Abstract
SARS-CoV-2 is an emerging human beta-coronavirus that caused the COVID-19 (Coronavirus Disease − 19) pandemic, the most significant health and social crisis in the last 100 years. SARS-CoV-2 is not only a respiratory virus; the symptoms of COVID-19 can include gastrointestinal, neurological, renal, cardiovascular and other complications. A large part of SARS-CoV-2 infected individuals are asymptomatic or have mild symptoms, around 20% of COVID-19 cases require hospitalization, and 5% can become critically ill. This review summarizes data on the biology of the virus and its pathological manifestations, antiviral immune response, information on the experimental models used in the related studies, treatment approaches and vaccine strategies.
Coronaviruses
General information
Coronaviruses (CoVs) belong to the subfamily Coronavirinae in the family Coronaviridae, order Nidovirales (according to the International Committee on Taxonomy of Viruses). CoVs are enveloped viruses, 65–125 nm in diameter, which possess a relatively large single-stranded, positive-sense RNA genome of around 27–32 kb - the largest known genomes for RNA viruses. Currently, the most famous virus from the coronaviridae family, SARS-CoV-2 has a genome length of 29.881 kb (GenBank No. MN908947) and encodes 9860 amino acids [Citation1]. The name “Corona” represents the crown-like spikes on the outer surface of the virus (the word ‘corona’ means crown in Latin).
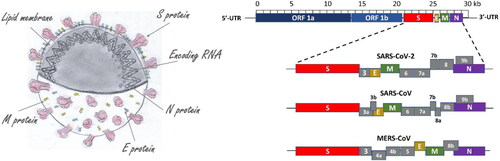
The morphology of coronavirus (left) and the organisation of the viral genomes of SARS-CoV, MERS-CoV and SARS-CoV-2 are illustrated in .
Figure 1. Coronavirus morphology along with the organization of the genome of SARS-CoV, MERS-CoV and SARS-CoV-2.
The spike (S) glycoprotein is responsible for the binding of the coronavirus to the host cell (this function is performed by the S1 part that contains the receptor-binding domain of the virus - RBD) and mediates membrane fusion and virus entry (the S2 domain plays the leading role in this process) after processing by cellular transmembrane serine protease 2 (TMPRSS2) [Citation2]. The S glycoprotein is also the main target for neutralizing antibodies [Citation3] (see ). The interaction of Spike’s RBD domain with the host receptor ACE2 is shown in .
Figure 2. The structure of SARS-CoV-2 Spike protein with neutralizing antibody (A) and the structure of the complex between Receptor ACE2 and RBD domain (B). Images were generated using Yasara View [Citation4].
![Figure 2. The structure of SARS-CoV-2 Spike protein with neutralizing antibody (A) and the structure of the complex between Receptor ACE2 and RBD domain (B). Images were generated using Yasara View [Citation4].](/cms/asset/b52b743a-1675-4fbd-a8c2-c8aa2ab87c80/tbeq_a_1847683_f0002_c.jpg)
Viral proteins
The understanding of the SARS-CoV viral proteins allows a rational approach to the development of more effective antiviral drugs [Citation5–7]. The proteins of SARS-CoV (presented in ) consist of two major polyproteins: ORF1a and ORF1ab (which cleave proteolytically to form 16 non-structural proteins); four structural proteins: spike (S), envelope (E), membrane (M) and nucleocapsid (N); and eight accessory proteins: ORF3a, ORF3b (NP _828853.1, not present in SARS-CoV-2), ORF6, ORF7a, ORF7b, ORF8a, ORF8b and ORF9b (NP _828859.1, not present in SARS CoV-2) [Citation5]. None of the accessory SARS-CoV proteins is essential for viral replication, but some of them play a role in viral pathogenesis, for example, some of them modulate interferon signalling pathways and the production of pro-inflammatory cytokines [Citation8]. Like SARS-CoV, SARS-CoV-2 lacks the haemagglutinin esterase gene found in human coronavirus (hCoV) HKU1 [8]. The spike protein [Citation2], envelope protein [Citation9,Citation10], membrane protein, nucleocapsid protein [Citation11], 3CL protease [Citation12], papain-like protease [Citation13], RNA polymerase [Citation14] and helicase [Citation15] have been proposed as viable targets for antiviral drugs [Citation16–18]. Examples of some viral proteins listed above are presented in .
Figure 3. Structures of some structural and non-structural proteins of SARS-CoV-2. Images were generated using Yasara View [Citation4].
![Figure 3. Structures of some structural and non-structural proteins of SARS-CoV-2. Images were generated using Yasara View [Citation4].](/cms/asset/33ee2db2-dbc2-434d-a9b1-90c3a48caefd/tbeq_a_1847683_f0003_c.jpg)
Table 1. A summary of SARS-CoV-2 proteins and their functions.
Family members
Based on their phylogenetic relationships and genomic structures, CoVs are divided into four genera: Alphacoronavirus (α), Betacoronavirus (β), Gammacoronavirus (γ) and Deltacoronavirus (δ) [Citation19–22]. The alpha-CoVs and beta-CoVs infect only mammals inducing mainly respiratory, gastrointestinal and neurological diseases. The gamma-CoVs and delta-CoVs affect birds, but some of them can also be pathogenic for mammals.
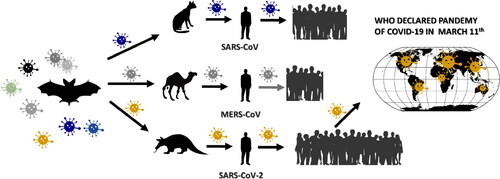
Currently, there are seven coronaviruses known to be pathogenic for humans (SARS-CoV, MERS-CoV, 229E, NL63, OC43, HKU1, as well as the new SARS-CoV-2) and all of them have a zoonotic origin from bats, mice or domestic animals. Four of them, HCoV-229E and HCoV-NL63 (α-CoVs) as well as HCoV-OC43 and HCoV-HKU1 (β-CoVs), induce mild upper respiratory diseases (common colds) in immunocompetent people [Citation23]. These viruses are endemic in the human populations, causing 15–30% of respiratory tract infections each year. They can result in more severe disease in infants, young children, the elderly, and in individuals with underlying illnesses (especially immunocompromissed individuals), with a greater incidence of lower respiratory tract infection in these people [Citation19, Citation21].
In this century, three highly pathogenic human beta-CoVs emerged: SARS-CoV, MERS-CoV and SARS-CoV-2. SARS-CoV caused the outbreak (2002-2003) of Severe Acute Respiratory Syndrome (SARS), which started in November 2002 in Guangdong province in China. The infected patients exhibited pneumonia symptoms with a diffused alveolar injury which led to acute respiratory distress syndrome (ARDS). There were more than 8000 infected individuals (mortality rate of around 9.6%) in more than 30 countries [Citation24]. A decade later, in 2012, MERS-CoV was identified to induce Middle East Respiratory Syndrome (MERS). MERS was spread mainly in the Middle East (almost 80% of the cases were in Saudi Arabia); the virus is endemic in this part of the world, but there was an outbreak in South Korea in 2015. According to the World Health Organization [Citation25], up to February 2020, there were >2500 laboratory-confirmed cases with a fatality rate of 34.4%. The affected individuals suffered pneumonia, followed by ARDS and renal failure [Citation26]. The SARS-CoV-2 outbreak began at the end of 2019 in Wuhan, Hubei province in China, and soon spread to many other countries. On January 30, 2020, the World Health Organization (WHO) declared “a public health emergency of international concern”; on March 11, the 2020 Covid-19 outbreak was announced a pandemic. SARS-CoV-2 has caused a global pandemic in only three months.
The first genomic sequence of SARS-CoV-2 was reported on January 10, 2020; the virus was isolated from a 41-year-old man who had worked at the animal market in Wuhan [Citation1]. SARS-CoV and SARS-CoV-2 are very similar, with 82% nucleotide sequence homology.
Cellular receptors, targeted tissues and organs
The most-reported cell receptor for SARS-CoV and SARS-CoV-2 is the cellular exopeptidase angiotensin-converting enzyme 2 (ACE2). The physiological role of ACE2 lies in the regulation of heart and kidney functions and blood pressure control. The main substrate for ACE2 is angiotensin II, and ACE2 acts as a negative regulator of the renin–angiotensin–aldosterone system (RAAS) by catalysing the hydrolysis of the active angiotensin II to the inactive angiotensin 1-7 [Citation27,Citation28]. The cell receptor for MERS-CoV is dipeptidyl peptidase 4 (DPP4) [Citation22, Citation29,Citation30]. The receptor-binding domains of both SARS-CoV and SARS-CoV-2 are reported to show high similarity; however subtle differences between the two account for a slightly higher binding affinity between SARS-CoV-2 and the ACE2 receptor compared to SARS-CoV [Citation31]. The SARS-CoV-2 spike binds the ACE2 receptor at least ten times more tightly than SARS-CoV does [Citation32]. This difference alone can only partially explain the higher transmissibility of SARS-CoV-2. As in the case of SARS-CoV, the cellular transmembrane serine protease 2 (TMPRSS2) is also required for SARS-CoV-2 entry into the cell – for the priming of spike S protein. The SARS-CoV-2 spike protein has been demonstrated to possess a sequence of amino acids (not present in SARS-CoV) that allows it to be recognized and cleaved by an enzyme called furin [Citation33,Citation34]. It is currently unclear whether and how this sequence is related to the virulence of SARS-CoV-2. However, from experience with other viruses (influenza viruses), we know that such sequences in the receptor-binding protein contribute to their virulence [Citation35].
Studies have detected the co-expression of transcripts encoding ACE2 and TMPRSS2 by single-cell RNA sequencing in various cell types, including nasal epithelial cells, type II alveolar cells, myocardial cells, proximal tubule cells of the kidney, ileum and oesophagus epithelial cells and bladder urothelial cells [Citation36,Citation37]. Autopsy studies have confirmed the presence of SARS-CoV-2 in multiple organs, such as the lungs, pharynx, heart, liver, brain, kidneys as well as in the blood, with the highest level found in the respiratory tract [Citation38].
ACE2 also determines the potential host range for SARS-CoV/SARS-CoV-2. These viruses can infect humans, cats, dogs, pigs, Chinese hamsters, civets, horseshoe bats, but not mice and rats [Citation39,Citation40].
Origin
Research has recognized bats as the natural reservoirs of a large variety of viruses, including the known human coronaviruses. SARS-CoV and MERS-CoV were further transmitted to humans via civet cats and camels, respectively [Citation41].
The novel coronavirus SARS-CoV-2 is also believed to have originated from bats, as its closest known relative (BatCoV RaTG13; GenBank: MN996532) was described in horseshoe bats (Rhinolophus affinis): the nucleotide homology between SARS-CoV-2 and BatCoV RaTG13 is 96.2% [Citation42–44]. However, its intermediate host before transmission to humans is not fully clarified yet. Genomic and evolutionary evidence suggests that this role can be played by pangolins – ant-eating mammals often used in traditional Chinese medicine. SARS-CoV-2 and CoV isolated from pangolins share 99% genetic homology [Citation40, Citation43, Citation45]. Additional investigations are required to confirm the intermediate zoonotic source/host(s) of SARS-CoV-2 that caused the transmission of the virus to humans, as this is crucial for our efforts to limit the spread of the virus.
Zoonotic transmission [Citation46] of the virus to humans in the cases of SARS-CoV, MERS-CoV and SARS-CoV-2 is shown in .
