?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cordyceps militaris is an edible and medicinal fungus that has been traditionally used as a crude drug for treatment of human disease. Meanwhile, the functional nutrient substances in C. militaris possess various physiological activities. In addition, re-utilizing the soybean curd residue as solid medium for producing edible and medicinal fungus is an efficient and commercial method to dispose of the soybean curd residue waste materials. In this study, soybean curd residue was utilized as solid medium raw material for fermentation by C. militaris mycelium. The crude polysaccharide (CSCPS) from fermented soybean curd residue and C. militaris mycelium mixture showed potent bioactivities, including antioxidant, antitumor and immunomodulatory activities. Based on five antioxidant assays, the CSCPS showed powerful antioxidant capacities at OH•, DPPH and ABTS radical scavenging assays. The CSCPS significantly activated the proliferation of RAW 264.7, and protected it from DOX-induced and LPS-stimulated cell damage. Furthermore, the CSCPS inhibited the proliferation of HT1080, Hela, A549, U-2 OS and MDA-MB-231 cells.
Introduction
Cordyceps militaris is an entomogenous fungus that has broadly been used as a crude drug and a folk tonic food in China, which has similar pharmacological activities to the well-known Chinese traditional medicine Cordyceps sinenses [Citation1]. The functional components found in C. militaris include polysaccharides, cordycepin, cordycepic acid, superoxide dismutase (SOD) and fibrinolytic enzyme (CMase) [Citation2, Citation3]. A number of reports have indicated that C. militaris fruit body possessed antitumor, antivirus, antifungal, antiphlogosis, antiarrhythmic, immunomodulatory, blood pressure reduction, and fibrinolytic activities [Citation4–7]. Because of its attractive beneficial effects and rarity in nature, C. militaris is considered as one of the most expensive mushrooms in China. In order to obtain sufficient quantities of the fruiting bodies for commercial applications, efforts have been made to develop cultivation techniques, including solid state fermentation for the formation of fruit body of C. militaris, or submerged culture for harvesting mycelium, which both can be utilized as ingredients in functional foods and supplements [Citation5, Citation7, Citation8]. Soybean curd residue (SCR) is the by-product produced by tofu and soymilk factories in East Asia. Up to now, several agro-industrial by-products have been utilized as inexpensive growth medium for economic production of different mycelial species [Citation9]. SCR contains nutrient substances characterized with low fat, high fiber content, protein, inorganic salts and so on. Therefore, SCR also could be utilized as the solid fermentation medium for mycelia. Polysaccharides, one of the most important active components in fungi, have become the focus of recent scientific research. Many natural polysaccharides and polysaccharide–protein complexes isolated from fungi can be used as a source of therapeutic agents that are relatively non-toxic and show no significant side effects. In recent studies, polysaccharides of C. militaris have been identified to possess antitumor [Citation10], immunomodulatory [Citation11], anti-oxidant [Citation12] and anti-hypolipidemic [Citation13] effects. In this study, the SCR was fermented by C. militaris mycelium using solid fermentation. In order to investigate the bioactivity of the C. militaris crude polysaccharides (CSCPS) from fermented SCR, the antioxidant activity, immunomodulatory activitiy and anti-tumor activity were studied in this experiment.
Materials and methods
Chemical and reagents
2,2-Diphenyl-1-picryl-hydrazyl (DPPH) was purchased from Sigma Aldrich, Inc. (Saint Louis, MO, USA). SOD Assay Kit-WST was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Dulbecco's modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin solution were purchased from Sigma Aldrich, Inc. (Saint Louis, MO, USA). A Cell Proliferation Kit (MTT) was purchased from Roche (Tokyo, Japan). Doxorubicin (DOX) was purchased from TopoGEN, Inc. (Florida, USA). All other chemical reagents were analytical grade.
Cell lines
The murine macrophage cell line RAW 264.7 was obtained from the Riken Cell Bank (Tsukuba, Japan) and maintained in DMEM medium containing 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL of streptomycin at 37 °C in a humidified 5% CO2 atmosphere (ESPEC CO2 Incubator). The cells were cultured for 2–3 days to reach the logarithmic phase and then were used for experiments. Hela, HT1080, A549, U-2 OS and MDA-MB-231 cells were obtained from RIKEN (Tsukuba, Japan). Cells were grown in DMEM containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Culture was maintained at 37 °C in a humidified 5% CO2 atmosphere (ESPEC CO2 Incubator). Cells were cultured for 2–3 days to reach the logarithmic phase and used for experiment.
Culture of Cordyceps militaris
The mycelium of C. militaris was purchased from Wuhan, China. The white mycelium was transferred to agar medium, which contained: sucrose 20 g/L, peptone 10 g/L, agar powder 20 g/L, MgSO4 1.5 g/L, KH2PO4 3 g/L. After 7 days, when white mycelium appeared on the surface of the medium, the mycelium was transferred into the liquid medium, containing: sucrose 20 g/L, peptone 10 g/L, potato powder 4 g/L, MgSO4 1.5 g/L, KH2PO4 3 g/L. The C. militaris mycelium was incubated in 200-mL flasks with 100 mL of PDA liquid medium, and the mixture was cultured stationarily for 7 days. After the culture, the SCR medium was inoculated by C. militaris mycelium for fermentation experiment.
