Abstract
Geum urbanum L. is a medicinal plant used from ancient time against gastro-intestinal disorders, bleeding and inflammation of mucous membranes, gums (gingivitis), etc. In our previous works, we demonstrated the antibacterial and antioxidant properties of ethyl acetate (EtOAc) extracts in vitro. The aim of this study was to evaluate the possibility of the EtOAc fraction from aerial parts to provoke acute and subacute toxicity by its internal daily administration (per os) in healthy H- albino male and female mice. We used three concentrations of treatment according to their biological activity potential of application − 20, 70 and 210 mg/kg. All animals were observed and recorded periodically for clinical signs, mortality and changes in their behavior (incl. hypo-activity, aggression, etc.). The body weight was measured at every 5 days up to the end of the experiment (a total 28 days). We studied the biochemical parameters (superoxide anion radical, hydrogen peroxide, superoxide dismutase, lipid peroxidation and damaged proteins in blood plasma and brain) and complete hematological parameters, and the histopathological findings in mouse spleens and Payer’s patches. The investigated extract did not show any signs of significant acute and subacute toxicity at the highest applied test-concentration, and therefore, we encouraged its possible application as food additive for various infectious diseases.
Introduction
Many medicinal plants have a variety of properties and are used as dietary supplement to treat various diseases and ailments [Citation1]. Of the about 750,000 plant species known in the word about 4100 species of higher plants grow in Bulgaria, of which 770 species (19%) possess medicinal properties. In comparison, about 2200–3100 species of higher plants are distributed in Central and Northern Europe. Due to the favorable geographical location, various climate and soil conditions, Bulgaria ranks first among the European countries and sixth in the word in the export of herbs [Citation2]. Currently, 200 plant species are used to obtain over 270 phytoproducts (about ¼ of all pharmaceuticals) with proven antioxidant properties, which are applied in the preventive health, medicine, cosmetics and food industry [Citation3, Citation4].
Reactive oxygen and nitrogen species (ROS/RNS) can be formed from exogenous sources (ultraviolet, X-ray and γ-ray irradiation, pollutants in the atmosphere) and endogenous sources (metal-catalyzed or electron transport reactions in neutrophils and macrophages during inflammation). Mitochondria are known to generate significant amounts of hydrogen peroxide (H2O2). Under physiological conditions, its production is estimated at about 2% of the total absorption of the superoxide anion radical (O2 ·) by the body. However, it is difficult to detect the occurrence of O2 · in intact mitochondria due to the high activity of superoxide dismutase (SOD). The O2 · resulting from metabolic processes is considered to be a ‘primary’ ROS that can interact with other molecules to generate a ‘secondary’ ROS, directly or predominantly through catalyzed enzymatic processes. The O2· is usually converted to H2O2 by SOD, which is an antioxidant enzyme, and H2O2 is then metabolized to two harmless molecules, H2O and O2, by catalase or to oxidized glutathione and two molecules H2O by glutathione peroxidase [Citation5]. The peroxide radicals (ROO·) are formed by the free-radical attack on unsaturated higher fatty acids. The detection and measurement of lipid peroxidation are often used as a marker for various diseases and toxicities [Citation6] and the termination occurs by enzymes or by the radical-scavenging activities of antioxidants [Citation7]. When formed in excess, the amounts of free radicals can overcome the capacity of the protective enzymes of the organism such as SOD, catalase and glutathione peroxidase, and cause cell aging, as well as induce apoptosis [Citation8], mutagenesis [Citation9], coronary heart disease [Citation10] and carcinogenesis [Citation5], probably by destabilizing the membranes [Citation11], inducing DNA damage [Citation9] and so forth. In order to maintain optimal cell homeostasis, regulation of cellular redox signaling is crucial [Citation5].
Phenolic compounds have been reported to have a capacity to scavenge free radicals. Phenolic antioxidants (PhOH) can function as terminators of free radical chains and as chelators of metal ions capable of catalyzing lipid peroxidation. PhOHs interact with ROO· and other molecules by donating a hydrogen atom to the radicals [Citation5, Citation12]. The resulting products of oxidation of polyphenols (PhO·) are relatively stable and act as terminators of the reaction chain by reaction with other radicals [Citation12].
Genus Geum (Rosaceae family) is represented by about 70 species [Citation13]. Eight species from the genus are distributed in Bulgaria, among which is the medicinal plant Geum urbanum L. [Citation14]. This plant, commonly known as wood avens or St. Benedict’s herb, has been used since ancient times in the traditional phytomedicine against gastro-intestinal disorders and bleeding from the gums. Since 1920, more than 200 compounds have been isolated from the genus Geum [Citation13], but the studies in the chemical composition of G. urbanum are limited. Only several studies have reported the isolation of chemical constituents of G. urbanum. To date, 16 flavonoids have been isolated from this plant [Citation15–18], 21 ellagitannins [Citation15–19], 15 phenylpropanoids [Citation15–18, Citation20, Citation21] and 2 triterpenoids [Citation18]. According to the literature data, the beneficial effects on the gastrointestinal tract are due to the high content of gallic and ellagic acids, while gemin A has a styptic effect [Citation22]. It has been also reported that G. urbanum [Citation15], G. rivale [Citation23], G. bulgaricum [Citation22, Citation24] and G. japonicum [Citation25] contain apigenin, quercetin and luteolin, which have demonstrated significant antineoplastic effects [Citation26, Citation27].