Coronavirus disease 2019 (COVID-19)
The initial site of infection of SARS-CoV-2 is the respiratory tract. The incubation period of SARS-CoV-2 is usually 1–14 days, mostly 3–7 days. However, it could also be longer depending on the age of the patients [Citation47]. The clinical picture of the disease ranges from asymptomatic via mild and moderate respiratory symptoms to severe and even potentially life-threatening cardiovascular and pulmonary complications [Citation48]. Fever is often the initial and major symptom of COVID-19, which can be accompanied by dry cough, shortness of breath, muscle ache, dizziness, headache, sore throat, rhinorrhea, chest pain, diarrhoea, nausea and vomiting. In severe cases, patients quickly progress to develop acute respiratory syndrome, septic shock, metabolic acidosis and coagulopathy [Citation22, Citation49,Citation50]. Nearly 20% of patients develop severe condition [Citation51], and the situation for 5% of them can become critical. Factors associated with increased mortality risk include male sex, advanced age, and presence of comorbidities, including hypertension, obesity, cardiovascular diseases, diabetes mellitus, cerebrovascular diseases [Citation52,Citation53]. Reportedly, about 2–11% of patients with COVID-19 have underlying chronic liver disease [Citation53].
Although COVID-19 is mainly associated with respiratory pathology, it can also lead to several extrapulmonary manifestations such as gastrointestinal, neurological, renal, cardiovascular and other complications. Hyperglycaemia and ketoacidosis, ocular and dermatologic symptoms are found in these patients [Citation54–56]. The diverse clinical picture is related to various factors, including the expression of the ACE2 receptor and protease TMPRSS2 in multiple cells, organs and tissues; endothelial damage and thromboinflammation, dysregulation of immune responses, disturbed functioning of RAAS and dysbalance between Angiotensin II and Angiotensin 1-7 physiological arms [Citation57].
Cardiovascular manifestations
There is abundant evidence that coronaviruses affect the cardiovascular system. [Citation52]. Studies have suggested that the target cardiac cells of SARS-CoV-2 are pericytes characterized by high expression of ACE2. The pericyte injury due to virus infection may result in capillary endothelial cells dysfunction [Citation58]. Cardiac complications of COVID-19 include acute myocarditis, arrhythmia, cardiogenic shock and even sudden death [Citation48, Citation52]. Some authors have hypothesized that SARS-Cov-2 may induce myocardial injury via Nox2-related production of reactive oxygen species (ROS) [Citation59]. Reports have demonstrated increased levels of troponin T and NT-proBNP, which are biomarkers of early myocardial damage, in SARS-CoV-2-infected patients [Citation58, Citation60,Citation61]. Individuals with COVID-19 often display underlying cardiovascular disease, and 19–33% of hospitalized patients experience cardiac injury [Citation62]. Elevated troponin levels in patients with COVID-19 are associated with high mortality [Citation63]. Moreover, studies have reported a sustained depression in left ventricular function in two-thirds of the patients recovered from a moderate to severe form of COVID-19 [Citation64,Citation65].
Kidney damage
Reports indicate that up to one in four patients in critical condition develop acute kidney injury (AKI). The pathophysiological developments of COVID-19 induced AKI can be both SARS-CoV-2 specific and non-specific. The COVID-specific mechanisms include cell damage caused by viral entry via the ACE2 receptor (which is characterized with a high renal expression), disruption of the RAAS, and elevated levels of pro-inflammatory cytokines. On the other hand, the non-specific pathways include hypovolemia, haemodynamic alterations, right heart failure, high levels of positive end-expiratory pressure (PEEP) in severe cases, where mechanical ventilation of the patient is required, elevated levels of pro-inflammatory cytokines as a result of the viral infection and thrombotic events, damage by nephrotoxic drugs and nosocomial sepsis. At this point, no specific treatment for COVID-19 associated AKI is available [Citation66].
Neurological manifestations
Neurological manifestations have been commonly reported in patients with COVID-19 [Citation57, Citation67,Citation68]. Practically around half of patients experience a reduction or complete loss of taste or smell [Citation69]. This fact is not surprising because respiratory viruses (including human coronaviruses) may disturb the central nervous system (CNS) due to their ability to induce direct damage in CNS as a result of replication and/or misdirected host immune responses that could be associated with autoimmunity in susceptible individuals. SARS-CoV-2 can affect the CNS via the olfactory tract in the early stages of infection by attacking the olfactory bulbs [Citation70]. Olfactory and taste disorders are part of the complaints in some patients with COVID-19 [Citation71]. Reports recognize neurological impairment and the demyelinating reaction as complications in case of severe COVID-19 [Citation67].
Liver complications
ACE2 receptors are expressed in cholangiocytes of the liver. Observations have shown hepatic dysfunction possibly related to the direct cytopathic effect of the virus, an uncontrolled immune reaction, sepsis or drug-induced liver injury, in 14-53% of patients with COVID-19, particularly in those with severe disease [Citation53, Citation72].
Pancreas
Pancreatic injury can occur in some COVID-19 patients [Citation73]. In another study, the authors report mild pancreatic injury patterns in patients with COVID-19 pneumonia that can be due to direct viral involvement of the pancreas or from secondary enzyme abnormalities in the context of severe illness without substantial pancreatic injury [Citation74]. On the other hand, there are reports of new-onset diabetes and severe metabolic complications of pre-existing diabetes. A global registry of patients with COVID-19-related diabetes has been established to clarify better the association between SARS-CoV-2 and diabetes (covidiab.e-dendrite.com).
Coagulopathy
Coagulopathy and thrombotic events can occur in association with SARS-CoV-2 infection. The origin of such events could be explained by various factors including direct viral effects, the disturbed balance between vasoconstrictor and vasodilator angiotensins, and the release of cytokines. There is an elevated production of pro-inflammatory cytokines interleukin 6 (IL-6), IL-17A, and tumour necrosis factor α (TNF-α) in the majority of patients with severe outcomes, and hypercoagulability is recognized as an essential hallmark of inflammation.
The initial coagulopathy in COVID-19 patients is associated with prominent elevation of D-dimer (fibrin degradation product, whose progressively increasing values indicate increased thrombin production and activation of fibrinolysis and can serve as a prognostic parameter indicating a worse outcome) and fibrin/fibrinogen-degradation products. Studies report a coagulopathy in up to 50% of patients with severe COVID-19 manifestations [Citation75–77]. COVID-19 frequently runs a more complicated course in patients with comorbidities at a more advanced age, and this group of individuals also have an age-related increased risk of thrombosis.
Reproductive system
One of the most critical questions is whether SARS-VoV-2 can affect (and in what way) the reproductive health of men and women and human fertility. Furthermore, can vertical (mother-to-fetus) and sexual transmissions occur? These concerns are based on the following facts: i) The ACE2 receptor is found in the vagina, ovaries, uterus and placenta. Moreover, ACE2 is involved in the regulation of follicle maturation, and ovulation affects changes in the endometrium and embryo development. The degree of expression of this receptor changes during the menstrual cycle; ii) ACE2 is also expressed in the testes, and, according to some reports, orchitis could be a complication of SARS-CoV infection [Citation76]. However, the viral load is thought to be too low to cross the blood-testis barrier, and the ACE2 concentration has been suggested to be insufficient to allow the virus to enter (for review of this and other routes of SARS-CoV-2 transmission see [Citation78]). To date, there is no evidence of damage to the female reproductive system in COVID-19 patients. Additional studies are needed to clarify better whether or not, and to what extent SARS-CoV-2 can affect male reproductive health [Citation79,Citation80]. Sexual and vertical transmission of SARS-CoV-2 has not been confirmed.
SARS-CoV-2 and children
Liguoro et al. [Citation81] have performed a systematic overview of the available data on clinical, laboratory and radiological findings in children with SARS-CoV-2 infection based on the analysis of sixty-two studies (conducted in the period Jan 1, 2020–May 1, 2020) and three reviews. The total number of children included is 7480, aged 0 to 18 years (mean age 7.6 years). This overview shows that infected children display mainly mild (42.5%) and moderate symptoms (39.6%), among which the most common are fever (51.6%) and cough (47.3%). Only 2% of children go to paediatric intensive care units. The estimated mortality is 0.08%. A higher proportion of more seriously ill children was found in newborns:- 12%, with the most common sign being shortness of breath (in 40% of cases). Laboratory and radiological findings in children were mainly non-specific [Citation81]. A multisystem inflammatory syndrome related to infection with SARS-CoV-2 has been reported in older children (known as MIS-C), manifested by severe abdominal pain, cardiac dysfunction and shock, with a phenotype resembling Kawasaki disease (KD). MIS-C can lead to severe and life-threatening illness in previously healthy children and adolescents [Citation82,Citation83]. The inflammatory response in MIS-C differs from the cytokine storm of severe acute COVID-19 and shares several features with KD [Citation84]. Compared with children with KD, those with MIS-C show differences in T-cell subsets, the level of IL-17A expression (it is lower) and biomarkers associated with arterial damage. Also, various autoantibodies have been suggested to be involved in the pathogenesis of MIS-C [Citation84].
Modes of transmission
SARS-CoV-2 can be transmitted through direct, indirect or close contact with infected people through secretions such as saliva and respiratory secretions or respiratory droplets (expelled during a cough, sneeze, speech, song or even a simple exhalation). The size of these respiratory droplets is critical because it determines how far they will travel and how long they will stay in the air. The so-called respiratory droplets are >5-10 μm in diameter, whereas those that are <5μm in diameter are referred to as droplet nuclei or aerosols [Citation85]. In the case of respiratory droplets, infection occurs at a distance within 1 m from the infected person, when they enter the mouth, nose or eyes of a susceptible individual. These larger droplets quickly fall to the ground, but being smaller and lighter aerosols can stay suspended in the air and travel a more extended way (it is not completely clear how much exactly) [Citation85–87]. Aerosol generation can occur during some medical procedures and is associated with increased risk of respiratory virus transmission (including SARS-CoV-2) to healthcare workers [Citation87,Citation88]. COVID-19 transmission through aerosol has been reported [Citation89–91].
Respiratory secretions (droplets and aerosols) can contaminate surfaces and objects, creating fomites [Citation85]. Viable SARS-CoV-2 virus and/or RNA detected by reverse transcription polymerase chain reaction (RT-PCR) can be found on those surfaces for various periods ranging from hours to days, depending on the ambient environment (including temperature and humidity), type of surface (the type of material and its relief - smooth, rough), exposure to sunlight. Literature reports surface persistence for up to 9 days, but disinfection can be achieved successfully using 70% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1-minute exposure. Other agents such as 0.05-0.2% benzalkonium chloride and 0.02% chlorhexidine digluconate are reported as less effective [Citation92]. There are no specific reports to demonstrate fomite transmission directly. This is not surprising because people who have come in contact with contaminated surfaces have usually also been in physical contact with the infectious person [Citation87].
Other possible modes of SARS-CoV-2 transmission have also been discussed. The virus detection in urine and stool has been documented [Citation50, Citation93–96]. SARS-CoV-2 RNA can be detected in feces for an extended period, even after respiratory samples have tested negative and patients are asymptomatic. According to the available literature, the transmission of SARS-CoV-2 in humans through feces or urine has not been confirmed [Citation87]. Currently, there is no evidence of SARS-CoV-2 transmission through breast milk [Citation97]. WHO recommends that mothers with suspected or confirmed COVID-19 should be encouraged to initiate or continue to breastfeed [Citation98].
Although SARS-CoV-2 has been detected in the amniotic fluid, in the cord blood and the placentas of infected women, the ability of this virus to undergo intrauterine or transplacental transmission from infected pregnant women to their foetuses has not been confirmed [Citation99,Citation100]. Experts warn that full clarification of this issue requires further research.
Viral transmission occurs during close and prolonged contact, such as a shared household or stays in enclosed places for 15 min or more with an infected person and without precautions [Citation101].
SARS-CoV-2 RNA can be detected in people 1–3 days before the onset of clinical symptoms. The highest viral loads (measured by RT-PCR) appear on the day of symptom onset and then gradually decrease over time [Citation86,Citation87, Citation102,Citation103]. One of the challenges for preventive control of SARS-CoV spreading is that people infect others before they became ill [Citation104–106].
