 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Peptic ulcer is a common disorder of the gastrointestinal system affecting people of all ages worldwide. Ethanol is one of the most common causative agents for peptic ulcer. The aim of this study was to evaluate the protective effect of two newly synthesized indole imines (389 and 393) against ethanol-induced gastric ulcer in rats. Animals were orally administered different treatments: group I–II (vehicle & ulcer controls, respectively); 0.5% aqueous CMC solution, group III (standard control; omeprazole; 20 mg/kg bw); groups IV–VI, test compound 389 (20, 40 and 60 mg/kg bw) and groups VII–IX, test compound 393 (20, 40 and 60 mg/kg bw). Various parameters including pH, gastric contents, ulcer index and percentage protection were determined and histopathological study of gastric mucosa was performed to evaluate the protective effect of the test compounds. Oral administration of the test compounds, i.e. 389 and 393 (20, 40 and 60 mg/kg bw), significantly (p < 0.05) reduced the ulcer index in a dose-dependant manner as compared to the ethanol-induced ulcer control group. The gastroprotective potential of the studied test compounds was supported by the histological examination of the gastric mucosa showing a decreased inflammatory response with less oedema and leucocyte infiltration. The study concluded that the synthesized test compounds possess antiulcerogenic potential possibly due to their anti-inflammatory effects.
Introduction
Peptic ulcer is a common disorder of the gastrointestinal system affecting 10% of the global population [Citation1,Citation2]. Gastric and duodenal ulcers occur because of disparity between the potential harmful and protective factors of the gastric mucosa. Excessive use of caffeine, alcohol, tobacco and stress also contribute to the development of gastric ulcer [Citation3]. Commonly used ulcer models include nonsteroidal anti-inflammatory drugs (NSAIDs) which cause destruction of mucosa through inhibition of prostaglandin synthesis [Citation4]. NSAIDs may cause tissue destruction through TNF-α, IL-1b, IL-8 and platelet activating factor with subsequent release of reactive oxygen species (ROS) and proteases [Citation5]. ROS disturb the natural antioxidant defence mechanism of the body leading to oxidative stress [Citation6]. On the other hand, ethanol may block the gastric mucosal microcirculation, causing hypoxia, ROS production and lipid peroxidation. Ethanol also produces indirect harmful effects through leucocytes recruitment, which drives inflammatory responses, oxidative stress, decrease in the PGE2 levels and leads to impairment in gastric mucosal integrity [Citation7].
Combination therapy including omeprazole, metronidazole and clarithromycin has been found effective to treat peptic ulcer disease [Citation3]. Proton pump inhibitors (PPIs) have been used as a part of a multidrug regimen for treatment of Helicobacter pylori and are also useful in the prevention and treatment of peptic ulcers caused by aspirin and other NSAIDs. Multiple medicinal plants and synthetic compounds have been evaluated for antiulcer effects in animal models. For example, ethanol-induced gastric mucosal damage was protected by 2-mercaptoethane sulfonate [Citation8]. Indole derivatives are reported to possess antimicrobial, anticonvulsant, anti-inflammatory, anticancer, antiulcer and antioxidant activities [Citation9]. A marked elevation in gastric pH was observed in experimental rats treated with 2,3-dimethylindole-1-acetamide,-1-propionamide and −1-butyramide [Citation10]. In view of the aforementioned antiulcer, antioxidant and anti-inflammatory activities of indole derivatives, it was worth screening some newly synthesized indole compounds for antiulcer activities. So, the present study was planned with the aim to evaluate and compare the antiulcer potential of newly synthesized indole imines against a standard drug (omeprazole) with the ultimate objective of exploring more efficacious, safe and possibly novel antiulcer agents in an ethanol-induced ulcer model.
Materials and methods
Synthetic compounds
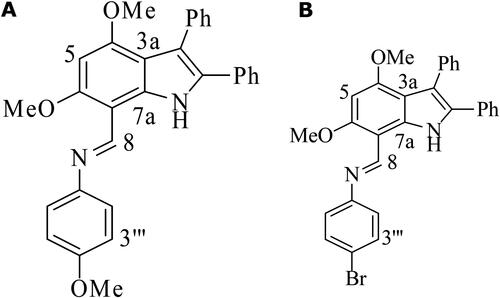
The two indole imines were newly synthesized by the Department of Chemistry, University of Sargodha, Sargodha, Pakistan. The compounds were coded as compound 389 and compound 393 and their chemical structures are depicted in .