In our previous study [Citation18], we showed the antimicrobial and antioxidant activities of several extracts obtained from G. urbanum L. and we concluded that the ethyl acetate extracts from roots (EtOAcR) and aerial parts (EtOAcAP) had the highest antibacterial effects against Staphylococcus aureus, S. epidermidis and Bacillus cereus strains at concentrations varying between 39 and 2500 µg/mL. Most likely their activities are due to the high content of polyphenolic compounds in the phytomixes (61% ± 9, respectively, 32% ± 3), compared with the other tested organic extracts, such as methanol (MeOH), n-butanol (n-BuOH) and petroleum ether. To clarify the antioxidant effect and antibacterial mechanisms of the extracts, we used two in vitro models (DPPH radical and superoxide anion scavenging activity methods). The EtOAcR fraction showed highest effect; therefore, we characterized its chemical composition by column (on silica gel and Sephadex LH-20) and thin-layer chromatography. We isolated two acetylated ellagic acid rhamnosides, new for the genus Geum – 3-O-methylellagic acid-3′-O-α-″-O-acetylrhamnopyranoside and 3-O-methylellagic acid-3′-O-α-2″-O- acetylrhamnopyranoside, and three compounds, new for the species G. urbanum: 3,3′-di-O- methylellagic acid-4-O-β-d-glucopyranoside and the triterpenoids tormentic acid and niga- ichigoside F1, but individually they had not shown any antimicrobial activity [Citation18]. In another study, we investigated the anti-quorum-sensing potential of EtOAc and n-BuOH fractions against Pseudomonas aeruginosa PA01 [Citation28].
It is known that most urinary tract and food poisoning infections often are associated with S. aureus [Citation29, Citation30], S. epidermidis [Citation31] and B. cereus [Citation32] in immunocompromised patients. Therefore, we suggest that per os administration of EtOAcAP extract could be of importance about bacterial infections. Due to scarce information about the toxicity profile of this plant, we turned our attention to its pharmacological application in Bulgaria and other European countries. Thus, the present study focuses on the safety assessment of EtOAcAP fraction through acute and subacute toxicity evaluations after oral administration using H-albino mice as the animal model. For this purpose, we investigated the effect of this extract on the biochemical, hematological and histopathological parameters, according to 3Rs principles (Replacement, Reduction and Refinement of animals).
Material and methods
Plant material and preparation of the extract
The dry aerial parts from G. urbanum were commercial products from the region of Veliko Tarnovo (Bulgaria), provided by herbal pharmacy ‘Sun’® in 2014 (batch No. 422). The EtOAc extract was prepared as described previously [Citation18, Citation28]. Briefly, 500 g aerial parts from the plant were macerated in 3 L MeOH two times for 18 h at room temperature. The crude MeOH extract was concentrated in a rotary evaporator under reduced pressure and the residue was extracted with petroleum ether. The organic solvent/water fraction was concentrated and re-extracted with EtOAc. Solvent solution was evaporated to obtain dried fraction.
Ethics statement
The study was approved by the Institutional Ethics and Animal Care Committee (Nr. 125/07.10.2015-07.10.2020). All efforts were made to minimize animal suffering during the experimental procedures involving animals.
Experimental animals
In the present study, 32 H-albino young virgin mice (25–37.8 g), 4- to 8-week old of either sex (16 male and 16 female) were purchased from the Institute of Neurobiology, Experimental and Breeding Base for Laboratory Animals (EBBLA) – Slivnitsa, Bulgarian Academy of Sciences. The animals were housed in separate cages at a room temperature of 24 ± 1 °C and relative humidity 56–66%, with 12 h light and 12 h dark cycle in the animal house facility of The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences. The basic animal feed was provided by Evro-Prot OOD (Sofia, Bulgaria). Mice were fed with pellets consisting of wheat, corn, sunflower meal, soybean meal, wheat bran, calcium carbonate, brazing agent, sodium chloride and premix. The analytical analysis of the pellets (TopMix, F70-1) showed that they contained crude protein 20.09%, crude fiber 5.99%, crude fat 2.15%, crude ash 5.26%, calcium 0.73%, phosphorus 0.55%, absorbed phosphorus 0.186%, sodium 0.13%, lysine 0.91% and methionine 0.35%. Water was available ad libitum to the mice throughout the study duration except during actual experiments. Animals were acclimated for 14 days before treatment.
Acute oral toxicity
The assay of acute toxicity was performed following the Organization for Economic Cooperation and Development (OECD) guideline 423 [Citation33]. The H-albino mice (16 male and 16 female mice) were divided into four groups (n = 4): one control − 50 µL double distilled water and three others with increasing concentrations of EtOAc extract pre-dissolved in water (20, 70 and 210 mg/kg). The volume of extract administration was above 50 µL. The animals were administered per os with the extract by pipette once a day for 14 days (a total of 14 doses). All animals were observed and recorded periodically for mortality and changes in mice behavior (incl. hypo-activity, aggression, etc.) at 2 h, 6 h, 12 h and 24 h in the first day after the sample administration, and then, daily for a total of 14 days. The body weight of each control and treated animal was measured at every 5 days. At the 14th day, half of the mice (eight male and eight female animals) were sacrificed by decapitation; blood and brain samples were collected for hematological parameters and biochemical estimations.