Fermentation of soybean curd residue (SCR) by C. militaris
The SCR was fermented by C. militaris mycelium. And the fermentation conditions were as follows: 20 g of soybean curd residue, 2 g of fructose, 0.6 g of Yeast extract, 80% of moisture content, 5.5 of pH, 5% of inoculum size (v/w), and 20 days of fermentation time. After fermentation, the fermented SCR (containing mycelium) was dried and ground into powder for further experiment.
Extraction and determination of CSCPS
The dried SCR-fermented preparation was extracted by boiling water for two hours. The water-soluble polysaccharide was precipitated by adding eight volumes of 99.5% ethanol and stored at 4 °C overnight. The precipitated polysaccharide (CSCPS) was collected by centrifuging at 7000 rpm (Beckman coulter, Allegra X-30R Centrifuge) for 30 min. After drying at room temperature, the precipitate was dissolved in distilled water. The protein was removed by Sevage method [Citation14]. Briefly, the CSCPS extract by chloroform and n-butyl alcohol (25:5:1, v/v/v) was shaken intensively for 20 min, followed by centrifugation to remove the denatured proteins. Finally, the extract was precipitated with eightfold volumes of ethanol. The CSCPS were collected by centrifugation, washed twice with ethanol, and then freeze-dried by lyophilizer. The total polysaccharide content was determined by the phenol-sulfuric acid method with some modifications. The color reaction was measured as follows: 1 mL of the various concentrations of CSCPS solutions, 0.5 mL of 5% phenol solution and 2.5 mL of concentrated sulfuric acid were mixed, and boiled for 15 min. After the reaction solutions cooled to room temperature, the optical density (OD) of the reaction solutions was determined at 490 nm and the polysaccharide content was calculated with D-glucose as the standard. The results were expressed as milligram of glucose equivalent per gram of the fermented SCR [Citation14].
Antioxidant activities of CSCPS
Hydroxyl free-radical scavenging activity
The reaction mixture (2.5 mL) contained 0.5 mL FeSO4 (1.5 mmol/L), 0.35 mL of H2O2 (6 mmol/L), 0.15 mL of sodium salicylate (20 mmol/L), and 1 mL of different concentrations of CSCPS solutions [Citation14]. Ascorbic acid was used as the positive control. After incubation for 1 h at 37 °C, the absorbance of the hydroxylated salicylate complex was measured at 562 nm. The percentage scavenging effect was calculated as:
where A0 was the absorbance of the solvent control, A1 was the absorbance of the sample or ascorbic acid, whereas A2 was the absorbance of the reagent blank without sodium salicylate. Ferrous metal ions chelating activity The method of determining Ferrous metal ions chelating activity was according to a literature procedure with a few modifications [Citation15]. Various concentrations of CSCPS solutions were mixed with ethylenediaminetetraacetic acid (EDTA) solution (1 mL), 50 μL of ferrous chloride (2 mmol/L) and 0.2 mL of ferrozine (5 mmol/L), shaken well, settled for 10 min at room temperature, and the absorbance of the mixture was determined at 562 nm. EDTA was included as the positive control. The ion chelating activity was calculated as:
where A0 was the absorbance of the control (without sample), A1 was the absorbance in the presence of the sample and A2 was the absorbance without ferrozine.
DPPH radical-scavenging activity
Aliquots (0.5 mL) of various concentrations of CSCPS solutions were mixed with 2 mL (25 μg/mL) of a methanol solution of DPPH. Then the mixture was shaken vigorously and allowed to stand in the dark for 30 min. The absorbance was measured at 517 nm against a blank [Citation16]. Ascorbic acid was used as the positive control.
DPPH free-radical-scavenging activity was calculated according to the following equation:
where A0 was the absorbance without samples and A1 was the absorbance in the presence of the samples.
ABTS radical-scavenging activity
Briefly, 2.45 mmol/L solution of potassium persulfate was prepared by PBS buffer (1 mol/L, pH = 7.4). Then 0.01 mol/L phosphate buffered saline (PBS, pH = 7.4) was added to prepared ABTS solution to adjust its absorbance to 0.70 ± 0.02 at 734 nm [Citation17]. CSCPS was formulated into a series of concentrations (1.0–5.0 mg/mL) in distilled water. Finally, the absorbance values were measured at 734 nm after incubation at room temperature for 10 min. ABTS radical-scavenging activity was formulated into a series of concentrations:
where, A is the absorbance of ABTS solution + sample/standard, B is the absorbance of potassium persulfate + sample/standard, C is the absorbance of ABTS solution + distilled water/methanol and D is the potassium persulfate + distilled water/methanol.