Figure 1. Molecular structures of Indole Imine(s) A: Compound 389; N-(4-Methoxyphenyl) (4,6-dimethoxy-2,3-diphenyl-1H-indol-7-yl) methanimine, B: Compound 393; N-(4-Bromophenyl) (4,6-dimethoxy-2,3- diphenyl-1H-indol-7-yl) methanimine.
The pH was recorded on a Hanna (HI-4221) digital pH-meter and centrifugation was carried out using an 800 D Desktop Electric Centrifuge (4000 rpm). The infrared (IR) and ultraviolet/visible (UV/Vis) spectra were recorded on a Shimadzu (Prestige 21) FT-IR spectrometer using KBr discs and a Thermo Spectronic (UV-1700) spectrophotometer in CHCl3/MeOH, respectively. All the chemicals and reagents of analytical grade were procured from Sigma-Aldrich Germany. For the synthesis of compounds (389 and 393), a suspension of 4,6-dimethoxy-2,3-dipheny l-1 H-indole-7-carbaldehyde (1.0 g, 2.8 mmol, 1 eq) and aniline (8.4 mmol, 3 eq) was refluxed in dry EtOH at 80 °C for 15–18 h. Upon the disappearance of carbaldehyde, the resulting solution was cooled to ambient temperature and crystallized out the required imine in good yield (76–81%).
Animals
Male rats (180–220 g) were procured from the animal house of the University of Agriculture Faisalabad, Pakistan. Animals were kept under controlled conditions (temperature; 25 ± 2 °C), humidity (60 ± 10%) and 12 h light/dark cycle. Animals were fed with standard diet and were provided with tap water ad libitum throughout the study duration [Citation7]. Prior to ulcer induction, the study animals were kept under fasting conditions for 22 h. Following each experiment, rats were euthanized under chloroform anaesthesia.
Ethics statement
All the procedures were carried out following the guidelines framed by the Institutional Animal Care and Use Committee of the University and the study plan was approved by the Advanced Studies and Research Board (ASRB) of Sargodha University. All care was taken to minimize animal suffering.
Experimental design and antiulcer activity
The assay was performed following the protocol described by Rahim et al. [Citation11] with a few modifications. The study animals were divided randomly into nine groups with five animals in each group (n = 5). The animals were orally administered with different treatments for eight consecutive days, with ulcer induction on the last day using absolute ethanol 5 mL/kg body weight (bw). Group I and II (vehicle and ulcer controls, respectively) received 5 mL/kg bw of 0.5% aqueous carboxy methyl cellulose (CMC). The animals in all groups except the vehicle control group were induced with ulcer by administering absolute ethanol (5 mL/kg bw) on the last day of animal trial, one hour following the administration of corresponding treatments. Group III (standard control) received omeprazole (20 mg/kg bw); groups IV–VI, test compound 389 (20, 40 and 60 mg/kg bw, respectively) and group VII–IX, test compound 393 (20, 40 and 60 mg/kg bw, respectively). After 1 h of ulcer induction, animals were sacrificed to collect the gastric contents and stomachs were washed and preserved for microscopic examination.
Various parameters including severity index, protection against ulcer, acidity and gastric contents were determined for disease assessment and evaluation of the antiulcer activity of the test compounds. The severity of ulcers was determined by calculating the ulcer score, which was ranked 0–5 according to the size of the lesion. Gastric contents were centrifuged and the supernatant was used for determination of pH and total acidity. Ulcer index (UI) was calculated by multiplying the total area of mucosal lesion (TAML) in mm2 by 100 and dividing by the total mucosal area (TMA) in mm2. Protection percentage (PP) was calculated by subtracting the UI of the treated sample from the UI of the control, then dividing by UI control and multiplying by 100 [Citation2].
The formulas for the calculation of UI and PP are given below.