Subacute oral toxicity
The 28-day subacute toxicity experiment was performed according to the OECD Guideline 407 [Citation34]. The other animals from the previous experiment (eight male and eight female mice), which were divided into four groups (n = 2), were administered per os with the extract by pipette once a day for further 14 days (a total 28 doses). All animals were observed and recorded periodically for mortality and changes in mice behavior (incl. hypo-activity, aggression, etc.) at 2 h, 6 h, 12 h and 24 h in the first day after the sample administration, and then, daily for a total of 14 days. The body weight was measured at every 5 days to the end of the experiment. At the 28th day, all animals were sacrificed by decapitation; blood and brain samples were immediately collected for hematological and biochemical analysis. Histological preparations were prepared from the spleens and Payer’s patches of animals.
Biochemical assays
At the 14th and 28th days of the experiments, half of the mice were sacrificed by decapitation. Blood collected in 0.5 mL EDTA.K3 vacutainer tubes (Golden Vac™) was centrifuged at 3000 × g for 15 min at 4 °C. For mouse brain tissue homogenization 10% (w/v) brain tissues were homogenized with 0.1 mol/L sodium phosphate buffer (pH 7.4) and were centrifuged at 14,250 × g for 10 min at 4 °C. The serum biochemical parameters such as O2 ·, H2O2, SOD, lipid peroxidation (i.e. malondialdehyde [MDA] content) and damaged proteins were analyzed. O2− production rate was measured using the method of superoxide dismutase-inhibitable reduction of cytochrome c [Citation35]. The method of Pick and Mizel [Citation36] was used for measurement of H2O2 production. SOD enzyme activity was determined using a Cayman chemical kit (Randox Labs, Crumlin, UK). The lipid peroxidation levels were analyzed by an Abbexa kit (Abbexa Ltd.Cambridge, UK). Oxidative damaged proteins were determined via protein carbonyl content using the 2,4-dinitrophenylhydrazine (DNPH) binding assay [Citation37], slightly modified by Adachi and Ishii [Citation38]. Protein content was determined by Lowry procedure [Citation39].
Hematological analysis
For the hematological assay, 0.5 mL EDTA.K3 vacutainer tubes (Golden Vac™) were used to collect blood. The blood samples were subjected to evaluation of the following parameters: white blood cell (WBC, 109/L), lymphocytes (LYM, 109/L), monocytes (MONO, 109/L), granulocytes (GRA, 109/L), percentage of lymphocytes (LYM%), percentage of monocytes (MONO%), percentage of granulocytes (GRA%), red blood cell counts (RBC, 1012/L), hemoglobin (HGB, g/dL), percentage of red blood cell (HCT%), mean corpuscular volume (MCV, fL), mean corpuscular hemoglobin (MCH, Pg), mean corpuscular hemoglobin concentration (MCHC, g/dL), percentage of red cell distribution width (RDW%), platelets (PLT, 109/L), percentage of procalcitonin (PCT%), mean platelet volume (MPV, fL) and the percentage of platelet distribution width (PDW%), by an automatic blood cell analyzer (Hema screen 10, MedWrench, USA).
Histopathological assay
After necropsy, spleen and Payer’s patches samples were fixed in 10% buffered formalin and stored at room temperature for histopathological examination. The paraffin sections were stained with Hematoxylin and Eosin (H&E) stain in order to demonstrate nucleus and cytoplasmic inclusions [Citation40–42]. Briefly, the sections were deparaffinized with xylene. The slides were passed through decreasing concentrations of ethanol (100%, 90%, 80%, 70%) and rinsed in water. Thereafter, they were stained for 5 min with filtered solution of the nuclear stain Harri’s hematoxylin. After rinsing in tap water (5 min), the sections were ‘blued’ by treatment with a weak alkaline solution. The nonspecific background was removed using a 1% HCl in 70% EtOH for 5 min; washed in running tap water, dipped in an alkaline solution (e.g. ammonia water) and washed again with tap water. The sections were further stained with an aqueous solution of eosin for 10 min and washed with tap water for 5 min. This stains many non-nuclear elements in different shades of pink. Following the eosin stain, the slides were dehydrated with increasing concentrations of EtOH and rinsed in several baths of xylene.
Statistical analysis
The data were presented as mean values with standard deviation (±SD) and were analyzed by using ANOVA (analysis of variance). Differences were considered statistically significant at a level of p < .05.
Results and discussion
In this study, we demonstrated the acute and subacute toxicity of EtOAcAP extract from G. urbanum L. rich in phenolic compounds on the hematological parameters, Payer’s patches, brain and spleen tissues in model H-albino mice. In addition, the antioxidant activity of the extract was studied in blood plasma and brain.
Evaluation of common signs of acute and subacute toxicity after administration of EtOAcAP extract
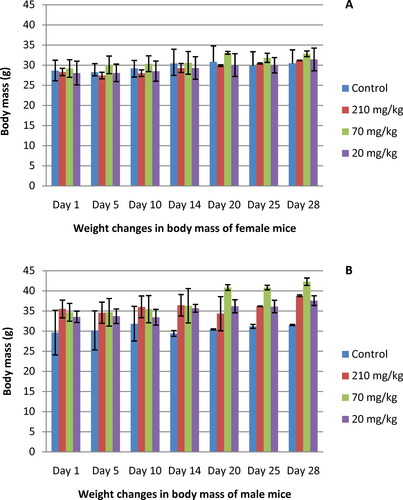
The results from the acute toxicity testing revealed that oral administration of a total of 14 doses (210, 70 and 20 mg/kg) of the EtOAcAP extract did not show any signs of morbidity or mortality in the treated animals during a period of 14 days. There were also no behavioral changes in male and female mice at the doses given during the whole treatment period. Interestingly, the body weight gain of female mice treated with 70 mg/kg of extract was more pronounced compared with the control group. The other two treatment groups (210 and 20 mg/kg) increased their body weight without significant differences (p < .05) to the control group (). The weight gain of all three treated groups of male mice was higher as compared to the control group (). No treatment-related morbidity or mortality was observed in the animals treated with the EtOAc extract 28 days after the first dose. No behavioral changes were found in the treated groups as well as control animals. At the end of the experiment, there was significant increase (p < .05) in the body weight of the groups treated with 210, 70 and 20 mg/kg EtOAc extract of both female and male mice (p < .05) as compared to the untreated control (). The strongest weight gain was observed in male mice at dose 70 mg/kg (p < .05; ).
