?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A polyphasic approach consisting of physical, chemical and molecular characterization was applied to three Alternaria species, A. alternata, A. chlamydospora and A. solani, to characterize and identify isolates producing and non-producing silver nanoparticles (AgNPs). Based on physical characters, the synthesized AgNPs were characterized by transmission electron microscopy and formation of spherical AgNPs sized 5 nm to 60 nm. Scanning electron microscopy (SEM) of the AgNPs did not show a uniform surface; the shape was irregular, like a coral reef. Energy Dispersive Spectroscopy (EDS) showed the presence of Ag element. Fourier transform-infrared spectroscopy (FTIR) confirmed the conversion of Ag ions to AgNPs by fungal metabolites. A. alternata PNU75 non-producing AgNPs showed negative results when characterized by physical devices. Chemical characterization involved the screening of three Alternaria species for fatty acids (FAs) with similar FAs composition while different in terms of relative concentration. The principal FAs were palmitic, stearic, oleic acid and linoleic acid, which comprised 90% or more of the total FAs composition of these species. Molecularly, the fungal protein pattern of the three Alternaria species showed changes against the control by finding 6 proteins associated with AgNPs-producing species. Inter simple sequence repeats (ISSR) analysis using ten primers indicated a high level of genetic variability of three Alternaria species producing and non-producing AgNPs. The results also indicated that the AgNPs synthesized by A. alternata at the concentration 100 mg/L gave the highest inhibition zone against Aspegillus flavus and Aaltenaria alternata, where the percentages of reduction were 77.1% and 87.6% respectively.
Introduction
Nanobiotechnology is one of the most challenging and fastest-growing sectors of nanotechnology [Citation1]. Biogenesis of nanoparticles has created a lot of enthusiasm for the scholarly community; it crosses over any barrier between core materials and atomic or molecular structures [Citation2]. Metallic nanoparticles are the cornerstone of nanomaterials for the foundation of many nanostructures so their biogenesis is considered as a starting point for a new class in material science [Citation3]. Silver nanoparticles (AgNPs) are one of the widest nanoparticles applied in a variety of fields, including electronics, mechanics, energy science, medicine and agricultural technology [Citation4,Citation5]. Biogenesis of AgNPs is a promising approach that depends on biological resources obtained from fungi, bacteria, algae, viruses, or plants [Citation6–9]. The use of fungi as biological agents in the biogenic process of silver nanoparticles is attractive due to safe, low-cost and high yield, easy handling and eco-friendliness in comparison with the physical and chemical synthesis procedures [Citation3]. Fungi are one of the best opportunities for biogenic AgNPs, due to secreting large quantities of enzymes, cellular oxidoreductase proteins, and fungal biomolecules some of which can be used for the fast, stable and sustainable synthesis of nanoparticles [Citation10]. Furthermore, fungi can be easily cultivated and provide good biomass as large-scale “nanofactories” and can produce NPs with controlled size and shape [Citation11]. Various species of fungi have been explored for the biogenesis of AgNPs such as Aspergillus [Citation1], Fusarium [Citation10] and Trichoderma [Citation12]. Fungi are one of the best options to produce AgNPs, due to the vast repertoire of proteins, enzymes, and other bioactive secondary metabolites that they produce and the redox activity they possess [Citation13,Citation14].
The characterization of fungi is based on the use of phylogenetic and chemotaxonomic markers [Citation15]. Chemotaxonomic markers used in the fungi species include various compounds such as fatty acids, amino acids and carbohydrates. In particular, fatty acids are frequently used as a chemotaxonomic tool for the identification of fungal species. DNA and/or RNA are established for characterization techniques in the field of mycology for the identification and classification of fungal species [Citation16]. The use of polymerase chain reaction (PCR) markers such as Random Amplification of Polymorphic DNA (RAPD) profiling, inter-simple sequence repeat (ISSR) [Citation17], amplified fragment length polymorphism (AFLP) [Citation18] and microsatellite markers [Citation19] have been proposed for the characterization of fungal species.
In the present study, we investigated the probable role of producing and non-producing silver nanoparticles by Alternaria species including A. alternata, A. chlamydospora and A. solani. Characterization of isolates based on a polyphasic approach involving physical, chemical and molecular patterns.