Histological examination
Excised stomachs were fixed with formalin (10%), dehydrated and cleared using paraffin and xylene. Then the tissues were cut into slices (4–5 mm) and stained using haematoxylin and eosin (H & E) dyes for histological examination under a light microscope [Citation12].
Statistical analysis
The obtained data are expressed as mean values with standard error of the mean (±SEM). Parametric data were assessed by one-way ANOVA (analysis of variance) followed by Dunnett’s test. Graphics and statistical hypothesis testing were done using Graph Pad Prism and IBM SPSS.
Results and discussion
Peptic ulcer is a global health problem with high rate of morbidity and substantial mortality [Citation9]. In recent years, advances in pharmacological studies have contributed to novel therapeutically active compounds that can be used for the development of new medicines [Citation10]. Ethanol is one of the most common causative factors for peptic ulcer. Ethanol-induced gastritis model can provide unique insights into the gastric pathology because ethanol stimulates acid secretion, neutrophil infiltration and accelerates gastric mucosal necrosis and apoptosis [Citation13]. Conversely, the decline in the leukocyte recruitment in the ulcerated mucosa triggers the protection against ulcers in animals [Citation14]. Ethanol treatment significantly alleviates the gastric acidity, pH and gastric lesions while reducing the mucus content [Citation1]. Therefore, we evaluated the antiulcer effects of the newly synthesized compounds (389 and 393) in a model of ethanol-induced ulcer in rats.
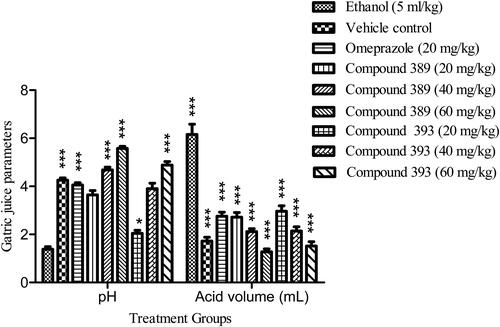
For the assessment of the antiulcer effects of the test compounds, we determined different parameters, i.e. number of gastric ulcer lesions, ulcer index, percentage protection, pH, volume of gastric secretion, total acidity and mucus contents. The results showed that both the test compounds significantly improved the gastric lesions in a dose dependent fashion (). The compound 389 in doses 40 and 60 mg/kg bw showed more pronounced effects than the reference drug, whereas the antiulcer effects of compound 393 were comparable to those of omeprazole.
Table 1. Effects of test compounds on acid output, gastric mucosal injury and mucus contents in ethanol-induced ulcer in rats.
Gastric ulcer was induced in rats upon treatment with ethyl alcohol, which was evident by the increase in gastric acid secretions () and mucosal lesions ().
Figure 2. Effect of test compounds on gastric juice parameters including pH and acid volume (mL) in ethanol-induced ulcer in rats.
Note: Values are expressed as Mean ± SEM (n = 5); asterisks indicate significant differences ***p < 0.001; **p < 0.01; *p < 0.05; ns, non-significant vs. control group.
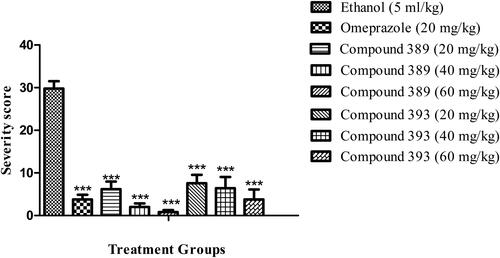
Pre-treatment of animals (1 h before ulcer induction) with test compounds 389 and 393 markedly reduced the ulcer index as shown in . The compound 389 substantially decreased the number of ulcer lesions (3 ± 0.84, 1.6 ± 0.68, 0.6 ± 0.4) when administered in doses (20, 40 and 60 mg/kg bw, respectively) as compared to the ulcer control (10.8 ± 1.02). Compound 393 also significantly reduced the number of lesions in a dose-dependent manner (). In addition, both test compounds led to a significant reduction in ulcer score when compared with the vehicle control (). Compound 389 showed a higher percentage of protection against ulcer (36.6, 54.8 and 70.6) than the compound 393 (33.7, 49.4 and 67.7) ().