Figure 1. Body weight gain of female (A) and male (B) mice treated orally with EtOAc extract of G. urbanum L. for 28 days.
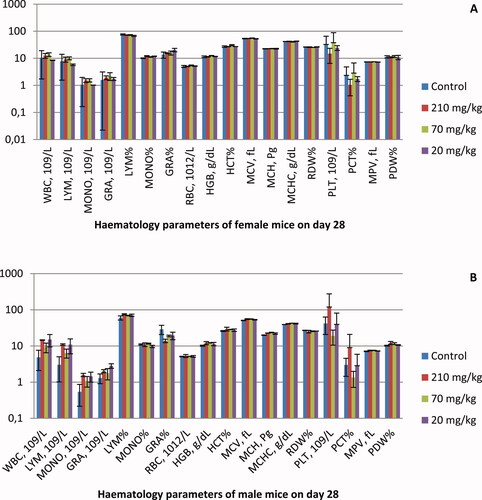
The hematological results are shown in . No significant differences in blood cell counts were observed at the 28th day in the treated female () and male () mice. Exceptions are the animals treated with the highest concentration (210 mg/kg). In the female group, the number of PLT and the percentage of PCT were halved compared to the control animals, but the differences were not significant (p > .5). In comparison, the same blood parameters were increased more than twice in male mice (p > .5). In all three treated groups of male animals, the values of WBC, LYM, MONO and GRA showed an increasing trend, but the differences were not significant (p > .5).
Histopathological evaluation of spleen preparations and Peyer's patches
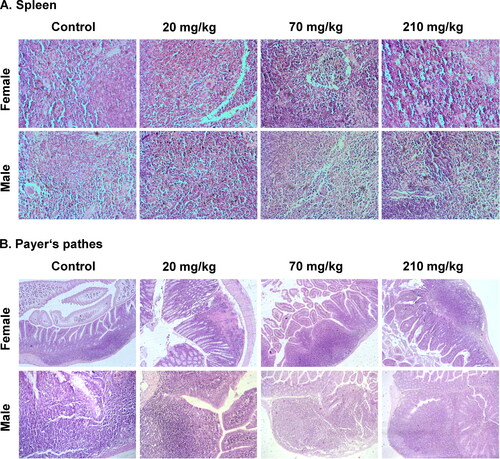
Histological sections of spleen and Payer’s patches samples of male and female mice were investigated for pathomorphological changes after treatment with the different biologically active doses (20, 70 and 210 mg/kg). As shown in (only representative sections of one female and one male animal are shown), the microscopic observation revealed no remarkable pathological changes in both tissues after oral administration with the tested doses.
Hystopathology of spleen preparations
In the experimental and control groups, there was a preserved spleen vascular structure. The scars of open circulation were identified in all groups of animals. Increased exposure dose showed no signs of cell loss or necrosis of the structures in the organs of the treated groups versus controls. The reticular skeleton (reticular framework) of the bodies was preserved in all groups. Groups treated with higher concentrations of the extract showed signs of low grade fibrosis. The alterations remained limited on the border between red and white pulp. This process showed no extension to the inside of both pulps. WBC, RBC, GRA and circulating mononuclear cells were visible between the fibers. In the exposure groups, the blood cell populations were presented in different ratios. Тhe ratio between the individual cell groups was preserved and did not deviate in any of these cell groups. Macrophage populations of varying density were detected in the experimental groups. In the groups with high dose exposure, these cells did not show different dynamics as compared to the other exposure groups and the control animals. Isolated signs of extra medullary hematopoiesis were found in the treated animals. The findings were similar among the treated groups and with lower values than those of the control groups. Combinations of erythroid, myeloid and megakaryocytic cells were also detected. Representation of hemoglobinogenic pigments, ceroid and lipofuscin was found in both, treated and control groups. The white and red pulp of the treated groups contained deposits of hemoglobinogenic pigments. Female animals from the treated groups showed a higher degree of hemosiderin pigmentation than male animals. Ceroid and lipofuscin synthesized in the process of lipid peroxidation were at low levels and in sporadic deposits. High concentrations of the treatment did not indicate a dynamic change in the volume of such accumulations. The spleen periarterial lymphoid system/PALS/was not marked in the treated groups. Small and irregularly shaped regions containing cells, corresponding to T cell-dependent areas, were observed. The zones appeared with a lower incidence in the control groups. In the treated groups, the follicles were not interrupted by PALS and were located at the points of separation of central arterioles. Isolated follicles with germinal centers were detected in the treated groups. These follicles were less intensely stained due to the presence of fewer cells, including macrophages and apoptotic B cells. Small groups of macrophages/subsets/were detected on the border of red pulp with PALS and follicles [Citation43]. The number of subsets varied in a narrow range (1.6% to 4%). Marginal zones were unevenly mixed with the red pulp. No correlation between the width of the area and the applied concentrations were observed between the treated groups of animals. The findings in populations of B cells confirmed that the marginal zones comprise a subset of noncirculating cells, which are phenotypically different from follicular B cells [Citation44]. Follicular and marginal zones were not clearly distinguished. Тhese areas were cellularly represented. The number of follicles had values close to the control groups. Individual animals show deviations relating to the representation of the smaller follicles and low levels in the distribution of lymphocytes. In the absence of cytotoxic effects on these populations, the most likely reason is related to age variations in the distribution of lymphocytes. The increase in the number of macrophages and reticular cells was accounted.