Determination of SOD-like activity
The levels of SOD-like activity of extracted polysaccharide were measured using the SOD Assay Kit-WST according to the technical manual provided by Dojindo Molecular Technologies, Inc [Citation18]. Briefly, using a 96-well plate, 20 µL of various concentrations of CSCPS solutions were added into each sample and blank 2 well, and 20 µL of double distilled water was added to each blank 1 and blank 3 well. Then 200 µL of WST working solution was added to each well. After mixing, 20 µL of dilution buffer was added to each blank 2 and blank 3 well, and 20 µL of enzyme working solution was added to each sample and blank 1 well. The plate was incubated at 37 °C for 20 min and the O.D. was determined at 450 nm. The SOD-like activity was calculated by the following equation:
where A blank 1, A blank 2, A blank 3, A sample were the absorbance of blank 1, blank 2, blank and sample, respectively.
Immunomodulatory activities of CSCPS
Bioactivity assay
The effect of CSCPS on the proliferation of RAW 264.7 cells was evaluated using the MTT assay. Briefly, 5 × 104 cells/mL of RAW 264.7 cells were seeded in a 96-well plate. After 24 h of incubation, RAW 264.7 cells were treated with different concentrations of CSCPS solutions (10, 20, 40, 80 μg/mL) for 24 h, 48 h or 72 h, 100 μL phosphate buffered saline (PBS) and 10 μL of MTT labeling reagent was added to each well after removed the medium for 4 h of incubation. Then 100 μL of the DMSO was added to each well, and the plate was incubated in a humidified atmosphere overnight. The absorbance of the sample was measure at wavelength of 570 nm.
Protective activity
RAW 264.7 cells were cultured in a 96-well plate at a density of 5 × 104 cells/mL for 24 h at 37 °C in a 5% CO2 atmosphere. Then cells were incubated with DOX (5 μmol/L) or LPS (1 μg/mL) in the presence or absence of various concentrations of CSCPS solutions (10, 20, 40, 80 μg/mL) for 24 h, 48 h and 72 h. After incubation, the medium was removed, then 100 μL of phosphate buffered saline (PBS) and 10 μL of MTT labeling reagent was added to each well for 4 h incubation. Then 100 μL of DMSO was added to each well, and the plate was incubated in a humidified atmosphere overnight. The absorbance of the sample was measured at wavelength of 570 nm.
Antitumor activities of CSCPS
Hela, HT1080, A549, U2OS and MDA-MB-231 cells were grown in DMEM at 37 °C in a 5% CO2 atmosphere to logarithmic phase, respectively. Cells were harvested, and an aliquot (100 μL) of cells suspension (5 × 104 cells/mL) were dispensed into a 96-well plate and pre-incubated at 37 °C in a 5% CO2 atmosphere for 24 h. Cells were treated with different concentrations of CSCPS solutions (5, 10, 20, 40 and 80 μg/mL) for 24 h, 48 h or 72 h. After removing the medium, 100 μL phosphate buffered saline (PBS) and 10 μL of MTT labeling reagent was added into each well. After 4 h of incubation, the solution of each well was removed, 100 μL of the solubilization solution was added into each well, and the plate was incubated in a humidified atmosphere overnight. The absorbance of the sample was measure at wavelength of 570 nm.
Data analysis
The experiments were conducted in triplicate and results were expressed as mean values with standard deviation (± SD). Two-tailed Student's t-test was used for the statistical analysis. Significant differences were considered significant at p < 0.1 (*), p < 0.05 (**), p < 0.01 (***).
Results and discussion
The yield of polysaccharide
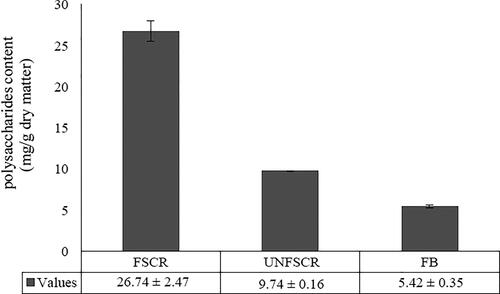
As shown in , the polysaccharide yield of fermented SCR was 26.74 ± 3.17 mg/g dry matter. Compared with unfermented soybean curd residue and fruiting body of C. militaris, polysaccharide content of fermented SCR was higher than that of unfermented soybean curd residue (9.74 ± 0.46 mg/g dry matter) and fruiting body (5.42 ± 0.27 mg/g dry matter). The results showed that the polysaccharide content of fungus was increased by fermentation method.
Antioxidant activity of CSCPS
Oxidative stress has been proven to be implicated in aging, chronic disease, cancer etc. Antioxidants could be useful in combating different processes and diseases [Citation19]. A large number of reports indicated that the polysaccharide of fungal origin has excellent antioxidant activity. The activities of polysaccharides are highly dependent on their chemical composition and structural characteristics such as glycosidic linkages [Citation20], monosaccharide composition [Citation21] and polymerization degrees [Citation22].