Factors associated with SARS-CoV-2 susceptibility and pathogenesis
One of the most intriguing questions is why people react differently to the virus: some people do not develop symptoms at all, or the clinical picture is mild, while others develop a severe clinical picture that can lead to a life-threatening condition. The answer to this question is undoubtedly ambiguous and includes factors from the virus, the host and the environment.
Host factors
The risk of a more severe course of COVID-19 and a fatal outcome is increased in individuals with underlying comorbidities (including obesity, diabetes, cardiovascular and lung conditions and cancer) and those with impaired immune system, regardless of age, and in older people who are more likely to have these conditions. Data show that men are more vulnerable than women.
Various hypotheses have been proposed to explain higher morbidity and mortality in the elderly. Potential roles of the hallmarks of ageing including immunosenescence, inflammation and inflammasomes, genomic instability, mitochondrial dysfunction, epigenetic alterations, biological clocks such as telomere attrition, impaired autophagy and several other pathobiochemical changes have been discussed in relation with COVID-19. Intensive studies have focussed on the differences between cellular/molecular mechanisms of biological responses between young, middle aged and older people as well as between males and females to improve our knowledge about host–coronavirus interactions and prophylactic and therapeutic strategies [Citation107,Citation108].
Severe COVID-19 clinical picture is observed (although less frequently) in younger people without known concomitant diseases. What is the role of our genetics and epigenetics? Analysis of the genome of about 4,000 people from Italy and Spain shows a correlation between specific genetic variants and the severity of COVID-19 [Citation109]. The study includes 835 patients and 1255 control participants from Italy and 775 patients and 950 control participants from Spain; all the patients had severe COVID-19 (pulmonary failure). In total, the authors analyzed 8,582,968 single-nucleotide polymorphisms and conducted a meta-analysis of the two case-control panels. As a result of their efforts, a 3p21.31 cluster of six genes (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1) was identified as the genetic susceptibility locus in patients with COVID-19 with respiratory insufficiency.
Further studies are needed to clarify better the significance of these genes for virus infection and the severity of the disease. It is interesting to note that the product of one gene (SLC6A20, which encodes the sodium-imino acid (proline) transporter 1 - SIT1) interacts with the ACE2 viral receptor. Other genes encode chemokine receptors (such as CC motif chemokine receptor 9 - CCR9, and the C-X-C motif chemokine receptor 6 - CXCR6) and are involved in the immune response against pathogens. The study also confirmed the potential importance of ABO blood group antigens for virus infection and disease: higher risk for blood group A and a lower one for blood group O as compared with other blood groups [Citation109]. There are data that specific HLA polymorphisms are related to the susceptibility and protection from SARS-CoV-1 infection [Citation110,Citation111]. Authors have suggested that epigenetic dysregulation of ACE2 and interferon-regulated genes is associated with increased COVID-19 susceptibility and severity in lupus patients [Citation112].
Viral factors
Although researchers have described thousands of mutations and different variants and subvariants of the virus, there is currently no evidence that any mutation or variant is responsible for the development of a more severe clinical picture and higher mortality or to be associated with a milder disease [Citation113,Citation114]. Environmental factors are poorly understood. However, there is some evidence that suggests a possible correlation between air pollution and a more severe clinical course of disease [Citation115].
Model systems
Suitable model systems are required to study virus biology and behaviour, various aspects of its pathogenicity, as well as to identify effective strategies for prevention and treatment. Cell cultures (primary cell cultures and permanent cell lines) have been used for many years in experimental and clinical virology as well as in the design and development of antiviral agents and vaccine production.
Cell lines established from African green, cynomolgus, rhesus monkey as well as buffalo green monkey kidney (such as BGM, CV-1, FRhK, LLC-Mk2, MA-104, pCMK, Vero E6) are susceptible to productive SARS-CoV infection. Reaching a high viral titer is not always accompanied by a specific cytopathic effect [Citation116].
The novel coronavirus SARS-CoV-2 was isolated in pathogen-free human airway epithelial (HAE) cells [Citation117] as well as from Vero E6 (kidney epithelial cells of African green monkey, clone E6) and Huh7 (human hepatoma) cell lines. An engineered TMPRSS2-expressing VeroE6 cell line that is highly susceptible to SARS-CoV-2 infection and useful for virus isolating and propagating has been obtained [Citation39].
Cell cultures are valuable model systems, but they also have their disadvantages and limitations. Thus, as a result of prolonged cultivation in laboratory conditions, many genetic and epigenetic changes can occur in the permanent cell lines, moving them away from the characteristics of the initial tissue/organ from which they are derived. Primary cell cultures represent better biological features of the starting material. However, their application is associated with other difficulties, including a limited capacity for cell division and short life, heterogeneity (it is not possible to obtain the same primary cell culture twice even following the same protocol), different expression levels of genes crucial to infectivity, including host receptors. Sometimes it is not easy to find source material which is the case, for instance, with donors for HAE cells. Also, the conventional monolayer (2 D) cell cultures do not reproduce adequately virus–host cell interactions. 3 D cell cultures, as well as organ-on-a-chip models, are improved next generation in vitro models that can represent better the properties of biological systems in vivo.
Animal models
Certain animal species have long been established as important model systems for investigating human pathology. Approximately 90% of the laboratory animals used in biomedical studies are mice, rats and other rodents [Citation118]. ACE2 of mice cannot interact with SARS-CoV-2, so these laboratory animals cannot be used as experimental models without genetic modifications (for example, transfection of human ACE2) [Citation40]. For a detailed review that recently focussed on animal models in SARS-CoV-2 research, see [Citation119]. Here we outline the most notable examples in brief.
Small animals
The high genetic similarity (>80%) between SARS-CoV and SARS-CoV-2 and the fact that both viruses use the same cellular receptor (ACE2) makes it possible to use the models developed for the investigations of SASR to study the new coronavirus and COVID-19. Among them are F344 rats where SARS-CoV was adapted to replicate and induce severe disease as a result of a mutation (single amino acid substitution) at spike S glycoprotein recognizing cellular ACE2 [Citation120]. Mouse-adapted models of SARS-CoV (SARS-CoV MA15) have also been developed [Citation121].
Genetically modified small animals can be applied to investigate the pathogenicity of novel coronaviruses as well as in the search for effective treatment options. Among the benefits of this type of models are their relatively low cost and high reproducibility of experiments as well as the availability of detailed information about their biological characteristics [Citation118, Citation122,Citation123]. A ferret and Syrian hamster model of SARS-CoV-2 infection and transmission that recapitulates aspects of human disease have been reported. These models are expected to be useful for understanding SARS-CoV-2 pathogenesis, as well as the search for new prophylactic and therapeutic approaches [Citation124,Citation125].
Non-human primates
Non-human primates (NHPs) provide highly valuable animal models for research related to human health and pathology because of at least two reasons: i) they have close phylogenetic relationship with humans; ii) among all other laboratory animals, they most closely resemble the development and maturation of the human immune system. What is more, the similarities in lung development and structure between human and NHPs that are not observed in rodents make them very suitable for studies in the field of lung pathologies and treatment [Citation118, Citation122,Citation123].
Designing a suitable model of COVID-19 is an essential next step towards a better understanding of the mechanisms associated with the pathogenesis of SARS-CoV-2, as well as easier identification of both preventive and treatment solutions.
Immunity
SARS-CoV-2 is a new virus, so the entire human population generally lacks specific immunity against it, and people are susceptible to infection.
The antiviral immune response involves two steps:
an early, non-specific phase (usually occurs in the first 5-7 days after infection) where a key role plays the synthesis and secretion of type I interferons (IFN-I) such as IFN-α and IFN-β. This first line of defense is essential because it is critical to the fate of the virus, i.e. whether it will be successfully controlled or spread to the host.
a later, antigen-specific phase involving adaptive immunity performed by T and B lymphocytes. Cytotoxic CD8+ T cells directly kill virus-infected cells. Through cytokine signalling, CD4+ T cells stimulate B cells directly to produce virus-specific antibodies and to support other defending cell populations indirectly [Citation126,Citation127].
Like other viruses, the new coronavirus implements different mechanisms to escape the host immune response. It can be assumed that the immune evasion mechanism of SARS-CoV-2 potentially resembles those of the other two highly pathogenic human beta coronaviruses SARS-CoV and MERS-CoV. Some of the proteins synthesized by SARS-CoV-2 (such as N protein, a novel short protein encoded by orf3b) may counteract the antiviral effect of IFN-β [Citation29, Citation128,Citation129].
Acute SARS-CoV-2 infection has been found to result in a reduction of T lymphocytes, natural killer cells, monocytes and dendritic cells [Citation130]. A decreased number of CD4+ and CD8+ T cells, as well as lower IFN-γ production that correlates with disease severity, has been reported in COVID-19 patients [Citation51].
The main target of neutralizing antibodies to coronaviruses, including SARS-CoV-2 is the S glycoprotein [Citation2]. Antibodies against the nucleoprotein are also generated [Citation131,Citation132].
IgM and IgG antibodies have been reported to show similar dynamics in COVID-19 disease. There are data that specific IgA response tends to appear earlier and is stronger and more persistent than the IgM response [Citation133–135]. IgA levels in severe cases were significantly higher than those in mild or moderate cases [Citation136].
Adequately regulated, both the innate and adaptive immune responses are needed to control the infection and clear the virus successfully. On the other hand, the excessive inflammation, local generation of a large number of free radicals and an exaggerated immune response can lead to the so-called “cytokine storm” and subsequent progression to acute lung injury/acute respiratory distress syndrome and in the worst scenario multi-organ failure and death.
Cytokine storm
The “cytokine storm” is associated with hyperactivation of the transcription factor NF-kappa B (NF-κB) and sudden acute release of different pro-inflammatory cytokines and chemokines such as IL-6, IL-1, tumour necrosis factor-alpha, and interferons. SARS-CoV-2 directly activated NF-κB via pattern recognition receptors [Citation137].
Downregulation of ACE2 due to the binding of SARS-CoV-2 to its cellular receptor leads to an increase in angiotensin II (Ang II). In addition to its vasoconstrictor function, angiotensin II also expresses pro-inflammatory effects via interaction with its AT1 receptor (AT1R). AngII-AT1R axis stimulated NF-κB and disintegrin and metalloprotease 17 (ADAM17), which leads to the following consequences: generation of epidermal growth factor receptor (EGFR) ligands and TNFα that are NF-κB stimulators; trans-signalling of IL-6-sIL-6Ra complex, in which the gp 130-mediated activation of STAT3 occurs in the lung epithelial cells [Citation61, Citation137–140].
The cytokine storm, which results in exacerbation of the severe acute respiratory distress syndrome reported in COVID-19 patients, is induced by NF-κB hyperactivation. Even though SARS-CoV-2 itself can activate NF-κB, hyperactivation of NF-κB is achieved by the IL-6 amplifier, through the simultaneous activation of both NF-κB and STAT3.
Increased levels of cytokines IL-1β, IL-7, IL-8, IL-9, IL-10, FGF (fibroblast growth factor), G-CSF (Granulocyte colony-stimulating factor), GM-CSF (Granulocyte-macrophage colony-stimulating factor), IFN-γ, IP-10 (Interferon gamma-induced protein 10), MCP-1 (monocyte chemoattractant protein-1), MIP-1A and MIP1-B (macrophage inflammatory proteins), PDGF (Platelet-derived growth factor), TNF-α and VEGF (vascular endothelial growth factor) have been reported in patients with severe COVID-16.
Such hypercytokinemia is a frequently occurring event also in other severe infections with SARS-CoV, MERS-CoV as well as H5N1 and H7N9 influenza viruses. It is often associated with disease progression and outcome [Citation61, Citation137–140]. The correlation between levels of inflammatory cytokines (G-CSF, IP10, MCP1, MIP1A, and TNF-α) and disease severity has been observed [Citation61]. There are data that the IL-6/IFN-γ ratio is higher in severe than in moderate COVID-19 patients and could be connected with an enhanced cytokine storm [Citation141].