Histopathology of Payer’s patches: inflammatory changes, epithelial changes and mucosal architecture structure
The density of leukocytes evaluated over the area of the basement membrane (assessment on the fields of high magnification) was without expressed representation. The evaluation of the leukocytes density in the treated groups relative to the control was 1 (minimum below 10%). In some animals, the density reached up to 10%, but without the dispersion of inflammatory cells. Rating 2 was not reached. Enlargement of the inflammatory infiltration included mucosal involvement (evaluation 1) and effect on mucosa and submucosal layer (evaluation 2). The affected areas had a minimal increase compared to the control animals. The results obtained did not differ from those of inflammation with low intensity and affected the individual structures of the intestinal wall. In the treated groups, the increase in epithelial cells in longitudinal crypts compared to the baseline number of epithelial cells in the crypt showed variations from 12% to 25%. None of the groups showed a value of over 25%, and the assessment of hyperplastic changes was 1. The loss of goblet cells was estimated as a reduced number of goblet cells compared to the baseline number of goblet cells per crypt. In the treated groups, the variation in this indicator was from 10% to 20%. The interval in the control group was 8–10%. The inflammatory cells did not infiltrate between the crypt epithelial cells, and the neutrophil leukocytes in the crypt lumens were single. These changes were not associated with loss of surface epithelium. There were negative findings on the indicators cryptitis, crypt-abscesses and erosion. No defects in the epithelium reaching and exceeding the muscle layer of the epithelium (lamina muscularis mucosae) were found in the treatment groups. Changes in the architecture of the crypts were sporadic. In each of the treated groups, non-parallel crypts were found. Variable diameters crypts, such as findings with very low frequency and not dependent on the dose and exposure time were observed. Isolated pseudopolyps and viliform, hypertrophic areas were found in the treated groups. They did not lead to minimal luminal reduction. The villous-crypt ratio was usually 2:1. In the groups treated with higher concentrations, the ratio was greater in the individual animals, but did not exceed 3:1. This determined the low (minimal) degree of villous blunting (evaluation 1). No troves of villous atrophy were found in the groups.
Assessment of small intestine and adjacent lymph nodes
We also investigated the potential of the EtOAcAP extract to affect the functions of the small intestine and the adjacent lymph nodes after 28 days of treatment. The number of lymph follicles was not increased in the treated experimental animals as compared to the control group. The changes were expressed in a sinus response (increased number of intrasinusoidal macrophages) at doses of 20 mg/kg and 70 mg/kg. There was no pronounced and severe lymphadenitis. No stimulated/reactive follicles (lack of large lymphoblasts, apoptotic lymphocytes and small number of corpus macrophages) were found in the treated groups. Paracortical areas retained their size and area relative to the control group. In the treated groups, the cell density was restored relative to the control group. Plasma cell representations remained in the medullary areas. The representation of plasma cell precursors (immunoblasts and plasmoblasts) was low. None of the cell populations showed infiltration into the cortical and capsule zones. No proliferative activity of residual sinusoidal macrophages was reported in the studied groups. Distinct macrophage aggregates were found in paracortical and medullary regions. The alterations were retained at the average doses (20 mg/kg and 70 mg/kg) and were missing at the highest dose. No lymphoid necrosis was accounted in any of the treated groups. Isolated signs of cellular edema and karyorexis were detected only in few cells within the treated groups. Their numbers were in a narrow range despite the increased exposure time. Sporadic apoptotic bodies were detected in the treated groups, in regions with reduced macrophage numbers. These areas corresponded to germline/germinative centers. The formation of secondary follicles was not observed and the findings were not repeatable (in the secondary follicles and their germinal centers, programmed cell death is a homeostatic mechanism). This process is triggered by stimuli at higher doses after a longer period of exposure. Lack of chronic necrosis and low apoptotic levels were associated with lack of lymphatic depletion in the treatment groups. The number and density of paracortical lymphocytes was similar to that in the control group. Limited vascular changes were observed in the treated groups. These include sinus hyperemia (congestion) and sinus erythrocytosis. The changes showed values similar to those of the control group. The lack of abundant deposits of hemosiderin further supported the lack of hemorrhage in the obtained results. An isolated finding was sinus ectasia, which was not related to repeated topography of changes. The finding was isolated in both paracortical and medullary sinuses. There were pigment deposits in the cytoplasm of sinusoidal macrophages. These findings were found in both control and treated animals. Findings of a limited deposition of amorphous, eosinophilic hyalinization and extracellular material were reported in the treated groups. The amendments were not found in all treated animals and there was no detectable dose-dependent progression of deposits. The topography of changes was strictly isolated in the subcapsular sinuses.