Hydroxyl free-radical scavenging activity
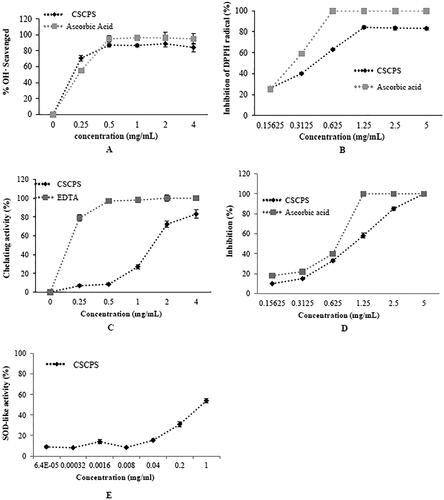
As shown in , ascorbic acid and CSCPS exhibited dose-dependence (0.25–2 mg/mL) of hydroxyl radical scavenging activity. The scavenging effect of ascorbic acid was higher than that of CSCPS, and the maximum inhibition achieved by ascorbic acid and CSCPS were 96.40 ± 3.36% and 88.8 ± 4.23% at 1 mg/mL and 2 mg/mL, respectively. The EC50 value of CSCPS of HO• scavenging activity was 0.238 mg/mL.
Figure 2. Antioxidant activities of CSCPS. (A) Hydroxyl radical scavenging activity; (B) DPPH radical-scavenging; (C) Chelating activity; (D) ABTS radical-scavenging capacity; (E) SOD-like activity. Note: All treatments were conducted in triplicate, and mean values were reported. The error bars represented the standard deviation of each data point. Ascorbic acid and EDTA were the positive controls.
It has been reported that hydroxyl radicals could not only react with biological macromolecules and hereby cause cell damage, but also promote the generation of ROS, which could induce severe oxidative damage of different tissues [Citation19, Citation23]. Thus, the removal of HO• is important for the protection of living systems. Compared with other reports, the CSCPS had the similar HO• scavenging activity with the polysaccharide of P70-1 and CBP-1 [Citation24, Citation25]. CBP-1 was extracted and purified from the fruiting body of cultured C. militaris by alkaline extraction and has strong hydroxyl radical scavenging activity with an EC50 value of 0.638 mg/mL [Citation24]. The P70-1 extract by boiling water from the fruiting bodies of cultured C. militaris was also found to possess hydroxyl radical-scavenging activity with an EC50 value of 0.548 mg/mL [Citation25].
DPPH radical scavenging activity
As shown in , it was generally observed that the DPPH radical-scavenging effect increased as the concentration of CSCPS increased, to a certain extent, and then leveled off, even with further increases in the concentration. For example, CSCPS at a concentration of 0.156–1.25 mg/mL exhibited 25.62 ± 1.29% to 83.89 ± 0.15% scavenging activity of DPPH radical, whilst its scavenging activity was 83.46 ± 0.21% at the concentration of 5 mg/mL. No significant increase in the DPPH radical scavenging effect was observed with further increases in dosage. The scavenging rate of ascorbic acid reached 100% at 0.625 mg/mL. The EC50 value of CSCPS of DPPH radical scavenging activity was 0.44 mg/mL.
DPPH• radical is a common and convenient model for testing the free-radical scavenging potential in vitro of polysaccharides [Citation26]. The antioxidants react with the stable free radical DPPH (deep violet color) and convert it to 1,1-diphenyl-2-picryl hydrazine with decoloration. For comparison, Shi et al. [Citation27] reported that the polysaccharide of fermented SCR by Ganoderma lucidum (GLPL) using a similar fermentation method, has an excellent DPPH• radical scavenging capacity with an EC50 value of 0.23 mg/mL; the result was higher than that of CSCPS. And compared with the polysaccharide of fermented SCR (CPS) by Preussia aemulans, which was separated from Cordyceps senensis fruiting body (in our previous research), the EC50 value of DPPH radical scavenging capacity of CPS was 0.48 mg/mL. Both CSCPS and CPS had similar scavenging activity of the DPPH radical in vitro [Citation16]. The antioxidant capacity may be related with the structure of polysaccharides [Citation28]. Some reports indicated that higher β-glucan content of polysaccharides has appreciable antioxidant activities [Citation29]. The polysaccharide of CMPs8, CMPs8_5 and CMPs9 with higher content of β-glucan extracted from C. militaris fruiting body showed more potent DPPH radical scavenging ability than others [Citation30].
Ferrous meatal ions chelating activity
The transition metals could magnify the cellular damage and are known as strong pro-oxidants due to their higher reactivity. Therefore, the Fe2+ chelation has been claimed to be one of the antioxidant mechanisms and widely applied to antioxidant research [Citation19, Citation31]. In the present study, CSCPS was compared to EDTA for their Fe2+-chelating capacity. The chelating actions of polysaccharides on ferrous ions increased in a dose-dependent manner (). At 4 mg/mL, polysaccharides chelated 83.10 ± 4.42% of ferrous ions. EDTA (the positive control) showed a high chelating ability of 100% at 2 mg/mL. The EC50 value of CSCPS for Ferrous meatal ions chelating activity was 1.276 mg/mL. We suggested that this moderate to high ferrous-ion chelating activity shown by fungi could be beneficial to human health.