Different strategies for reducing the cytokine storm in the treatment of COVID-19 are discussed. Among them is the application of artificial-liver blood purification [Citation142] and angiotensin receptor blockers [Citation143].
Antibody-dependent enhancement
Antibody-dependent enhancement (ADE) is a paradoxical phenomenon in host–pathogen biology, in which the presence of specific antibodies can be beneficial to the virus. It is crucial not only for the understanding of viral pathogenesis but also for the development of adequate antiviral strategies, especially vaccines.
The ADE is a process in which suboptimal virus-specific antibodies enhance the entry of the virus, and in some cases, the replication of the virus, into monocytes/macrophages and granulocytic cells through interaction with Fc and/or complement receptors. ADE has been reported in vitro and in vivo for various viruses of public health and veterinary importance, including coronaviruses [Citation144,Citation145].
ADE has been observed in some preclinical animal models vaccinated with experimental SARS-CoV vaccines [Citation146,Citation147]. Avoiding this reaction is one of the significant challenges in developing the vaccine against COVID-19.
Some authors have suggested that ADE of SARS-CoV-2 infection due to prior exposure to other locally circulating coronaviruses with similar antigenic epitopes may explain the severity of cases observed in the Hubei province of China [Citation148]. It is not yet clear whether ADE is linked to SARS-CoV-2 infection, but finding an answer to this question is one of the hot topics in biomedicine at the moment [Citation149].
Pre-existing immunity
Undoubtedly, one of the most intriguing questions is whether the presence of a cross-immune response against the human coronaviruses inducing common colds (hCoV-ICC) - 229E, NL63, OC43 and HKU1, can contribute to the milder course of SARS-CoV-2 infection.This hypothesis is based on the following facts: i) hCoV-ICC, on the one hand, and SARS-CoV-2, on the other, share many common characteristics, including transmissibility and the ability to infect and multiply in the upper respiratory tract; ii) 229E, NL63, OC43 and HKU viruses are widely distributed and each of us has probably encountered them many times in our lives – it has been estimated that ≥90% of adults have IgG antibodies specific to these four viruses [Citation150].
We do not yet have a definitive answer to this question. However, pre-existing SARS-CoV-2-cross-reactive T cell responses (directed against S glycoprotein and other proteins) have been proven in healthy individuals not exposed to SARS-CoV-2, indicating some potential for pre-existing immunity in the human population [Citation150,Citation151]. One thing is for sure, studying the immune response to hCoV-ICC will help us to be better oriented in the situation with SARS-CoV-2 and the COVID-19 pandemic.
Effectivity of the immune response
We do not yet understand the durability of the immune responses to SARS-CoV-2. The available experience with human coronaviruses that cause common colds indicates that they do not always induce long-lasting antibody responses, which, in turn, makes the reinfection of a person with the same virus possible [Citation152,Citation153]. Specific antibodies were found in individuals that survived SARS-CoV or MERS-CoV infections 2–3 years later [Citation154,Citation155] and even after a more extended period in the case of MERS survivors [Citation153] but it is not clear whether they are enough to prevent reinfection.
Our knowledge about possible reinfection by SARS-CoV-2 is limited, but it is well known that reinfections by coronaviruses occur. For hCoV-ICC, the reinfection times ranged between 6 and 105 months and frequently occurred at 12 months after the initial infection [Citation156]. Such information on the duration of protective immunity is essential to predict a possible second wave of an epidemic.
Both CD4+ (T helper cells) and CD8+ (cytotoxic, or killer, T cells) lymphocytes have been reported to play an important role in protective immunity against SARS-C0V-2 infection and are associated with milder disease. Low amount of naive T cells (which may be due to ageing) are recognized as immunological risk factors predisposing for severe COVID-19 [Citation157]. Individuals who have recovered from COVID-19 have been reported to possess high levels of both neutralizing antibodies and T cells. In contrast to severe cases, milder cases of COVID-19 show more significant numbers of memory CD8+ T cells in the respiratory tract [Citation150, Citation158,Citation159].
Antiviral antibodies against SARS-CoV-2 were reported not to decline within four months after diagnosis [Citation160]. It is still unclear what titer of neutralizing antibodies is sufficient to confer protection against infection
Some sex differences in immune responses that underlie COVID-19 disease outcomes have been described, including higher plasma levels of IL-8 and IL-18 in male patients, whereas females demonstrate more pronounced T cell activation [Citation161]. Such differences can at least partially explain the more severe symptoms and higher mortality among men than among women.
Estimates show that a large part (40–75%) of SARS-CoV-2 infected people are asymptomatic or have mild symptoms. These individuals seem to develop low levels of antibody-mediated immunity which poses a significant challenge for establishing herd immunity [Citation162–164].
Genetic stability and mutations
Coronaviruses, including SARS-CoV-2, possess an RNA proofreading machine (ExoN) that can protect them from the increased mutation rate typical of other RNA viruses [Citation165,Citation166]. The following reasons can at least partially contribute to explain the genetic stability of SARS-CoV-2: i) It is new to humankind and therefore does not meet with an already existing immune response against it; ii) We do not have widespread specific anti-coronavirus agents to treat the infection; hence, there is little selective pressure. SARS-CoV-2 mutation rate is thought to be twice as low as that of the influenza virus.
However, the virus is changing, and SARS-CoV-2 today is certainly not what it was at the beginning of the pandemic. The genome of the virus was first sequenced at the beginning of 2020, and on January 11, 2020, it was published in a specialized database for genetic information, then immediately found a place in "Nature" journal [Citation1]. What has happened to the virus in the past nine months?
The first news in this regard was announced by Tang et al. [Citation167]. According to the data available at that time, at least 149 sites of mutations were identified across the genome of 103 sequenced strains of SARS-CoV-2 allowing the identification of two viral subtypes, termed L and S subtype [Citation167]. Later, the phylogenetic network analysis of 160 complete genomes of human SARS-Cov-2 revealed the presence of three variants designated as A, B and C [Citation168]. These subtypes/variants seem to have different geographical distribution and transmission ability.
A more recent study conducted by Mercatelli and Giorgi [Citation113] from the University of Bologna, Italy, also attempts to answer this question. The authors systematized the genomic analyses of 48 635 virus samples isolated and sequenced in laboratories around the world and available because of the collective endeavour of the GISAID consortium.
Although a total of 353 341 mutation events were identified compared to the reference viral genome NC_045512.2 Wuhan, the results show that the number of mutations that each sample carries is relatively low (average 7.23 per sample) and more than 15 mutation events are detected in very few isolates. Interestingly, the samples originating mainly from Asia (256 samples in total) did not differ from the reference one, while the remaining 48,379 samples carry at least one mutation. There was a difference in the average number of mutations per sample between countries, but not between continents.
The authors identify six phylogenetic groups G (named after the Spike D614G mutation), GH, GR, S, V and L with different characteristic mutations, frequency of occurrence and geographical distribution:
The original lineage “L,” corresponds to the reference genome NC_045512.2. Clade S appeared in early 2020; clades V and G, after mid-January; and GR and GH, in late February 2020.
Clade G and its derivatives GH and GR are the most common: they represent about 74% of all analyzed samples. The G and GR clades are most common in Europe and Italy. This study [Citation113] did not detect GH in Italy, but it was often found in France and Germany. GH is the most common variant in North America, while GR predominates in South America. In Asia, the prevalence of G, GH and GR is also increasing. Clade S is identified in some areas in the USA and Spain, and L and V are gradually disappearing.
Another study presents data based on the genome analysis of more than 90,000 SARS-CoV-2 isolates. The results suggest that every two viruses isolated from any two individuals in any two parts of the world differ on average by only ten nucleotides [Citation114].
Despite the slow rate of mutation, scientists have identified and categorized more than 12,000 mutations. One of them is the most common and is detected in around 97% of the cases; this is the mutation D614G (aspartate to glycine change in protein position 614 of the S protein, presented in ). This mutation is not located within the receptor-binding region of the S protein. A recent study of different SARS-CoV-2 S protein variants using pseudoviruses (see ) linked the D614G mutation alone to higher infectivity but did not observe it to result in a change of sensitivity to neutralizing antibodies [Citation169].
Figure 5. Mutation D614G stabilizes the open state of the S protein of SARS-CoV-2. The S-protein in the open “active” state (PDB ID:6xs6) is more accessible for interaction with ACE2 than in the closed state (PDB ID:6vxx). This key mutation may be a reason for increased viral infectivity.
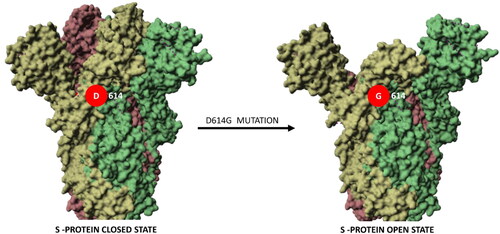
Table 2. Mutations of S protein and their effect on viral infectivity and sensitivity to neutralizing monoclonal antibodies (mAbs)/sera from convalescent patients [Citation169].
It is not excluded that different viral variants may cause COVID-19 with various rates of severity. Up to now, however, there is no mutation associated with a higher risk to public health.
Treatment strategies
SARS-CoV-2 is a new virus, so at the moment there is no specific and universally accepted treatment and vaccine for it. Supportive care is also crucial to the treatment provided to COVID-19 patients and includes approaches to managing clinical symptoms (such as fever), fluid management, oxygen therapy, optimal management of complications (including antibiotic and/or antifungal treatment) and underlying comorbidities.
The wide spread of the disease all over the world raises the urgency of the search for a targeted anti-SARS-CoV-2 agent. Currently, the efforts of specialists are focussed in two directions:
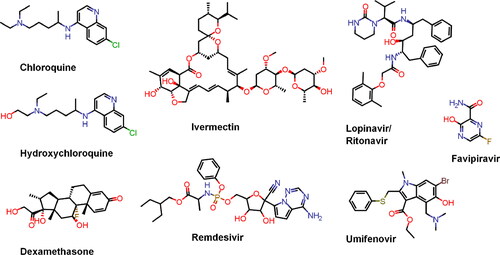
Drug repurposing. The choice is based on the following factors: registered drugs with known pharmacokinetics, pharmacodynamics and toxicological profiles; some of them showed an effect in the treatment of SARS and/or MERS; promising activity against COVID-19 has been observed in small clinical trials (in China and other countries); they can be produced quickly and in large quantities. Examples of such medications are chloroquine and hydroxychloroquine, lopinavir/ritonavir, remdesivir, favipiravir (see ). In general, the repurposed agents can be divided into two main groups: i) drugs that are expected to attack the replication and life cycle of the SARS-CoV-2 directly, and ii) those that affect the immune response by stimulating innate immunity or suppressing unregulated abnormal immune/inflammatory reactions and prevent or relieve the induced damage. These drugs have been included in large clinical trials initiated by the WHO and by France, the United Kingdom, Canada, etc. Brief information about some of them is presented in . The interaction of these treatments with other drugs used in the patients should be monitored carefully [Citation22, Citation48, Citation177, Citation193, Citation200]. The regimen of hydroxychloroquine in combination with azithromycin has been suggested to be promising for individuals with SARS-CoV-2 infection [Citation170, Citation201], but later WHO discontinued these clinical trials due to lack of benefit for hospitalized patients.
Table 3. Repurposed therapeutic agents for the treatment of COVID-19 currently in clinical trials.