Oxidative stress in brain tissue and blood plasma after administration of EtOAcAP extract
ROS formed as a result of aerobic metabolism, when present in excess, can attack biomolecules, leading to cellular or tissue damage associated with degenerative diseases [Citation45]. O2·– is involved in the formation of more potent and dangerous hydroxyl radical as well as singlet oxygen, which contribute to oxidative stress in the body [Citation46]. In our previous research, we found that EtOAcA extract acted as a strong O2·– scavenger, which correlates well with the total phenolic content [Citation18]. Recent studies showed that the polyphenolic compounds from medicinal plants contributed to scavenge O2·– [Citation46, Citation47].
Antioxidant enzymes are the first line of defense against oxygen radicals and play a crucial role in maintaining oxidative balance. Their activities are regulated by many different factors [Citation48]. Some authors reported that plant extracts have many antioxidant and antimicrobial components that provide a wide range of antioxidant activity [Citation49].
The brain is the main target of oxidative stress, which is associated with oxidative damage [Citation50, Citation51]. In the experiments testing the acute toxicity of the EtOAcAP extract, no significant differences in O2·– (male mice: 34.5, 19.8, 11.8 and female mice: 16.4, 21.37, 11.8 nmol) and H2O2 (male mice: 14.7, 15.2, 18.5 and female mice: 16.4, 11.8, 16.9 nmol) level in brain tissue were observed, compared to the control group (male mice: O2·– =22.9 nmol, H2O2=26.4 nmol and female mice: O2·– =23 nmol, H2O2 = 22.8 nmol). Superoxide radical levels in the blood plasma of female and male model mice treated with concentrations of 20, 70 and 210 mg/kg, decreased compared to the control group (). Similar results were observed in experiments with rats. The plant extracts of Teucrium polium, Cyperus rotundus, Anethum graveolens and Nasturtium officinale showed inhibitory effects against ROS generation [Citation52]. Jackie et al. [Citation53] reported that the extract of Etlingera elatior has antioxidant effect in rats. We established the augmentation of the level of H2O2 in the blood plasma of male and female mice after their exposure to the EtOAcAP fraction. This was probably associated with higher lipid peroxidation (male mice: 0.1, 0.7, 0.7; and female mice: 0.6, 0.7, 0.4 MDA nmol/mL) and protein carbonyl content levels (male mice: 0.8, 0.8, 0.9; and female mice: 0.7, 0.9, 0.8 nmol/mg protein) compared to the control group (male mice: LP = 0.5 MDA nmol/mL, DP = 0.3 nmol/mg protein and female mice: LP = 0.3 MDA nmol/mL, DP = 0.3 nmol/mg protein). In our experiments, male and female brain tissues supplemented with G. urbanum L. extract showed a significant increase in SOD activity. Bhattacharya et al. [Citation54] showed that the extract of Bacopa monniera Linn induced an increase in SOD activity in rat brain.
Figure 4. Biochemical analysis results of blood plasma in female (A) and male (B) mice and of brain serum in female (C) and male (D) mice treated orally with EtOAc extract of G. urbanum L. for 14 days.
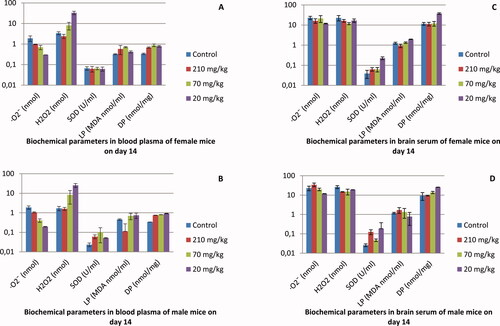
The results of the experiment with acute toxicity testing of EtOAc extract demonstrated a decrease in the levels of O2·– in blood plasma. At the same time stimulation of the H2O2 production rate was observed. As is shown in , the SOD activities in the blood plasma of male mice were higher. The levels of SOD in brain tissues were higher both in male and female mice. There was a rise in the lipid peroxidation in blood plasma (). No significant changes in damaged protein levels in blood plasma and brain were observed.
In the experiment with subacute toxicity (), stimulation of O2·–· production when we used 70 and 210 mg/kg EtOAc extract of G. urbanum L. was observed. This trend was valid both in the male and female mice. The H2O2 production rate did not change in all extract doses used in these experiments. Different doses of the extract did not affect the production of O2·and H2O2 () The O2·– levels were comparable to the controls in the brain tissues. Treatment with 210 mg/kg EtOAc extract of G. urbanum stimulated the SOD activity in brain tissues of female mice. The levels of lipid peroxidation in all tested variants were comparable to those of the control variant. No significant changes in carbonyl content were observed in blood and brain tissues both in female and male mice.
Figure 5. Biochemical analysis results of blood plasma in female (A) and male (B) mice and of brain serum in female (C) and male (D) mice treated orally with EtOAc extract of G. urbanum L. for 28 days.