ABTS radical-scavenging activity
ABTS free-radical assay is also a rapid method to evaluate the antioxidant capacities of polysaccharides that can donate an electron and hydrogen atom to an unstable ABTS cation radical to form a stable ABTS free radical, and the color reduction of blue-green ABTS free radical solution can be detected at 734 nm [Citation32]. In the present study, the extent of scavenging of ABTS.+ was considered as an indicator to test the concentration of antioxidant. As shown in , CSCPS scavenged ABTS.+ radicals in a dose-dependent manner. At the concentration of 0.15625–0.625 mg/mL, the CSCPS and ascorbic acid had similar scavenging activities. When the concentration increased to 1.25 mg/mL, the scavenging rate of ascorbic acid reached to 100%. At 5 mg/mL, the ABTS radical-scavenging activity of polysaccharide was the highest, with 97.42 ± 0.64%. The EC50 value of CSCPS of ABTS radical-scavenging activity was 1.05 mg/mL. Compared with GLPL the polysaccharide from fermented SCR by Ganoderma lucidum (EC50 = 1.21 mg/mL), we also found that both GLPL and CSCPS had similar ABTS.+ radical-scavenging capacities in high level. These indicated that the polysaccharide of edible mushrooms mycelium has significant antioxidant activity [Citation27].
SOD-like activity
All living bodies have a complex antioxidant defense system that includes various antioxidant enzymes, such as superoxide dismutase and catalase. A rapid and facile method for the assay of SOD-like activity, based on the ability to inhibit the auto-oxidation of pyrogallol, is widely used to evaluate antioxidant capability. In the present study, we found that the SOD-like activity increased with the concentrations of polysaccharide. And the SOD-like activity was 53.99 ± 1.87%, while the concentration of polysaccharide was 1 mg/mL ().
Immunomodulatory activities of CSCPS
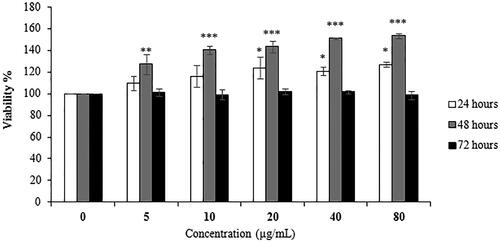
The stimulatory effect of CSCPS extracted from the fermented SCR on the proliferation on macrophages cells was tested. The results showed the exposure of CSCPS activated the proliferation of the macrophages (). CSCPS activated the proliferation of RAW 264.7 cells in a dose-dependent manner from 24 h to 48 h treatment. At the concentration of 80 μg/mL for 48 h treatment, the stimulatory effect reached maximum, which was 153.41 ± 2.29%. Actually the longer treatment (72 h) lost the proliferation effect on RAW 264.7 cells.
Figure 3. Effect of CSCPS treatment on proliferation of RAW 264.7 cells. Note: Each value represents mean ± SD of at least three independent experiments, and each experiment was performed in triplicate. *p < 0.05, **p < 0.01 and *** p < 0.001.
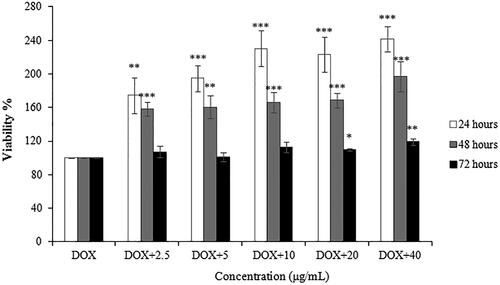
In , CSCPS showed significant protective effect on RAW 264.7 cells by DOX-induced damage in a dose- and time-dependent manner (2.5–80 μg/mL, 24 h–48 h). The maximum cell viability of DOX-induced RAW 264.7 cells with polysaccharide treatment was 241.61 ± 13.70% at 40 μg/mL for 24 h. For 72 h treatment, there is no significant difference between survival rates of DOX-induced RAW 264.7 cells and that with polysaccharide treatment.
Figure 4. Protective effect of CSCPS on DOX-induced RAW 264.7 cells proliferation. Note: Each value represents mean ± SD of at least three independent experiments, and each experiment was performed in triplicate. *p < 0.05, **p < 0.01 and *** p < 0.001.
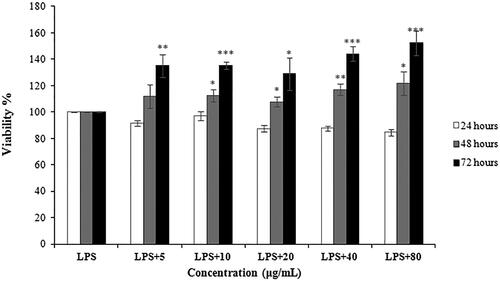
In , the results significantly indicated that CSCPS could protect RAW 264.7 cells by LPS-induced cell damage. For 48 h and 72 h polysaccharide treatment, CSCPS could increase the proliferation of LPS-stimulated RAW 264.7 cells in a dose- and time dependent manner. After 72 h with 40 μmol/L polysaccharides treatment, the cell viability was increased into 152.36 ± 9.09%.
Figure 5. Protective effect of CSCPS on LPS-stimulated RAW 264.7 cells proliferation. Note: Each value represents mean ± SD of at least three independent experiments, and each experiment was performed in triplicate. *p < 0.05, **p < 0.01 and *** p < 0.001.