Figure 3. Effect of test compounds on ulcer severity in ethanol-induced ulcer in rats.
Note: Values are expressed as Mean ± SEM (n = 5); asterisks indicate significant differences ***p < 0.001; **p < 0.01; *p < 0.05; ns, non-significant vs. control group.
Mucus creates an undisturbed layer on the mucosal surface, preserves neutral pH and supports the healing process [Citation15]. In the present study, the pre-treatment with test compounds increased the mucus production. The compound 389 significantly improved the mucus secretion in all doses compared to the ulcer control as summarized in . In contrast, compound 393 did not show significant improvement in mucus secretion at 20 mg/kg bw dose, however, at 60 mg/kg dose it caused a considerable increase in mucus secretion. Both the test compounds significantly increased the gastric pH in a dose-dependent manner compared to the control group (). Omeprazole (standard drug) also markedly increased the pH of gastric juice followed by a decrease in total acidity. The volume of gastric juice was significantly decreased by the oral administration of test compounds. The outcomes of the current study were found to be consistent with a previously reported study wherein a new Schiff base derived manganese (II) complex showed an increase in mucus content in ethanol-induced acute gastric mucosal lesions in rats [Citation16].
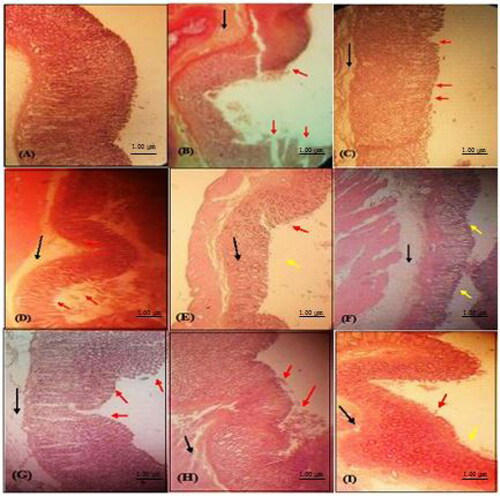
The findings of the present investigation revealed intact mucosa in animals treated with test compounds 389 and 393 at high dose (60 mg/kg), while a mild epithelial damage at lower doses presumably due to inhibition of leucocyte infiltration. The morphological changes including discontinuity in mucosal lining and necrotic lesions in the gastric mucosa were caused by ethanol administration (as shown in ). Severe oedema was also seen in the histological examination of animal stomachs. The pre-treatment of animals with test compounds 389 and 393 led to improvement in the gastric mucosal integrity. The tissue samples from the rats treated with 60 mg/kg of compounds 389 and 393 showed intact mucosa (, respectively). The rats treated with 40 mg/kg dose of compounds showed mild discontinuity in the surface epithelium with some signs of healing (€ and (G), respectively). The rats pre-treated with 20 mg/kg dose of compound 389 showed moderate discontinuity in mucosa (). In addition, the animals administered with compound 393 (20 mg/kg) showed deep mucosal damage, severe oedema and leucocyte infiltration. The administration of the standard drug, omeprazole, led to reduction of submucosal oedema, and the tissues showed only a mild discontinuity in mucosal epithelium as shown in . Pre-treatment with the test compounds produced a significant decrease in ulcer index demonstrating their mucoprotective effects and suggested the likely role of prostaglandins in antiulcer activity. The results obtained in the present investigation agreed well with the available literature [Citation8]. We in our previous study evaluated the gastroprotective potential of synthetic indole imines 8 and 9 in ethanol- and indomethacin-induced gastric ulcers and found promising results. In our previous study, we found no distortion in epithelial cells in normal control group rats, whereas discontinuity in the mucosal lining was observed in ulcer induced tissues. Tissue samples of rats treated with omeprazole and synthetic indole imines revealed intact mucosa with signs of healing at 60 mg/kg bw, while no remarkable signs of healing were observed in lower doses [Citation17]. Our results from the present study are in agreement with the findings of our previous study [Citation17]. The effects of compound 389 and 393 in the present study were similar to the effects of compound 8 and 9 investigated in our previous study.