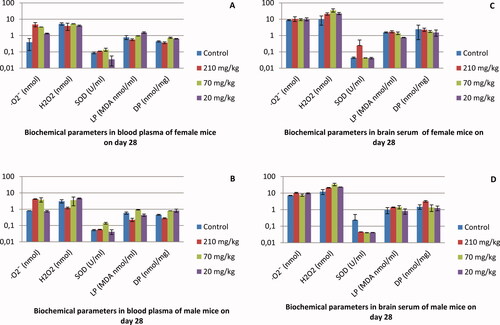
Lipids and proteins are the main targets for ROS [Citation55]. ROS production leading to lipid peroxidation and protein carbonylation was measured as MDA and carbonyl groups in male and female mice brain and blood plasma ( and ). No significant differences in lipid peroxidation and carbonyl content in the brain tissue of male and female mice after G. urbanum treatment were observed. There was a weak increase in lipid peroxidation and carbonyl content in male and female mice blood plasma after plant extract treatment. Oxidative stress causes damage to lipids, forming oxidized molecules (peroxides). Lipid peroxidation is a suitable endpoint for the assessment of oxidative stress [Citation56]. Lipid peroxidation is initiated by the presence of free radicals, which normally attack unsaturated fatty acids, and is propagated by a lipid chain reaction cycle that is catalyzed by a divalent metal ion. The latter can degrade the hydroperoxides contained in the membrane lipid to reactive alkoxy and peroxy radicals. The quercetin isolated from buckwheat hull [Citation57] and previously found in G. japonicum [Citation25], has been shown to be an antioxidant substance that interrupts this chain reaction and is a metal-chelating agent [Citation57]. Investigation of subacute toxicity showed that after 28-day treatment with G. urbanum EtOAcAP extract, the generation of O2·– and H2O2 in male and female brain tissue did not change (). The enzyme activities were significantly decreased in G. urbanum-treated mice compared to the controls. On day 28, the SOD activity in male and female brain tissue was significantly lower in treated animals, but was elevated on treatment at day 14. This could be interpreted as being a result of a correlation between G. urbanum treatment and antioxidant status in brain tissue. Despite lower SOD activity in male and female brain tissue on day 28, there were no significant differences in O2·– and H2O2 levels between day 14 and day 28 days of treatment. The levels of carbonyl content in the male and female brain tissue were significantly lower in the treated animals on day 28 () compared with those on day 14 (). On day 28, the levels of lipid peroxidation in male and female brain tissues remained almost unchanged (). At the same period of treatment, O2·– generation increased in male and female blood plasma compared to the 14-day group. Administration of G. urbanum for 28 days resulted in a significant change in superoxide level in female and male mice (4.8 and 4.2 nmol, respectively) treated with concentrations of 210 mg/kg and 70 mg/kg (3.4 and 3.8 nmol, respectively) compared to the control groups (1.8 and 0.8 nmol, respectively). No significant difference in H2O2 level, lipid peroxidation and carbonyl content in blood plasma of treated mice with the tested extract were observed.
Conclusions
No behavioral changes or animal death was registered in the mice treated with of G. urbanum extract during the total 28 days of observation. Interestingly, at a concentration of 70 mg, a slight increase in appetite was observed in male animals. No exposure-related changes in hematological parameters were noted. No significant histopathological changes were observed in the spleen and Payer’s patches tissues of the control and treated groups with the EtOAc extract. Therefore, it could be assumed that the dose ≥210 mg/kg of EtOAcAP extract is promising for application as food additive in various infectious diseases.
Disclosure statement
The authors declare that they have no known competing financial interests and personal relationships that could have appeared to effect negative the present study in this paper. No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Silva MG, Aragão TP, Vasconcelos CF, et al. Acute and subacute toxicity of Cassia occidentalis L. stem and leaf in Wistar rats. J Ethnopharmacol. 2011;136(2):341–346.
- Doncheva S. Bulgaria is first in the export of herbs [in Bulgarian] 2016. Available from: http://uspelite.bg/bylgariq-e-purvenec-v-evropa-i-na-chelno-miasto-v-sveta-po-iznos-na-bilki
- INCF. The medicinal plants-natural resources, lightweight and livehood. Handbook for journalists [in Bulgarian]. Sofia; 2015. (Foundation IaNC, editor.).
- Pamukoff D. The Science of Biomedicine and Phytotherapy 2017. Available from: http://dr-pamukoff.com/index.php?option=com_content&view=article&id=5&Itemid=6&lang=en
- Valko M, Rhodes C, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40.
- Abdel-Daim MM, Ghazy EW. Effects of Nigella sativa oil and ascorbic acid against oxytetracycline-induced hepato-renal toxicity in rabbits. Iran J Basic Med Sci. 2015;18(3):221–227.
- Velioglu Y, Mazza G, Gao L, et al. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46(10):4113–4117.
- Sastre J, Pallardó FV, Viña J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49(5):427–435.
- Takabe W, Niki E, Uchida K, et al. Oxidative stress promotes the development of transformation: involvement of a potent mutagenic lipid peroxidation product, acrolein. Carcinogenesis. 2001;22(6):935–941.
- Khan MA, Baseer A. Increased malondialdehyde levels in coronary heart disease. J Pak Med Assoc. 2000;50(8):261–264.
- Mora A, Paya M, Rios J, et al. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem Pharmacol. 1990;40(4):793–797.
- Kiselova-Kaneva Y. Biological effects of oxidative stress and herbs as a means of countering [in Bulgarian]. 2013. (Antida, Varna, editor). ISBN: 978-619-90019-4-3 33.
- Cheng XR, Jin HZ, Qin JJ, et al. Chemical constituents of plants from the genus Geum. Chem Biodivers. 2011;8(2):203–222.
- Assyov B, Petrova A, Dimitrov D, et al. Conspectus of the Bulgarian vascular flora: distribution maps and floristic elements, Fourth revised and updated edition. Sofia; 2012. (Foundation Bb, editor.).
- Paun G, Neagu E, Albu C, et al. Inhibitory potential of some Romanian medicinal plants against enzymes linked to neurodegenerative diseases and their antioxidant activity. Pharmacogn Mag. 2015;11(Suppl 1):S110–S116.