The immunomodulatory activities of polysaccharides from C. militaris fruiting body and mycelium had been reported in previous studies. The crude polysaccharide named CMP extracted from cultured C. militaris fruiting body could significantly improve the immunomodulatory capacities in cyclophosphamide-treated mice [Citation33]. In the research of Liu et al. [Citation34], the polysaccharide of CMP obtained from C. militaris fruiting body could increase the spleen and thymus indices, the spleen lymphocyte activity, total quantity of white blood cells and IgG function in mice. The structural feature of polysaccharides is also important for their immunomodulatory activities. The SDQCP-1 extracted from C. militaris fruiting body cultivated on hull-less barley could significantly enhanced the Cytokine secretion level of IL-10, IL-6 and TNF-α. The SDQCP-1 was elucidated to be a glucogalactomannan with a backbone composed of (1 →2)- α-D-Manp (48.4%) and (1 →4)-β-D-Glcp (1.2%) residues [Citation35]. Another polysaccharide (CMP-III) was extracted and purified from C. militaris with high molecular weight of 4.796 × 104 kDa, and the main linkage type was (1→4)-α-D-Glc (70.08%), (1→ 4,6)-α-D-Man (9.59%), (1→)-α-D-Man (10.79%) and (1→2,6)-α-D-Gal (3.93%). It could significantly promote macrophage phagocytosis and the secretion of NO, TNF-α and IL-6 [Citation36].
The antioxidant and immunomodulatory activity of CSCPS could be related with the structure of polysaccharide. Polysaccharides bind to cell membrane receptors, mediate the intracellular signal cascades, and play an antioxidant or immunomodulatory role. The structure of polysaccharides, especially their monosaccharide composition, is closely related to the recognition of cell surface receptors [Citation37]. Antioxidants could maintain the membrane fluidity of cells, which is beneficial for them to exert the immune responses [Citation38]. Therefore, the immunomodulatory polysaccharide usually has significant antioxidant activities.
In our studies, based on the results of macrophages proliferation and Protective activity assay, the CSCPS could enhance the proliferation ability of RAW 264.7 cells, and protect RAW 264.7 cells induced by DOX at low added dosage; furthermore CSCPS could also protect RAW 264.7 cells against LPS-induced cell damage. Therefore, the potent antioxidant and immunomodulatory activity of CSCPS could be related with the structure of the polysaccharides; the CSCPS should be purified and the structure should be investigated in our further research.
Inhibitory effect of CSCPS on several cancer cells
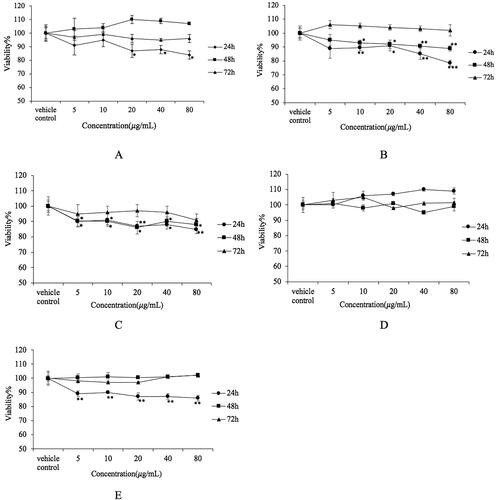
showed CSCPS inhibited the proliferation of Hela cells in a dose-dependent manner only for 24 h treatment; 48 h and 72 h treatment did not show significant inhibitory effect on Hela cells. The minimum survival rate of Hela cells was 84.55 ± 3.34% at 80 μmol/L for 24 h treatment. CSCPS inhibited the proliferation of HT1080 cells in a dose-dependent manner. However, with increasing the treatment time, the cell viability of HT1080 cells increased. Therefore, the effective treatment time was 24 h (78.15 ± 1.59%). After 24 h incubation, the inhibitory effect of CSCPS disappeared (). CSCPS inhibited the proliferation of A549 cells in a dose-dependent manner (). The cell viability for 24 h treatment was lower than that of 48 h treatment. However for 72 h treatment, cell viability was slightly increased. On the other hand, the minimum survival rate of A549 cells was 84.84 ± 3.57% at 80 μg/mL for 24 h treatment. shows the effect of CSCPS on U-2 OS cells. However, there was no significant inhibit activity on U-2 OS cells. The minimum survival rate of U-2 OS cells was 95.10 ± 2.61% at 40 μg/mL for 48 h treatment. shows that CSCPS inhibited the proliferation of MDA-MB-231 cells in a dose-dependent manner for 24 h treatment. The cell viability for 24 h treatment decreased to 85.81 ± 2.83%. However, for 48 h and 72 h treatment, there was no significant inhibitory effect.
Figure 6. Inhibitory effect of CSCPS on several cancer cells. (A) Hela cells; (B) HT1080 cells; (C) A549 cells; (D) U-2 OS cells; (E) MDA-MB-231 cells. Note: Each value represents mean ± SD of at least three independent experiments, and each experiment was performed in triplicate. *p < 0.05, **p < 0.01 and *** p < 0.001.