Figure 4. Histological examination of rat stomach (H & E stained) from different treatment groups: (A) Normal vehicle control group; (B) Ulcer control (ethanol 5 mL/kg) showing mucosal damage; (C) Standard drug (omeprazole treated; 20 mg/kg bw); (D) Compound 389 treated (20 mg/kg bw); (E) Compound 389 (40 mg/kg bw); (F) Compound 389 (60 mg/kg bw) showing normalization of morphological changes; (G) Compound 393 (20 mg/kg bw); (H) Compound 393 (40 mg/kg bw); (I) Compound 393 (60 mg/kg bw) showing normalization of morphological changes. Red arrow (disrupted epithelium), black arrow (oedema and neutrophil infiltration), yellow arrow (intact epithelium).
Conclusions
It can be concluded from the above discussion that two newly synthesized compounds significantly improved the mucosal damage in the ethanol-induced gastric ulcer models of experimental rats in a dose-dependent manner and possessed anti-secretory properties demonstrating gastroprotective effects with potential association of prostaglandins and mucus production. The gastroprotective effects were also confirmed by histomorphological studies while compound 389 was found to be more potent than compound 393. However, more studies are required to elucidate the exact gastroprotective mechanisms before entering into a clinical trial phase.
Acknowledgement
The authors are thankful to the College of Pharmacy, University of Sargodha, Sargodha, Pakistan for financial assistance and providing support in completing the experimental work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
All data that support this study are available from the corresponding authors [AM and MR] upon reasonable request.
References
- Al Mofleh IA, Alhaider AA, Mossa JS, et al. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J Gastroenterol. 2008;14(3):128.
- Guzmán-Gómez O, García-Rodríguez RV, Quevedo-Corona L, et al. Amelioration of ethanol-induced gastric ulcers in rats pretreated with phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients. 2018;10(6):763.
- Amirshahrokhi K, Khalili A-R. Gastroprotective effect of 2-mercaptoethane sulfonate against acute gastric mucosal damage induced by ethanol. Int Immunopharmacol. 2016;34:183–188.
- Lichtenberger LM, Zhou Y, Dial EJ, et al. NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol. 2006;58(11):1421–1428.
- Bell MR, Zalay AW, Oesterlin R, et al. Experimental antiulcer drugs. 1. Indole-1-alkanamides and pyrrole-1-alkanamides. J Med Chem. 1977;20(4):537–540.
- Riaz M, Mahmood Z, Shahid M, et al. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int J Immunopathol Pharmacol. 2016;29(3):421–425.
- Clark JD, Gebhart GF, Gonder JC, et al. The 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38(1):41–48.
- Santos F, Rao V. 1,8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats . Dig Dis Sci. 2001;46(2):331–337.
- Périco LL, Emílio-Silva MT, Ohara R, et al. Systematic analysis of monoterpenes: advances and challenges in the treatment of peptic ulcer diseases. Biomolecules. 2020;10(2):265.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–1614.
- Rahim NA, Hassandarvish P, Golbabapour S, et al. Gastroprotective effect of ethanolic extract of Curcuma xanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. Biomed Res Int. 2014;2014:416409.
- Sistani NK, Arzi A, Rezaie A, et al. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina (Kaunas, Lithuania). 2019;55(3):64.
- Kengkoom K, Tirawanchai N, Angkhasirisap W, et al. Omeprazole preserves the RER in chief cells and enhances re‐epithelialization of parietal cells with SOD and AQP‐4 up‐regulation in ethanol‐induced gastritis rats. Exp Ther Med. 2017;14(6):5871–5880.
- Al Batran R, Al-Bayaty F, Al-Obaidi MMJ, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PloS One. 2013;8(5):e64751.
- Santin JR, Lemos M, Klein-Júnior LC, et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(2):149–158.
- Ibrahim MY, Hashim NM, Dhiyaaldeen SM, et al. Acute toxicity and gastroprotection studies of a new schiff base derived Manganese (II) complex against Hcl/ethanol-induced gastric ulcerations in rats. Sci Rep. 2016;6(1):26819.
- Akhtar MS, Raza AR, Aziz M, et al. Synthesis and gastroprotective evaluation of new synthetic indole imines on animal models. Pharm Chem J. 2020;54(1):26–35.