Currently, the results of only several trials investigating the potential benefits of remdesivir in the fight against COVID-19 are available [Citation171, Citation178–181]. On May 1, 2020 the U.S. Food and Drug Administration (FDA) [Citation202] granted Veklury (remdesivir) authorization for emergency use for the treatment of suspected or laboratory-confirmed COVID-19 in hospitalized adult and paediatric patients with severe disease. This decision was based on the preliminary results of the randomized, double-blind clinical placebo-controlled trial of 1062 patients by Beigel et al. [Citation181] (ACTT-1 trial), which concluded that remdesivir shortened recovery time from 15 to 11 days (later revised to 10 days in the final report). The emergency use authorization was later expanded on August 28, 2020 to include all hospitalized COVID-19 patients regardless of severity of disease [Citation203]. On October 22, 2020 Veklury was approved by the FDA [Citation204] for treatment of hospitalized COVID-19 patients aged 12 and older and weighing 40 kg or more. The analysis of the evidence of remdesivir’s effectiveness, leading to its approval, included the findings from the trials by Beigel et al. [Citation181], Goldmann et al. [Citation179] and Spinner el al. [Citation180]. However, the interim results from the larger SOLIDARITY trial, which is coordinated by the WHO and aims to assess the effectiveness of remdesivir, hydroxychloroquine, lopinavir/ritonavir and interferon-β1a, has concluded that none of the investigated drugs had a significant clinical benefit (with regards to hospital stay duration, overall mortality and initiation of ventilation) compared to standard-of-care without a drug [Citation171]. The apparent disagreement between the findings of the reported trials may be at least partially explained by differences in trial design, patient heterogeneity and stage of disease progression of patients at enrolment. Large multicenter trials also need to account for the differences which arise from studying patients/volunteers from different countries/geographic locations, such as variations in the provided medical care and potential pharmacogenetic and other variability of local populations. As such, at the time of writing of this review, it is still early to know for certain if remdesivir will gain widespread approval outside of the USA, and more research is needed to carefully evaluate its clinical effects.
Evidence has demonstrated that administration of dexamethasone is effective for suppression of excessive immune response in critically ill patients [Citation205]. On July 16, 2020, FDA added dexamethasone to COVID-19 temporary compounding lists.
Anticoagulant therapy with heparin was also associated with better prognosis in severe COVID-19 patients [Citation206]. In the search for practical approaches to prevent COVID-19-associated thrombosis and complications and to improve treatment results, scientists have proposed several antithrombotic drugs, including heparin, FXII inhibitors, fibrinolytic drugs, nafamostat and dipyridamole, many of which with pleiotropic anti-inflammatory or antiviral effect [Citation207].
Nevertheless, in the urgency and early stages of anti-SARS-CoV-2 drug discovery, one should be careful in promoting new indications for known drugs. Namely, the American Heart Association has recognized chloroquine and hydroxychloroquine as agents which can cause direct myocardial toxicity. Furthermore, studies compromising chloroquine and hydroxychloroquine activity against SARS-CoV-2 have been published [Citation208]. Similarly, other drugs such as favipiravir and kaletra (lopinavir/ritonavir) as well as several antibiotics commonly used for the treatment of patients with COVID-19, for instance, azithromycin, can prolong the QT interval [Citation62, Citation209] and cause other difficulties [Citation210].
Design and development of effective new anti-SARS-CoV-2 agents. Drug design can be directed at several major targets (spike protein, envelop protein, membrane protein, protease, nucleocapsid protein, haemagglutinin esterase and helicase) [Citation16, Citation211]. Despite requiring a more extended development and approval process, the search for novel therapeutic agents may yield more specific and compelling treatment options and possibly a common drug solution for coronaviruses [Citation212]. For a broad-spectrum coronavirus antiviral to be developed, it must be able to target either viral or host components, which are vital to the replication cycle of the virus or the disease development in the host. Of course, the targeted factors must be present and highly conserved within a broader range of CoVs to ensure efficacy in future emergent CoVs [Citation122].
Brief information about other strategies proposed for the treatment of COVID-19 patients is presented below.
Plasma therapy
Though new to the current pandemic, serum therapy has been used (in various forms) since the 1890s when it was introduced in medicine by Emil von Behring for the treatment of diphtheria and tetanus – the first Nobel Prize in physiology and medicine in the twentieth century was awarded in 1901 for this achievement. A direct antiviral effect of virus-neutralizing antibodies in convalescent plasma is believed to be the main mechanism of action, but indirect effects may also contribute to improved viral clearance, inflammation and organ function [Citation123, Citation213]. Many clinical trials are currently underway to determine the benefit of plasma therapy compared to standard treatment with COVID-19 or other alternative therapy. They are also expected to answer several important questions related to the application of plasma therapy, including the accuracy and predictive value of antibody testing of donors and patients, optimal donor and patient selection, optimal timing and schedule of application [Citation123, Citation213]. Plasma therapy is thought to be more appropriate for patients with moderate symptoms before the virus has severely damaged tissues and organs.
Monoclonal antibodies
Studies have reported human monoclonal antibodies, as well as single-domain antibody fragments isolated from llama and alpaca that can recognize and neutralize SARS-CoV-2. Such biotechnologically derived antibodies/antibody fragments are expected to assist in the diagnosis, prevention and treatment of this infection [Citation214–218].
Of interest are the results obtained with two neutralizing monoclonal antibodies (COV2-2196 and COV2-2130), which recognize and bind simultaneously to regions of the S protein of SARS-CoV-2 virus in a synergistic manner. In two models of SARS-CoV-2 infection in mice, passive transfer of COV2-2196, COV2-2130, or a combination of both antibodies, protected the animals from weight loss and reduced viral load and levels of inflammation in the lungs. Moreover, application of ACE2-blocking monoclonal antibodies (COV2-2196 or COV2-2381) as monotherapy protects rhesus macaques from SARS-CoV-2 infection [Citation219].
Tocilizumab and sarilumab are monoclonal antibodies that inhibit interleukin-6 (IL-6) and are approved for the treatment of rheumatoid arthritis. Currently, they are also being investigated as therapeutic agents for COVID-19 [Citation220,Citation221].
Interferon
Due to its antiviral and immunomodulatory properties, interferon is also an attractive strategy for the treatment of SARS-CoV-2 infections, alone or in combination with other therapeutic agents [Citation222–225].
Modulation of renin-angiotensin-aldosterone system
Agonists of the ACE2-angiotensin1-7-Mas receptors signaling pathway are thought to have promising therapeutic potential in COVID-19 patients. This fact is not surprising, since the binding of SARS-CoV-2 to its cellular ACE2 receptor expresses at least two adverse effects on human health. First, it marks the beginning of a viral infection. Secondly, it prevents a cascade of reactions, including the formation of angiotensin 1-7 (angiotensin 1-7 is derived from angiotensin II with the involvement of ACE2, and ACE2 is already "occupied" by the virus, resulting in a lack of angiotensin 1-7 and an excess of angiotensin II).
By binding to specific receptors (so-called Mas receptors - MasR), angiotensin 1-7 counteracts the damaging effects resulting from the interaction of angiotensin II with its AT1 receptor (AT1R), including the stimulation of inflammation and fibrosis. This mechanism has been recognized as the driving force not only of acute respiratory distress syndrome and lung fibrosis (including fibrosis that is not aetiologically related to SARS-CoV-2) but also of several other socially significant pathological conditions, including diabetes, hypertension, cardiovascular and kidney diseases. Therefore, the use of agonists in the ACE2 - Angiotensin 1-7 - MasR pathway has been recognized as a promising option for the prevention of severe COVID-19 and other pathological conditions [Citation226,Citation227].
Mesenchymal stem cells
The idea of administering mesenchymal stem cells (MSCs) in COVID-19 patients is based on their immunomodulatory properties, which may contribute to the prevention of cytokine storm, as well as their regenerative capacity, which will be beneficial in lung recovery. MSC therapy has several benefits that make it advantageous compared to other stem cell treatment strategies [Citation228], namely: i) An intensive proliferative capacity and possibility to obtain MSCs in sufficient numbers; ii) MSCs are available in adult individuals and can be obtained from different tissues (adipose tissues, dental pulp, bone marrow, menstrual blood, etc.); iii) Avoidance of ethical, legal and social problems; iv) Being multipotent cells, MSCs are able to self-renew and differentiate into several cellular lineages. For a more in-depth review, see [Citation229]. In general, various clinical trials have documented MSC therapy to be a safe and effective treatment approach [Citation230].
Vitamin D
Vitamin D has been suggested to play a beneficial role in reducing inflammation in COVID-19 patients by increasing the anti-inflammatory cytokines and decreasing the levels of the pro-inflammatory cytokines [Citation231]. Treatment of vitamin D deficiency leads to reduced expression of DPP4/CD26 – a molecule that has been recognized as a possible co-receptor of SARS-CoV-2 and is essential for its virulence [Citation231,Citation232]. This explains the increased interest of scientists in inhibitors of DPP4/CD26.
Ribavirin
Ribavirin, a guanosine analogue that interferes with the replication of RNA and DNA viruses, has been expected to be potentially beneficial for the treatment of COVID-19, since it was approved for treating respiratory syncytial virus (RSV) infection and was used extensively during the SARS and MERS outbreaks (usually in combination with interferon, i.e. IFN-α or IFN-β). However, this drug shows the undesirable adverse effect of reducing haemoglobin, which is harmful to patients in respiratory distress. The therapeutic efficacy of ribavirin in SARS-CoV-2 infection has not been confirmed yet [Citation200,Citation201, Citation233,Citation234].
Protease inhibitors
Clinically approved anti-Hepatitis C virus drug boceprevir as well as a pre-clinical inhibitor against feline infectious peritonitis coronavirus (FIPV), GC376, have been reported to inhibit SARS-CoV-2 in cell cultures. The mechanism of action of both compounds is based on their ability to affect the coronavirus main protease (Mpro, also called 3CLpro). This protease is recognized as a promising drug target, as it is essential for processing and maturation of the viral polyprotein. What is more, a sterilizing additive effect has been observed as a result of the combined application of GC376 with remdesivir [Citation235].
Ivermectin
Ivermectin is an FDA approved antiparasitic agent discovered in 1978 with more than 2.5 billion doses distributed in the last 30 years. A Nobel Prize in Physiology or Medicine in 2015 was awarded to William C. Campbell and Satoshi Ōmura for the discovery of ivermectin [Citation236]. Antimicrobial and antiviral effects, as well as immunomodulatory and anticancer activities of ivermectin, have also been reported [Citation236–238]. Ivermectin inhibits the in vitro replication of some RNA viruses (including dengue virus, Zika virus, yellow fever virus, Avian influenza A virus, etc.) and DNA viruses,(such as Equine herpes type 1, BK polyomavirus, pseudorabies, porcine circovirus 2 and bovine herpesvirus 1 [Citation237,Citation238]. This drug has been found to inhibit SARS-CoV-2 in cell culture and is proposed in COVID-19 as a candidate for new drug use, immediately attracting the interest of the scientific community [Citation239].
Specialists are faced with two questions: 1) Whether ivermectin will show its antiviral activity in vivo; 2) And if so, whether this will happen in doses that are safe for humans [Citation239–241]. Studies have explored whether ivermectin should not be administered in children aged less than 5 years and/or weighing less than 15 kg. It is also not advised for use in patients with liver or kidney dysfunction [Citation242].
Phytochemicals and natural products
Various phytochemicals have been reported to possess antiviral and immunomodulating properties. Among them are phytochemicals such as flavonoids. Some of them (i.e. luteolin, myricetin, quercetin, apigenin, etc.) modulate the activity of NLRP3 inflammasome influencing in this way the inflammatory response to SARS viruses [Citation193]. Chinese herbal drugs from traditional medicine have also been considered as an alternative approach for prevention of COVID-19 in high-risk populations. The increased interest in Chinese traditional medicine (CTM) is not accidental, because the SARS outbreak in 2002-2003 provided a lot of experience with its application. One of the reasons for the dramatic decline in SARS mortality since the end of May 2003 in Beijing is believed to be the use of CTM as a supplement to conventional therapy. The efficacy of CTM in COVID-19, however, has not yet been clinically proven [Citation201, Citation233].