C. militaris is known for its antitumor activities because of the cordycepin content has remarkable antitumor capacity. According to different literature reports, not only cordycepin but also polysaccharide in fruiting body and mycelium of C. militaris has excellent anti-tumor activity. In the report of Yang et al. [Citation39] and Jing et al. [Citation29], the polysaccharide from C. militaris mycelium and cultured C. militaris fruiting body showed significant antitumor activities against Hela and HepG2 cells in vitro. In another report of Park et al. [Citation6], the polysaccharide from C. militaris fruiting body could inhibit the tumor size in nude mice. In recent studies of Liu et al. [Citation40], the polysaccharide of CMPS-II and CBPS-II extracted from mycelium and fruiting body of C. militaris could induce the apoptosis of cancer cells by increasing the protein and mRNA expression level of apoptosis factors caspase-3, caspase-9 and p53. In our studies, the CSCPS also has excellent antitumor activities on several tumor cells. CSCPS inhibited the Hela cells and HT1080 cells to some extent, the inhibition rate being about 20%. However, we investigated that the inhibitory effect of CSCPS occurred in a dose-dependent manner, which means we could increase the concentration to do the MTT assay. Otherwise, CSCPS might specifically inhibit other cancer cells. These problems are waiting to be investigated in our future research.
The crude polysaccharide CSCPS had significant antioxidant and antitumor activities in this research. These results could demonstrate that polysaccharides may be selectively toxic to tumor cells by affecting the redox status, inducing the apoptosis or necrosis of tumor cells. Furthermore, recent advances demonstrated that mushroom polysaccharides were able to interfere with redox homeostasis of tumor cells [Citation41]. In the future, the crude polysaccharide CSCPS should be purified by ion exchange chromatography such as DEAE cellulose chromatography into different purified fractions, and the structure of each fraction which has more antioxidant, antitumor and immunomodulatory activity should be determined by Nuclear Magnetic Resonance Spectroscopy. The structure-activity relationship between the purified polysaccharides and antioxidant, antitumor, immunomodulatory activity and their molecular mechanism will be the focus of our future research.
Conclusions
This study can reutilize some food processing waste materials as solid fermentation medium to produce antioxidant and anti-tumor health food supplements or a candidate cancer therapy drug; at the same time we can utilize disposed food processing waste materials to avoiding environmental pollution. On the other hand, this study is the first to reutilize the C. militaris mycelium to ferment soybean curd residue and investigate the bioactivities of polysaccharide of the fermented product. The results showed that the CSCPS had antioxidant capacities in five antioxidant assays. Especially hydroxyl free-radical scavenging activity showed a scavenging rate near to that of ascorbic acid (positive control). The CSCPS significantly activated the proliferation of RAW 264.7 cells and protected cells from DOX-induced and LPS-stimulated cell damage. Furthermore, the CSCPS inhibited the proliferation of HT1080, Hela, A549 and MDA-MB-231 cells to some extent at 24 h treatment time in a dose-dependent manner. Therefore, the reutilization of soybean curd residue for fermenting by C. militaris, not only can reduce soybean curd residue waste, but also can produce a new functional food or food additive for enrichment of food diversity.
Authors’ contributions
Yiting Li: Suggested the research plan and contributed to the writing of the manuscript. Linbo wang: Designed the research plan, participated in all experiments, and analyzed all the data. Shili Meng: Suggested the research plan and modified the manuscript. Liang Huang, Ning Sun, Hongpeng Yang, Yu Wang: Modified the manuscript. Litong Ban: Provided technical guidance, coordinated the writing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The data that support the findings from this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Zhu SJ, Pan J, Zhao B, et al. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J Ethnopharmacol. 2013;149(3):713–719.
- Cheung JK, Li J, Cheung AW, et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: signaling cascade and induction of cytokines. J Ethnopharmacol. 2009;124(1):61–68.
- Reis FS, Barros L, Calhelha RC, et al. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem Toxicol. 2013;62:91–98.
- Choi D, Cha WS, Park N, et al. Purification and characterization of a novel fibrinolytic enzyme from fruiting bodies of Korean Cordyceps militaris. Bioresour Technol. 2011;102(3):3279–3285.
- Das SK, Masuda M, Sakurai A, et al. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81(8):961–968.
- Park SE, Kim J, Lee YW, et al. Antitumor activity of water extracts from Cordyceps militaris in NCI-H460 cell xenografted nude mice. J Acupunct Meridian Stud. 2009;2(4):294–300.
- Rao YK, Fang SH, Wu WS, et al. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator's production and human cancer cell proliferation. J Ethnopharmacol. 2010;131(2):363–367.
- Dong JZ, Ding J, Yu PZ, et al. Composition and distribution of the main active components in selenium-enriched fruit bodies of Cordyceps militaris link. Food Chem. 2013;137(1-4):164–167.
- Hatvani N. Antimicrobial effect of the culture fluid of Lentinus edodes mycelium grown in submerged liquid culture. Int J Antimicrob AG. 2001;17(1):71–74.
- Nurmamat E, Xiao H, Zhang Y, et al. Effects of Different Temperatures on the Chemical Structure and Antitumor Activities of Polysaccharides From Cordyceps Militaris. Polymers. 2018;10(4):430.
- Bi S, Jing Y, Zhou Q, et al. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 2018;9(1):279–293.
