MS, Rakha BA, Akhter S. Effect of L-cysteine in extender on post-thaw quality of Sahiwal bull semen. Anim Sci Pap Rep. 2011;29:197–203.
- Kichev G, Danov D. New protein – glycolipoprotein complex formation in diluted semen. Comptes rendus de L’Academie Bulgare des Sci. 1975;4:537.
- Ivanova MG, Gradinarska DG, Genov MS, et al. Changes in the phospholipid asymmetry of buffalo sperm plasma membrane in relation to cryopreservation technology. Pak Vet J. 2019;39(2):289–292.
- Maia MS, Bicudo SD, Sicherle CC, et al. Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen cryopreserved in extenders with antioxidants. Anim Reprod Sci. 2010;122(1–2):118–123.
- Maurya K, Singh OP, Srivastava S. Seminal plasma ascorbic acid level and its relationship to sperm characteristics in Murrah buffalo bulls. Ind J Ani Sci. 2013;83(5):498–501.
- Sandeep A, Singh P, Virmani M, et al. Efffect of vitamin C on the seminal and biochemical parameters of murrah buffalo bull semen during different stages of freezing. Haryana Vet. 2015;54(1):15–18.
- Yamaguchi S, Funahashi H. Effect of the addition of beta-mercaptoethanol to a thawing solution supplemented with caffeine on the function of frozen-thawed boar sperm and on the fertility of sows after artificial insemination. Theriogenology. 2012;77(5):926–932.
- Andrabi SMH, Ndrabi MS, Ansari NU. Effect of non-enzymatic antioxidants in extender on post-thaw pquality of buffalo (BUBALUS BUBALIS) bull spermatozoa. Pak Vet J. 2008;28(4):159–162.
- Askari HA, Check JH, Peymer N, et al. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl. 1994;33(1):11–15.
- Memon AA, Wahid H, Rosnina Y, et al. Effect of antioxidants on post thaw microscopic, oxidative stress parameter and fertility of Boer goat spermatozoa in Tris egg yolk glycerol extender. Anim Reprod. Sci. 2012;136(1–2):55–60.
- Raina VS, Gupta AK, Singh K. Effect of antioxidant fortification on preservability of buffalo semen. Asian Australas J Anim Sci. 2002;15(1):16–18.
- Shang JH, Huang YJ, Zhang XF, et al. Effect of β-mercaptoethanol and buffalo follicular fluid on fertilization and subsequent embryonic development of water buffalo (Bubalus bubalis) oocytes derived from in vitro maturation. Italian J Anim Sci. 2007;6(sup2):751–754.
- Lecewicz M, Strzeżek R, Kordan W, et al. Effect of extender supplementation with low-molecular-weight antioxidants on selected quality parameters of cryopreserved canine spermatozoa. J Vet Res. 2018;62(2):221–227.
- Foote RH, Brockett CC, Kaproth MT. Motility and fertility of bull sperm in whole milk extenders containing antioxidants. Anim Reprod Sci. 2002;71(1–2):13–23.
- Fernandez-Santos MR, Martinez-Pastor F, Garcia-Macias V, et al. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and non-enzymatic antioxidants. J Androl. 2006;28(2):294–305.
- Songsasen N, Apimeteetumrong A. Effects of -mercaptoethanol on formation of pronuclei and developmental competence of swamp buffalo oocytes. Anim Reprod Sci. 2002;71(3–4):193–202.
- Rekha DK, Tripathi Y, Raghuveer CV, et al. Role of vitamin C an antioxidant in cadmium chloride induced testicular damage. Int J Appl Biol Pharmaceut Technol. 2011;2(3):484–488.
- Pradieé J, Cardoso T, Silva EF, et al. Effect of β-mercaptoetanol and cysteine on post-thawing quality and oxidative activity of ram sperm and on the viability of vitrified sheep embryos. Arq Bras Med Vet Zootec. 2016;68(5):1309–1315.
- Tuli RK, Holtz W. Effect of glycerolization procedure and removal of seminal plasma on post-thaw survival and GOTrelease from Boer goat spermatozoa. Theriogenology. 1994;42(3):547–555.
- Rasul Z, Ahmad N, Anzar M. Changes in motion characteristics, plasma membrane integrity, and acrosome morphology during cryopreservation of Buffalo spermatozoa. J Androl. 2001;22:278–283.
- Watson H. Biological membranes. Essays Biochem. 2015;59:43–69.
- Bilodeau JF, Blanchette S, Gagnon C, еt al. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology. 2001;56(2):275–286.
- Bucak MN, Ateşşahin A, Yüce A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze-thawing process. Small Ruminant Res. 2008;75(2–3):128–134.
- Sinha MP, Sinha AK, Singh BK, et al. The effect of glutathione on the motility, enzyme leakage and fertility of frozen goat semen. Anim Reprod Sci. 1996;41(3-4):237–243.
- Grunewald S, Said TM, Paasch U, et al. Relationship between sperm apoptosis signalling and oocyte penetration capacity. Int J Androl. 2008;31(3):325–330.
- Ishii T, Bannai S, Sugita Y. Mechanism of growth stimulation of L1210 cells by β-mercaptoethanol in vitro: role of the mixed disulfide of β-mercaptoethanol and cysteine. J Biol Chem. 1981;256:12387–12392.
- Kadirvel G, Periasamy S, Kumar S. Effect of cryopreservation on apoptotic-like events and its relationship with cryocapacitation of Buffalo (Bubalus bubalis) sperm. Reprod Domest Anim. 2012;47(1):143–150.
- Almadaly EA, Tawfik FS, El-Kon II, et al. Effect of different cryoprotectants on the post-thaw sperm characteristics and in vivo fertility of buffalo (Bubalus bubalis) bull semen. Slov Vet Res. 2019;56(22):541–551.
- Mahmoud KS, Roos MEA, Ghaffar ADA, et al. Sperm characteristics in cryopreserved buffalo bull semen and field fertility. Iran J Appl Anim Sci 2013;3:777–783.
- Glander H-J, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryostorage. Mol. Hum. Reprod. 1999;5(2):109–115.