Bacteriophages
The potential use of bacteriophages in the fight against COVID-19, in particular in the treatment of antibiotic-resistant secondary bacterial infections, is discussed [Citation243].
Colchicine
Colchicine is a known drug used nowadays for treatment of gout, rheumatologic diseases and chronic pericarditis. It has a prominent anti-inflammatory activity by interacting with neutrophil inflammasome complex, thus inhibiting interleukin, IL-6 [Citation244]. As such, it has a potential to decrease the effects of the cytokine storm induced by SARS-CoV-2. Two recent studies have reported the effects of colchicine on hospiralized COVID-19 patients. The first one used a 7-point scale to assess the effectiveness of colchicine to reduce disease worsening, and observed that worsening occured significantly less in the colchicine group (p = 0.02) [Citation245]. The second study observed a significant reduction of mortality in colchicine-treated COVID-19 patients compared to those under treatment regimens without colchicine (16.3% vs. 37.1%, p = 0.001) [Citation246]. Nevertheless, these promising results should be further scrutinized and confirmed in larger randomized trails.
Vaccines
The development and introduction of a successful vaccine is an important long-term strategy to prevent COVID-19 outbreaks in the future if the virus becomes endemic and causes periodic seasonal epidemics. It is targeted at front-line healthcare professionals, as well as people at increased risk of SARS-CoV-2 infection and more severe disease: elderly and individuals with comorbidities (such as hypertension, cardiovascular and lung diseases, obesity, diabetes, etc.).
Immunogenic peptides that can serve as potential targets for a SARS-CoV-2 vaccine have been identified by in silico design [Citation247]. Different options regarding candidate antigens (such as whole virion, spike S protein, nucleocapsid N protein, membrane M protein, envelope E protein), adjuvants, model systems and technologies have been discussed in the choice of vaccine strategy. Various types of vaccines have been under consideration, including inactivated vaccines, live-attenuated vaccines, subunit, mRNA and DNA vaccines, vectored vaccines, synthetic peptide or epitope vaccines [Citation248]. Еach vaccine platform and strategy has its immunological and technological advantages and disadvantages. The presence of various vaccine candidates based on different approach is valuable for at least two reasons: i) this is a new human virus, the immune response against it has not yet been completely studied; ii) the vaccine is expected to work in people of different ages, including the elderly and with various comorbidities. Different groups of people may benefit from different types of vaccines.
Three months after the SARS-CoV-2 genome sequencing (announced on January 11, 2020), at least 78 candidates for the vaccine against COVID-19 have been developed, and five of them were already in the first phase of the clinical trial for human safety, tolerability and immunogenicity [Citation153]. As of October 19, 2020, there were 44 candidate vaccines for COVID-19 in clinical evaluation (10 of them in Phase III of clinical trials) and 149 vaccines in preclinical development [Citation249].
The vaccines that are currently in Phase III clinical trials [Citation249] are prepared by:
Oxford University, UK, and Astra Zeneca: a chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19, also known as AZD-1222) expressing the SARS-CoV-2 spike protein [Citation250]. The development of ChAdOx1 nCoV-19 is based on promising human studies with adenoviral vectored vaccines against MERS (ChAdOx1-MERS vaccine) [Citation250] and tuberculosis (ChAdOx1-TB vaccine) [Citation251];
American biotechnological company Moderna, which has experience with mRNA-based MERS vaccines, developed jointly with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, the first COVID-19 vaccine that entered clinical trial in humans, on March 16, 2020: mRNA encoding a prefusion-stabilized SARS-CoV-2 S protein encapsulated in lipid nanoparticles (mRNA-1273);
CanSino Biological Inc/Beijing Institute of Biotechnology: a vaccine based on adenovirus type 5 vector expressing S protein (Ad5-nCoV);
BioNTech/Fosun Pharma/Pfizer: mRNA–lipid nanoparticle vaccine encoding the S protein RBD (known as BNT162b1);
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation: heterologous vaccine consisting of a recombinant adenoviruses types 26 and 5, both of them carrying the gene coding for spike glycoprotein of SARS-CoV-2 (rAd26-S + rAd5-S) [Citation252];
Janssen Pharmaceutical Companies: Ad26COVS1 vaccine based on adenovirus 26 vector expressing S1;
SARS-CoV-2 inactivated vaccines produced by Sinovac Research & Development Co LTD; Wuhan Institute of Biological Products/Sinopharm; Beijing Institute of Biological Products/Sinopharm.
Novavax Inc. – A full-length recombinant SARS-CoV-2 glycoprotein nanoparticle vaccine (NVX-CoV2373) that contains Nanovax`s patented saponin-based adjuvant [Citation253].
To be able to enter clinical practice, the vaccine against SARS-CoV-2 must meet a number of norms and standards such as quality, safety and efficacy: i) to be safe; ii) to provide adequate immunity preventing viral infection and disease; iii) to be useful also against possible variants of the virus that can appear.
Being safe. Vaccine administration should not be accompanied by immune pathology (eosinophil infiltration; increased susceptibility to infection due to ACE) that has been observed in animal models treated with some SARS-CoV and MERS-CoV vaccines [Citation146,Citation147, Citation254–256].
There are many factors which must be taken into account when considering the safety of a vaccine. They form two main groups: 1) resulting from the vaccine design, such as the choice of vaccine platform and adjuvants; 2) vaccine-specific attributes, e.g. age, pre-existing vaccination and others [Citation257].
Providing adequate immunity to prevent viral infection and disease. Several pieces in the puzzle of the SARS-CoV-2 immunity are still missing. Among the most intriguing questions to be answered is the involvement of the humoral and cellular immune response, as well as the duration of immunological memory and its protective value. It is not yet clear what immunity is sufficient to ensure the efficacy of the vaccine, and whether it will prevent infection or just alleviate clinical manifestation. Vaccines have been developed for some animal coronaviruses that cause significant economic losses to the swine industry, such as transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhoea virus (PEDV) [Citation258]. Although these vaccines protect against the pathogen, they do not answer the question of how long the created immunity would last, since the targeted farm animals are raised for food production and usually do not live long enough.
What is more, the vaccine for SARS-CoV-2 has to be able to induce a stable, protective immune response in people of all ages, including the elderly (≥ 60 years of age). Some data indicate that because of the so-called “immunosenescence”, elderly people have increased sensitivity to infections and reduced response to immunizations [Citation259].
Being useful also against possible variants of the virus that can appear. The data available at the moment show that the genome of the virus is stable and does not pose a difficulty for diagnosis as well as for the development of drugs and vaccines [Citation113,Citation114]. As mentioned before, due to their RNA proofreading machine (ExoN), coronaviruses, including SARS-CoV-2, are less prone to mutations compared to other RNA viruses [Citation165,Citation166]. Besides, as SARS-CoV-2 is widespread throughout the world, indicating that its strategy is successful, we can assume that it is not subjected to extreme mutations. Moreover, people now do not have an immune response to this new virus and specific antiviral agents are not available, i.e. there are no factors to provoke the evolution towards a higher variability of the virus. Monitoring the genomic stability of the virus is one of the major tasks of biomedical science to provide adequate diagnosis, prevention and treatment. Over 114,000 viral genomic sequences of SARS-CoV-2 have been shared until September 25, 2020, via GISAID (see www.gisaid.org).
A fundamental question to consider is how to create an effective vaccine in the face of possible virus mutation. There are generally two approaches to this: 1) Targeting the conservative regions of the immunogenic part of the viral genome; 2) Creating a universal vaccine platform, which would allow substitution of the target viral nucleotide sequence, thus allowing the necessary adjustments to be made to ensure guaranteed effectiveness with time.
The existence of an association between immunization with a tuberculosis Bacille Calmette-Guérin (BCG) vaccine and a lower COVID-19 incidence/mortality rate is also discussed [Citation260–262]. At present, this hypothesis has not been confirmed. Experts warn that conclusions in this regard can be made only based on concrete evidence obtained from targeted research, and not only from the analysis of retrospective data. This is because there are considerable differences between countries such as socio-economic status, demographic structure, time of onset of the epidemic, national control strategies to limit the spread of COVID-19, etc. [Citation263]. Clinical studies are underway to establish a causal relationship between BCG vaccination and protection against severe COVID-19, especially for front-line healthcare workers [Citation260].
However, there are data indicating the non-targeted protective effects of BCG vaccine (reduced morbidity and mortality) against various RNA and DNA viruses in humans (such as Respiratory syncytial virus; RSV) and animals (for example herpes and influenza viruses in mice). BCG vaccine may be involved in non-specific immunotherapy of cutaneous and genital warts induced by human papillomaviruses (HPV) and has been associated with improved response to vaccines against influenza virus and hepatitis B virus. These activities can be at least partially explained by stimulation of cross-reactive T-cell response as well as antibody response against secondary viral infections. In addition, interleukin IL-1β, a pro-inflammatory cytokine with important function for antiviral immunity, can be secreted by epigenetically modified monocytes/macrophages [Citation264]. BCG remains the standard immunotherapy treatment for patients with high-risk non-muscle-invasive bladder cancer globally for more than 40 years [Citation260].
Some authors have proposed a possibility that vaccines for other diseases such as measles, mumps, rubella (MMR vaccine) can create cross-resistance for SARS-CoV-2 to explain at least partially the higher resistance of children to COVID-19 as compared to the elder population [Citation123, Citation265]. This proposition can be rationalized through a 30 amino acid sequence homology between the Spike (S), Fusion (F1) and Envelope (E1) glycoprotein of SARS-CoV-2, measles and rubella viruses, respectively. In silico investigation suggests the sequence mentioned above as antigenic epitopes in measles and rubella, which in turn could encourage the idea that immunity acquired through the MMR vaccine may as well give some protective advantage against SARS-CoV-2 [Citation266].
Almost all the vaccines developed are for parenteral administration. The respiratory mucosal vaccine strategy has also been suggested to be promising for the early control or clearance of SARS-CoV-2, especially in the high-risk elderly population [Citation149].
When discussing the vaccine, along with the scientific and technological challenges, we must not forget one more important thing - once created, the vaccine must reach billions of people around the world, and as soon as possible. The cooperation and interaction between science and business, between countries and institutions, are needed to achieve this goal. An excellent example in this direction is the mechanism set up by Global Alliance for Vaccines and Immunization (GAVI), Coalition for Epidemic Preparedness Innovations (CEPI) and WHO (COVID-19 Vaccines Global Access - COVAX Facility) to raise funds to provide vaccines to residents in countries with different incomes, ensuring coordinated, rapid, transparent and equitable access for all of them. COVAX will maintain a buffer of doses of vaccines for emergency humanitarian use, including for dealing with severe outbreaks before they go out of control.
Conclusions
The COVID-19 pandemic has presented enormous medical, scientific, social, economic and moral challenges to the world. The new disease was announced at the very end of 2019, without anyone at that moment suspecting the trials that lie ahead for all of us. At the time of writing this paper (October 30th, 2020), there were 44 592 789 confirmed cases and 1 175 553 deaths from COVID-19 globally.
Over the past ten months, through the unprecedented efforts of medical doctors and scientists from all countries and various specialties, a huge (but still far from sufficient) step has been made towards a better understanding of the biology and behaviour of the virus and the immune response against it, alongside with creating and improving diagnostic methods and treatment protocols. Clinical trials are underway with repurposed therapeutic agents (two of them, dexamethasone and remdesivir, have already been approved for the treatment of severe cases) and vaccine candidates (at least ten of which are already in the third phase of clinical trials). Experts have focussed their efforts on the development of specific new anti-coronaviral drugs; the gemome of the virus is carefully monitored; the scientific community is looking for the factors that determine the susceptibility to a viral infection and its severity. Yes, the COVID-19 pandemic is a challenge in many ways, and the price paid by humanity is measured in pain, suffering and lost lives. But COVID-19 is also an opportunity for the triumph of the human spirit, professionalism and cooperation, of empathy and mercy; of everything that makes us people.