- Chen X, Wu G, Huang Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int J Biol Macromol. 2013;58:18–22.
- Huang Z, Zhang M, Zhang S, et al. Structural characterization of polysaccharides from Cordyceps militaris and their hypolipidemic effects in high fat diet fed mice. RSC Adv. 2018;8(71):41012–41022.
- Li YT, Meng SL, Shi M, et al. Bioactivity evaluation of crude polysaccharide from rice bran fermented by Preussia aemulans and the changes in its nutritional contents. J Food Biochem. 2016;40(5):664–672.
- Li SH, Wang LB, Song CF, et al. Utilization of soybean curd residue for polysaccharides by Wolfiporia extensa (Peck) Ginns and the antioxidant activities in vitro. J Taiwan Inst Chem E. 2014;45(1):6–11.
- Li YT, Meng SL, Shi M, et al. Physiological active substance changes of soybean curd residue fermented by Preussia aemulans and its bioactivity. Am J Biochem Biotechnol. 2016;12(1):26–35.
- Li YT, Meng SL, Wang LB, et al. Antioxidative activity of exopolysaccharide extract from fermented wheat distillers’dried grains using UV-irradiation degradation pretreatment by Preussia aemulans. AJFST. 2014;6(9):1067–1075.
- Hu HH, Zhang ZY, Lei ZF, et al. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J Biosci Bioeng. 2009;107(1):42–48.
- Yuan J, Chen S, Wang L, et al. Preparation of purified fractions for polysaccharides from Monetaria moneta Linnaeus and comparison their characteristics and antioxidant activities. Int J Biol Macromol. 2018; 108:342–349.
- Lo TCT, Chang CA, Chiu KH, et al. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr Polym. 2011;86(1):320–327.
- Lo TCT, Jiang YH, Chao ALJ, et al. Use of statistical methods to find the polysaccharide structural characteristics and the relationships between monosaccharide composition ratio and macrophage stimulatory activity of regionally different strains of Lentinula edodes. Anal Chim Acta. 2007;584(1):50–56.
- Liu W, Wang H, Pang X, et al. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 2010;46(4):451–457.
- Sun YX, Liu JC, Yang XD, et al. Purification, structural analysis and hydroxyl radical-scavenging capacity of a polysaccharide from the fruiting bodies of Russula virescens. Process Biochem. 2010;45(6):874–879.
- Yu R, Yin Y, Yang W, et al. Structural elucidation and biological activity of a novel polysaccharide by alkaline extraction from cultured Cordyceps militaris. Carbohydr Polym. 2009;75(1):166–171.
- Yu R, Yang W, Song L, et al. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris. Carbohydr Polym. 2007;70(4):430–436.
- Zhang Z, Kong F, Ni H, et al. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr Polym. 2016;144:106–114.
- Shi M, Yang YN, Hu XS, et al. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014;155:50–56.
- Chen R, Jin C, Li H, et al. Ultra high pressure extraction of polysaccharides from Cordyceps militaris and evaluation of antioxidant activity. Sep Purif Technol. 2014;134:90–99.
- Jing Y, Cui X, Chen Z, et al. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr Polym. 2014;102:288–296.
- Liu Y, Li Y, Zhang H, et al. Polysaccharides from Cordyceps miltaris cultured at different pH: Sugar composition and antioxidant activity. Int J Biol Macromol. 2020;162:349–358.
- Zhang Z, Wang X, Zhao M, et al. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydr Polym. 2014; 112:578–582.
- Yuan Y, Xu X, Jing C, et al. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: functional properties and bioactivities. Carbohydr Polym. 2018;181:902–910.
- Wang M, Meng XY, Yang RL, et al. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr Polym. 2012;89(2):461–466.
- Liu JY, Feng CP, Li X, et al. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int J Biol Macromol. 2016;86:594–598.
- Zhang Y, Zeng Y, Cui YS, et al. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohyd Polym. 2020;235(1):59–69.
- He BL, Zheng QW, Guo LQ, et al. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int J Biol Macromol. 2020;145:11–20.
- Leung MYK, Liu C, Koon JCM, et al. Polysaccharide biological response modifiers. Immunol Lett. 2006;105(2):101–114.
- Puertollano MA, Puertollano E, Cienfuegos G, et al. Dietary antioxidants: immunity and host defense. Curr Top Med Chem. 2011;11(14):1752–1766.
- Yang S, Jin L, Ren XD, et al. Optimization of fermentation process of Cordyceps militaris and antitumor activities of polysaccharides in vitro. J Food Drug Anal. 2014;22(4):468–476.
- Liu XC, Zhu ZY, Liu YL, et al. Comparisons of the anti-tumor activity of polysaccharides from fermented mycelia and cultivated fruiting bodies of Cordyceps militaris in vitro. Int J Biol Macromol. 2019;130:307–314.
- Wang XY, GaO AN, Jiao YD, et al. Antitumor effect and molecular mechanism of antioxidant polysaccharides from Salvia miltiorrhiza Bunge in human colorectal carcinoma LoVo cells. Int J Biol Macromol. 2018;108:625–634.