- Granica S, Kłębowska A, Kosiński M, et al. Effects of Geum urbanum L. root extracts and its constituents on polymorphonuclear leucocytes functions. Significance in periodontal diseases. J Ethnopharmacol. 2016;188:1–12.
- Gstirner F, Widenmann H. Uber Inhaltsstoffe des Rhizoms von Geum urbanum L. Sci Pharm. 1964;32:98–104.
- Dimitrova L, Zaharieva MM, Popova M, et al. Antimicrobial and antioxidant potential of different solvent extracts of the medicinal plant Geum urbanum L. Chem Cent J. 2017;11(1):113.
- Piwowarski JP, Granica S, Kosiński M, et al. Secondary metabolites from roots of Geum urbanum L. Biochem Syst Ecol. 2014;53:46–50.
- Kosman VM, Blinova KF, Zenkevich IG. Identification and quantitative determination of eugenole in the underground organs of Geum urbanum L. Rastitel'nye Resursy. 1995;31:93–96.
- Psenak M, Jindra A, Stano J, et al. Vicianose from the root of Geum urbanum. Planta Med. 1972;22(1):93–96.
- Nikolova M, Valyovska-Popova N, Dimitrova M, et al. High-mountain Bulgarian plants- free radical scavenging activity and flavonoid composition. J BioSci Biotechnol. 2014:29–33. Paris: OECD Publishing. https://doi.org/10.1787/9789264070684-en.
- Panizzi L, Catalano S, Miarelli C, et al. In vitro antimicrobial activity of extracts and isolated constituents of Geum rivale. Phytother Res. 2000;14(7):561–563.
- Kaminska J, Assenow I. Phytochemical studies of Geum bulgaricum Panc. Acta Pol Pharm. 1971;28(2):201–206.
- Yean MH, Kim JS, Hyun YJ, et al. Terpenoids and phenolics from Geum japonicum. Korean J Pharmacogn. 2012;43(2):107–121.
- Nijveldt RJ, Van Nood E, Van Hoorn DE, et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–425.
- Fotsis T, Pepper MS, Aktas E, et al. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57(14):2916–2921.
- Dimitrova L, Popova M, Bankova V, et al. Anti-quorum sensing potential of Geum urbanum L. C R Acad Bulg Sci. 2019;72(3):341–349.
- Walker JN, Flores-Mireles AL, Pinkner CL, et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc Natl Acad Sci USA. 2017;114(41):E8721–E8730.
- Fisher EL, Otto M, Cheung GY. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front Microbiol. 2018;9:436.
- Upadhyayula S, Kambalapalli M, Asmar BI. Staphylococcus epidermidis urinary tract infection in an infant. Case Rep Infect Dis. 2012;2012:1–2.
- Schoeni JL, Wong ACL. Bacillus cereus food poisoning and its toxins. J Food Prot. 2005;68(3):636–648.
- OECD/OCDE. OECD Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing. doi:10.1787/9789264071001-en.
- OECD/OCDE. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing. doi:10.1787/9789264070684-en.
- Hassan HM, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196(2):385–395.
- Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226.
- Levine RL, Garland D, Oliver CN. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478.
- Adachi H, Ishii N. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2000;55(6):B280–B285.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- Kiernan JA. Histological and histochemical methods: theory and practice. 4 ed. UK: Scion; 2008. (Bloxham, editor.).
- LeicaBiosystems. http://www.leicabiosystems.com/specimen-preparation/tissue-processing/details/product/leica-tp1020/ [Internet]; 2013 [cited 2013 Nov 5].
- Spector G, editor. Preparation of cells and tissues for fluorescence microscopy. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2006.
- Matsuno K, Ezaki T, Kotani M. Splenic outer periarterial lymphoid sheath (PALS): an immunoproliferative microenvironment constituted by antigen-laden marginal metallophils and ED2-positive macrophages in the rat. Cell Tissue Res. 1989;257(3):459–470.
- Van Rees E, Sminia T, Dijkstra C. Structure and development of the lymphoid organs. Pathobiol Aging Mouse. 1996;1:173–187.
- Amarowicz R, Pegg R, Rahimi-Moghaddam P, et al. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84(4):551–562.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12(1):221.
- Gangwar M, Gautam MK, Sharma AK, et al. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: an in vitro study. Sci World J. 2014;2014:1–12.
- Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9.
- Sonam KS, Guleria S. Synergistic antioxidant activity of natural products. Ann Pharmacol Pharm. 2017;2(16):1–6.
- Fontella FU, Siqueira IR, Vasconcellos APS, et al. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005;30(1):105–111.
- Lucca G, Comim CM, Valvassori SS, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 2009;54(5–6):358–362.
- Bahramikia S, Ardestani A, Yazdanparast R. Protective effects of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chem. 2009;115(1):37–42.
- Jackie T, Haleagrahara N, Chakravarthi S. Antioxidant effects of Etlingera elatior flower extract against lead acetate-induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats. BMC Res Notes. 2011;4(1):67–68.
- Bhattacharya S, Bhattacharya A, Kumar A, et al. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14(3):174–179.
- Kayali R, Çakatay U, Tekeli F. Male rats exhibit higher oxidative protein damage than females of the same chronological age. Mech Ageing Dev. 2007;128(5–6):365–369.
- Ito F, Sono Y, Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8(3):72.
- Mukoda T, Sun B, Ishiguro A. Antioxidant activities of buckwheat hull extract toward various oxidative stress in vitro and in vivo. Biol Pharm Bull. 2001;24(3):209–213.