COVID-19 is neither the first nor the last pandemic. It is worth mentioning that the time intervals between the SARS, MERS and COVID-19 outbreaks are 10 and 7 years, respectively.
Moreover, in a situation like the present one, it is important for each of us and all of us as a society, as well as for governments and international organizations, to be able to learn from this experience. Importantly, to invest in medicine, science, education and new technologies; to rethink our attitude towards nature; to learn to discover the possibilities and solutions that lie in even the most significant problems.
Sharing knowledge and ideas is one of the essential steps towards overcoming the COVID-19 crisis.
Acknowledgement
We thank Lucija Podlipnik for the artwork of the SARS-CoV-2 virus. The article is also the result of cooperation within the open science project COVID.SI.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269.
- Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. e6.
- Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456.
- Krieger E, Vriend G. YASARA View - molecular graphics for all devices - from smartphones to workstations. Bioinformatics. 2014;30(20):2981–2982.
- Yoshimoto FK. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020;39(3):198–216.
- Panda PK, Arul MN, Patel P, et al. Structure-based drug designing and immunoinformatics approach for SARS-CoV-2. Sci Adv. 2020;6(28):eabb8097.
- Calligari P, Bobone S, Ricci G, et al. Molecular investigation of SARS–CoV-2 proteins and their interactions with antiviral drugs. Viruses. 2020;12(4):445.
- Liu DX, Fung TS, Chong KK, et al. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109.
- Chernyshev A. Pharmaceutical targeting the envelope protein of SARS-CoV-2: the screening for inhibitors in approved drugs. ChemRxiv. 2020;
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69
- Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527(3):618–623.
- Chen Y, Wang G, Ouyang L. Promising inhibitors targeting M pro: an ideal strategy for anti-SARS-CoV-2 drug discovery. Sig Transduct Target Ther. 2020;5(1):1–2.
- Shin D, Mukherjee R, Grewe D, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587(7835):657-662
- Hillen HS, Kokic G, Farnung L, et al. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584(7819):154–156.
- Habtemariam S, Nabavi SF, Banach M, et al. Should we try SARS-CoV-2 helicase inhibitors for COVID-19 therapy? Arch Med Res. 2020; 51(7):733–735.
- Prajapat M, Sarma P, Shekhar N, et al. Drug targets for corona virus: A systematic review. Indian J Pharmacol. 2020;52(1):56–65.
- Zumla A, Chan JF, Azhar EI, et al. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347.
- Trezza A, Iovinelli D, Santucci A, et al. An integrated drug repurposing strategy for the rapid identification of potential SARS-CoV-2 viral inhibitors. Sci Rep. 2020;10(1):13866
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23.
- Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502.
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192.
- Tu YF, Chien CS, Yarmishyn AA, et al. A review of SARS-CoV-2 and the ongoing clinical trials. IJMS. 2020;21(7):2657. doi: 10.3390/ijms21072657.
- Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019 [published January 24, 2020]. N Engl J Med. 2020; 382(8):727–733.
- Cheng VC, Lau SK, Woo PC, et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694.
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). 2020. Available on: https://www.who.int/emergencies/mers-cov/en/.
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848.
- Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res. 2009;32(7):533–536.
- Kuster GM, Pfister O, Burkard T, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41(19):1801–1803.
- Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236.
- Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590.
- Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220.
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263.
- Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic cleavage site in the spike protein of SARS-CoV-2 Is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–784 e5.
- Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. doi: 10.26508/lsa.202000786.
- Progress report on the coronavirus pandemic. Nature. 2020;584(7821):325.
- Sungnak W, Huang N, Becavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687.
- Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192.
- Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592.
- Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci USA. 2020;117(13):7001–7003.
- Luan J, Jin X, Lu Y, et al. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J Med Virol. 2020;92(9):1649–1656. doi: 10.1002/jmv.25817.
- Hu B, Ge X, Wang LF, et al. Bat origin of human coronaviruses. Virol J. 2015;12:221.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273.
- Cagliani R, Forni D, Clerici M, et al. Computational inference of selection underlying the evolution of the novel coronavirus, severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94(12):e00411. doi: 10.1128/JVI.00411-20.
- Matyášek R, Kovařík A. Mutation patterns of human SARS-CoV-2 and Bat RaTG13 coronavirus genomes are strongly biased towards C > U transitions, indicating rapid evolution in their hosts. Genes. 2020;11(7):761.
- Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol. 2020;30(7):1346–1351.e2.
- Ye ZW, Yuan S, Yuen KS, Fung SY, et al. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–1697.
- Qin J, You C, Lin Q, et al. Estimation of incubation period distribution of COVID-19 using disease onset forward time: A novel cross-sectional and forward follow-up study. Sci Adv. 2020;6(33):eabc1202.
- Dhakal BP, Sweitzer NK, Indik JH, et al. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29(7):973–987.
- Yi Y, Lagniton PNP, Ye S, et al. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766.
- Patel KP, Patel PA, Vunnam RR, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128:104386.
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629.
- Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840.
- Jothimani D, Venugopal R, Abedin MF, et al. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–1240.
- Hong N, Yu W, Xia J, et al. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020;98(5):e649–e55. doi: 10.1111/aos.14445.
- Amesty MA, Alió del Barrio JL, Alió JL. COVID-19 disease and ophthalmology: an update. Ophthalmol Ther. 2020;9(3):1–12.
- Baj J, Karakuła-Juchnowicz H, Teresiński G, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753.
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032.
- Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45(3):230–232.
- Violi F, Pastori D, Pignatelli P, et al. SARS-CoV-2 and myocardial injury: a role for Nox2? Intern Emerg Med. 2020;15(5):755–758.
- Sorodoc V, Sorodoc L, Ungureanu D, et al. Cardiac troponin T and NT-proBNP as biomarkers of early myocardial damage in amitriptyline-induced cardiovascular toxicity in rats. Int J Toxicol. 2013;32(5):351–357. doi: 10.1177/1091581813503888.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5.
- Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. 2020;9(3):215–221.
- Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950.
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiology. 2020;5(11):1265–1273;
- Nagel E, Puntmann VO. Errors in statistical numbers and data in study of cardiovascular magnetic resonance imaging in patients recently recovered From COVID-19. JAMA Cardiology. 2020:5(11):1307-1308.
- Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348.
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690.
- Huang J, Zheng M, Tang X, et al. Potential of SARS-CoV-2 to cause CNS infection: biologic fundamental and clinical experience. Front Neurol. 2020;11:659
- Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723–728.
- Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014.
- Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890.
- Rubino F, Cohen RV, Mingrone G, et al. Bariatric and metabolic surgery during and after the COVID-19 pandemic: DSS recommendations for management of surgical candidates and postoperative patients and prioritisation of access to surgery. Lancet Diabetes Endocrinol. 2020;8(7):640–648. doi: 10.1016/S2213-8587(20)30157-1.
- Liu F, Long X, Zhang B, et al. ACE2 expression in pancreas may cause pancreatic damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130. e2.
- Wang F, Wang H, Fan J, et al. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159(1):367–370.
- Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040.
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15(8):1375–1387. doi: 10.1007/s11739-020-02432-x.
- Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:1076029620938149
- Tatu AL, Nadasdy T, Nwabudike LC. Observations about sexual and other routes of SARS-CoV-2 (COVID-19) transmission and its prevention. Clin Exp Dermatol. 2020;45(6):761–762.
- Fraietta R, Pasqualotto FF, Roque M, et al. SARS-COV-2 and male reproductive health. JBRA Assist Reprod. 2020;24(3):347–350.
- Li R, Yin T, Fang F, et al. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod Biomed Online. 2020;41(1):89–95.
- Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179(7):1029–1046.
- Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453–454.
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346.
- Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968–981.e7. doi: 10.1016/j.cell.2020.09.016.
- World Health Organization. Infection Prevention and Control of Epidemic-and Pandemic-prone Acute Respiratory Infections in Health Care. 2014. Available on: https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=41AA684FB64571CE8D8A453C4F2B2096?sequence=1.
- Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for infection prevention and control (IPC) precaution recommendations. 2020. Available on: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions.
- Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567.
- Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251.
- Kim JM, Kim HM, Lee EJ, et al. Detection and isolation of SARS-CoV-2 in serum, urine, and stool specimens of COVID-19 patients from the Republic of Korea. Osong Public Health Res Perspect. 2020;11(3):112–117.
- Peng L, Liu J, Xu W, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020;92(9):1676–1680. doi: 10.1002/jmv.25936.
- Chen L, Lou J, Bai Y, et al. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol. 2020;115(5):790
- Ding S, Liang TJ. Is SARS-CoV-2 Also an enteric pathogen with potential fecal-oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159(1):53–61.
- Centeno-Tablante E, Medina-Rivera M, Finkelstein JL, et al. Transmission of SARS-CoV-2 through breast milk and breastfeeding: a living systematic review. Ann N Y Acad Sci. 2020. doi: 10.1111/nyas.14477.
- World Health Organization. Breastfeeding and COVID-19. 2020. Available on: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19.
- Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA.
- Auriti C, De Rose DU, Tzialla C, et al. Vertical transmission of SARS-CoV-2 (COVID-19): are hypotheses more than evidences? Am J Perinatol. 2020;37(S 02):S31–S8.
- U.S. Centers for Disease Control and Prevention. Contact Tracing for COVID-19. 2020. Available on: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/contact-tracing.html.
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675.
- Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412.
- Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–1761.
- Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090.
- Tong Z-D, Tang A, Li K-F, et al. Potential Presymptomatic Transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052–1054.
- Salimi S, Hamlyn JM. COVID-19 and crosstalk with the hallmarks of aging. J Gerontol A Biol Sci Med Sci. 2020;75(9):e34–e41.
- Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020;12(10):9959–9981.
- Severe Covid GG, Ellinghaus D, Degenhardt F, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534.
- Ovsyannikova IG, Haralambieva IH, Crooke SN, et al. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol Rev. 2020;296(1):205–219.
- Pisanti S, Deelen J, Gallina AM, et al. Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of Covid-19. J Transl Med. 2020;18(1):352.
- Sawalha AH, Zhao M, Coit P, et al. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. 2020;215:108410. doi:.
- Mercatelli D, Giorgi FM. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol. 2020;11:1800.
- Callaway E. The coronavirus is mutating - does it matter? Nature. 2020;585(7824):174–177.
- Becchetti L, Conzo G, Conzo P, et al. Understanding the heterogeneity of adverse COVID-19 outcomes: the role of poor quality of air and lockdown decisions. Available at SSRN 3572548. 2020. doi: .
- Kaye M. SARS-associated coronavirus replication in cell lines. Emerg Infect Dis. 2006;12(1):128–133.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8.
- Phillips KA, Bales KL, Capitanio JP, et al. Why primate models matter. Am J Primatol. 2014;76(9):801–827.
- Johansen MD, Irving A, Montagutelli X, et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13(6):877–891.
- Nagata N, Iwata N, Hasegawa H, et al. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J Virol. 2007;81(4):1848–1857.
- Roberts A, Deming D, Paddock CD, et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1):e5.
- Totura AL, Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expert Opin Drug Discov. 2019;14(4):397–412.
- Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98.
- Kim YI, Kim SG, Kim SM, et al. Infection and rapid transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020;27(5):704–709 e2.
- Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA. 2020;117(28):16587–16595.
- Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16(2):141–147.
- Crowe JE. Host defense mechanisms against viruses. In book: Fetal and Neonatal Physiology. 5th ed. Elsevier. 2017;1175–1197.e7.
- Kopecky-Bromberg SA, Martinez-Sobrido L, Frieman M, et al. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. 2007;81(2):548–557.
- Lu X, Pan J, Tao J, et al. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45.
- Zhou R, To KK, Wong YC, et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–877.e5.
- Liu W, Liu L, Kou G, et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(6):e00461. doi: 10.1128/jcm.00461-20.
- Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848.
- Paces J, Strizova Z, Smrz D, et al. COVID-19 and the immune system. Physiol Res. 2020;69(3):379–388.
- Yu HQ, Sun BQ, Fang ZF, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56(2):2001526. doi: 10.1183/13993003.01526-2020.
- Padoan A, Sciacovelli L, Basso D, et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta. 2020;507:164–166.
- Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17(7):773–775.
- Hirano T, Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733.
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
- Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446.
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607–613.
- Lagunas-Rangel FA, Chavez-Valencia V. High IL-6/IFN-gamma ratio could be associated with severe disease in COVID-19 patients. J Med Virol. 2020;92:1789–1790.
- Zhang Y, Yu L, Tang L, et al. A promising anti-cytokine-storm targeted therapy for COVID-19: the artificial-liver blood-purification system. Engineering (Beijing). 2020. doi: 10.1016/j.eng.2020.03.006.
- Elkahloun AG, Saavedra JM. Candesartan could ameliorate the COVID-19 cytokine storm. Biomed Pharmacother. 2020;131:110653.
- Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69–86.
- Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly. 2020;150:w20249.
- Weingartl H, Czub M, Czub S, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676.
- Czub M, Weingartl H, Czub S, et al. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23(17-18):2273–2279.
- Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–73.
- Jeyanathan M, Afkhami S, Smaill F, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632.
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. e15.
- Brown M. Immune correlates of SARS-CoV-2 protection. Nat Rev Immunol. 2020;20(10):593
- Callow KA, Parry HF, Sergeant M, et al. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435–446.
- Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306.
- Liu W, Fontanet A, Zhang PH, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795.
- Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564.
- Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26(11):1691–1693.
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: .
- Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844.
- Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971–977.e3.
- Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734.
- Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020. doi: 10.1038/s41586-020-2700-3.
- Van Vinh Chau N, Lam VT, Dung NT, et al. The natural history and transmission potential of asymptomatic severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2020;ciaa711. doi: 10.1093/cid/ciaa711.
- Poletti P, Tirani M, Cereda D, et al. Probability of symptoms and critical disease after SARS-CoV-2 infection. arXiv preprint arXiv:200608471. 2020;
- Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv. 2020;
- Denison MR, Graham RL, Donaldson EF, Eckerle LD, et al. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8(2):270–279.
- Hofer U. Viral evolution: fooling the coronavirus proofreading machinery. Nat Rev Microbiol. 2013;11(10):662–663.
- Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036.
- Forster P, Forster L, Renfrew C, et al. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117(17):9241–9243.
- Li Q, Wu J, Nie J, et al. The impact of mutations in SARS-CoV-2 Spike on viral infectivity and antigenicity. Cell. 2020;182(5):1284–1294 e9.
- Torjesen I. Covid-19: Hydroxychloroquine does not benefit hospitalised patients, UK trial finds. BMJ. 2020;369:m2263
- Pan H, Peto R, Karim QA, et al. Repurposed antiviral drugs for COVID-19 – interim WHO SOLIDARITY trial results. medRxiv. 2020.
- Sinha N, Balayla G. Hydroxychloroquine and COVID-19. Postgrad Med J. 2020;96(1139):550–555.
- Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949
- Sahraei Z, Shabani M, Shokouhi S, et al. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. 2020;55(4):105945
- RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. New Engl J Med. 2020;383(21):2030–2040.
- Srinivas P, Sacha GL, Koval C. Antivirals for COVID-19. Cleve Clin J Med. 2020. doi: 10.3949/ccjm.87a.ccc030.
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9.
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. New Engl J Med. 2020;383(19):1827–1837.
- Spinner CD, Gottlieb RL, Criner GJ, GS-US-540-5774 Investigators, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 — final report. New Engl J Med. 2020;383(19):1813–1826.
- Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653.
- Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60(5):1648–1661.
- Mulangu S, Dodd LE, Davey RT, Jr., et al. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303.
- Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382(24):2327–2336.
- Drugs and Lactation Database (LactMed). Bethesda (MD): National Library of Medicine (US). Remdesivir. 2006. 2020.
- Qazi NA, Morlese JF, Pozniak AL. Lopinavir/ritonavir (ABT-378/r). Expert Opin Pharmacother. 2002;3(3):315–327.
- Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4(5):1023–1033.
- Horby PW, Mafham M, Bell JL, et al. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4.
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463.
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512
- Du Y-X, Chen X-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108(2):242–247. doi: 10.1002/cpt.1844.
- McKee DL, Sternberg A, Stange U, et al. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859.
- Blaising J, Polyak SJ, Pécheur E-I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94.
- Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e3.
- Wang X, Cao R, Zhang H, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6(1):1–5.
- Theoharides TC, Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents. 2020;34(3):1241–1243.
- Mahase E. Covid-19: Low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422
- Johnson RM, Vinetz JM. Dexamethasone in the management of covid -19. BMJ. 2020;370:m2648
- Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5):e00399. doi: 10.1128/AAC.00399-20.
- Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443.
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. 01 May 2020. Available on: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment.
- U.S. Food and Drug Administration. FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19. 28 Aug 2020. Available on: https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized.
- U.S. Food and Drug Administration. FDA Approves First Treatment for COVID-19. 22 Oct 2020. Available on: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19.
- RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 — preliminary report. New Engl J Med. 2020. doi: 10.1056/NEJMoa2021436.
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099.
- McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4):571–587.
- Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;382(25):2411–2418.
- Page RL, O'Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32–e69.
- Kotecha P, Light A, Checcucci E, et al. Repurposing of drugs for Covid-19: a systematic review and meta-analysis. Panminerva Med. 2020. doi: 10.23736/S0031-0808.20.04024-0.
- Zhu Y, Yu D, Yan H, et al. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J Virol. 2020;94(14):e00635-20. doi: 10.1128/JVI.00635-20.
- Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–331.
- Mucha SR, Quraishy N. Convalescent plasma for COVID-19: Promising, not proven. Cleve Clin J Med. 2020;87(11):664–670. doi: .
- Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2251
- Pinto D, Park YJ, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295.
- Tai W, Zhang X, He Y, et al. Identification of SARS-CoV RBD-targeting monoclonal antibodies with cross-reactive or neutralizing activity against SARS-CoV-2. Antiviral Res. 2020;179:104820
- Dong J, Huang B, Jia Z, et al. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg Microbes Infect. 2020;9(1):1034–1036.
- Hanke L, Vidakovics Perez L, Sheward DJ, et al. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat Commun. 2020;11(1):4420
- Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449.
- Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568
- Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79(10):1277–1285.
- Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b Treatment for COVID-19 . Front Immunol. 2020;11:1061
- Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol. 2020;20(10):585–586.
- Rahmani H, Davoudi-Monfared E, Nourian A, et al. Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020;88:106903
- Andreakos E, Tsiodras S. COVID-19: lambda interferon against viral load and hyperinflammation. EMBO Mol Med. 2020;12(6):e12465
- Shete A. Urgent need for evaluating agonists of angiotensin-(1-7)/Mas receptor axis for treating patients with COVID-19. Int J Infect Dis. 2020;96:348–351.
- Wu Y. Compensation of ACE2 function for possible clinical management of 2019-nCoV-induced acute lung injury. Virol Sin. 2020;35(3):256–258.
- Golchin A, Farahany T Z. Biological products: cellular therapy and FDA approved products. Stem Cell Rev and Rep. 2019;15(2):166–175. doi: 10.1007/s12015-018-9866-1.
- Rajarshi K, Chatterjee A, Ray S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep. 2020;26:e00467 doi: 10.1016/j.btre.2020.e00467.
- Golchin A, Farahany TZ, Khojasteh A, et al. The Clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. CSCR. 14(1):22–33. doi: 10.2174/1574888X13666180913123424.
- Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601–604.
- Solerte SB, Di Sabatino A, Galli M, et al. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57(7):779–783.
- Yang Y, Islam MS, Wang J, et al. Traditional chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int J Biol Sci. 2020;16(10):1708–1717.
- Khalili JS, Zhu H, Mak NSA, et al. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92(7):740–746.
- Fu L, Ye F, Feng Y, et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun. 2020;11(1):4417
- Tambo E, Khater EIM, Chen J-H, et al. Nobel prize for the artemisinin and ivermectin discoveries: a great boost towards elimination of the global infectious diseases of poverty. Infect Dis Poverty. 2015;4(1):58
- Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo). 2020;73(9):593–602.
- Chaccour C, Hammann F, Ramon-Garcia S, et al. Ivermectin and COVID-19: Keeping Rigor in Times of Urgency. Am J Trop Med Hyg. 2020;102(6):1156–1157.
- Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787
- Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnol Biotechnol Equip. 2020;34(1):469–474.
- Schmith VD, Zhou JJ, Lohmer LRL. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin Pharmacol Ther. 2020;108(4):762–765. doi: 10.1002/cpt.1889.
- Levy M, Martin L, Bursztejn AC, the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique, et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br J Dermatol. 2020;182(4):1003–1006. doi: 10.1111/bjd.18369.
- Górski A, Międzybrodzki R, Żaczek M, et al. Phages in the fight against COVID-19? Future Microbiol. 2020;15:1095–1100.
- Papadopoulos C, Patoulias D, Teperikidis E, et al. Colchicine as a potential therapeutic agent against cardiovascular complications of COVID-19: an exploratory review. SN Compr Clin Med. 2020;2(9):1–11.
- Deftereos SG, Giannopoulos G, Vrachatis DA, GRECCO-19 investigators, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136
- Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–1289. doi: 10.1136/annrheumdis-2020-217712.
- Lee CH, Koohy H. In silico identification of vaccine targets for 2019-nCoV. F1000Res. 2020;9:145.
- Zhang J, Zeng H, Gu J, et al. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel). 2020;8(2):153. doi: 10.3390/vaccines8020153.
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. 19 October 2020. Available on: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4.
- Wilkie M, Satti I, Minhinnick A, et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime - MVA85A boost in healthy UK adults. Vaccine. 2020;38(4):779–789.
- Burki TK. The Russian vaccine for COVID-19. Lancet Respir Med. 2020;8(11):e85–e86.
- Novavax, Inc. Novavax to Present COVID-19 Vaccine Data at World Vaccine Congress Europe 2020. 20 Oct 2020. Available on: https://ir.novavax.com/news-releases/news-release-details/novavax-present-covid-19-vaccine-data-world-vaccine-congress.
- Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215.
- Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351–2356.
- Callaway E. Coronavirus vaccines: five key questions as trials begin. Nature. 2020;579(7800):481
- Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963
- Gerdts V, Zakhartchouk A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet Microbiol. 2017;206:45–51.
- Aspinall R, Del Giudice G, Effros RB, et al. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9
- Koti M, Morales A, Graham CH, et al. BCG vaccine and COVID-19: implications for infection prophylaxis and cancer immunotherapy. J Immunother Cancer. 2020;8(2):e001119. doi: 10.1136/jitc-2020-001119.
- Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat Rev Urol. 2020;17(6):316–317.
- Kumar J, Meena J. Demystifying BCG vaccine and COVID-19 relationship. Indian Pediatr. 2020;57(6):588–589. doi: 10.1007/s13312-020-1872-0.
- Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine-induced protection from COVID-19 infection, wishful thinking or a game changer? medRxiv. 2020;
- Mohapatra PR, Mishra B, Behera B. BCG vaccination induced protection from COVID-19. Indian J Tuberculosis. 2020. doi: .
- Fidel PL, Jr., Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? mBio. 2020;11(3):e00907-20. doi: 10.1128/mBio.00907-20.
- Sidiq KR, Sabir DK, Ali SM, et al. Does early childhood vaccination protect against COVID-19? Front Mol Biosci. 2020;7:120