Materials and methods
Isolates of Alternaria spp
Four isolates were used for the biosynthesis of silver nanoparticles. They were obtained from Dr. Mohamed A. Mahmoud Molecular Markers Laboratory, Plant Pathology Research Institute, Agricultural Research Center, Giza 12619, Egypt.
Genomic DNA extraction
Alternaria spp. isolates were cultured on double layer media in 50 mm Petri dishes, one liquid peptone yeast glucose (PYG, 1200 μL) and the other solid (potato dextrose agar as a film). DNA extraction was completed according to the protocol described in [Citation20].
Molecular identification and sequencing of Alternaria ssp. isolates
The amplification of the ITS1 region in PCR was accomplished using two primers, namely Dir1ITSAlt (5′-TGTCTTTTGCGTACTTCTTGTTTCCT-3′) and Inv1ITSAlt (CGA CTT GTG CTG CGC TC −3′). The PCR reaction was performed in a Techne TC-312 (Techne, United Kingdom). The protocol of PCR was according to [Citation21]. All experiments were repeated three times. The PCR products obtained from the of the ITS1 region were sequenced (Intron Biotechnology, South Korea). In addition, the nucleotide sequences obtained using the local BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were examined and compared with similar sequences in the GenBank database. The amplified PCR product was sequenced using ITS-1 ITS-4 primers and using an automated ABI-Prism 377 DNA Sequencer (Applied Biosystems Inc., CA, USA) at the DNA Sequencing Facility, King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia. Alignment of nucleotide sequences was done using ClustalW of the MEGA6 software program [Citation22]. A phylogenetic tree was generated based on the percentage difference between the sequences using the neighbor-joining method with the same program.
Fungal biomass preparation for AgNPs biosynthesis
To prepare biomass for biosynthesis studies, the fungus was grown in 250 mL Erlenmeyer flasks aerobically containing 100 mL liquid medium containing KH2PO4 7.0 g/L; K2HPO4 2.0 g/L; MgSO47H2O 0.1 g/L; (NH4)2SO4 1.0 g/L; yeast extract 1.0 g/L and glucose 15.0 g/L. The flask was incubated in a shaker at 150 rpm at 28 °C. After 72 h of growth, the fungal mycelium biomass was harvested by sieving through a Whatman filter paper no.1, followed by extensive washing with deionized water to remove any medium adhering to the biomass. The obtained biomass (20 g, fresh weight) was mixed with 100 mL of 10−3 M silver nitrate solution in a 250 mL Erlenmeyer flask for 72 h at 28 °C on a shaker at 150 rpm. The control sample was 100 mL of 1 mmol/L pure silver nitrate solution (without biomass) [Citation23].
Ultraviolet-visible (UV–vis) spectrophotometry
UV–Vis spectrophotometry is a common method of detection of AgNPs [Citation24]. In the case of bioreduction of AgNPs in AgNO3 solution, this is the main step of the reaction and occurs with changing the color of the solution. Color change in the reaction mixture (cell-free filtrate and silver nitrate) was the initial indicator of the formation of AgNPs. The solution was monitored by using double beam UV–Vis spectrophotometer, Cintra10e (GBC, Victoria, Australia). The absorption spectra of the supernatants were taken between 300 and 700 nm. Scanning was performed after reaction times ranging from 6 h to 96 h.
Transmission electron microscopy (TEM)
TEM was performed on a JEOL model JEM-1010 (Tokyo, Japan) within accelerating voltage of 80 kV after drying of a drop of aqueous AgNPs on the carbon coated copper grid. TEM grid samples were dried and kept under vacuum in desiccators before loading on a specimen holder. The TEM procedure was performed as described by [Citation25].
Scanning electron microscopy (SEM)
SEM was used for morphological characterization of the AgNPs. The powdered nanoparticles were scanned on scanning electron microscope (SEM) using a JEOL (JSM-6380 LA) instrument [Citation23].
Fourier transform-infrared spectroscopy
For Fourier transform-infrared (FTIR) spectroscopy measurements, the bio-transformed products present in the cell-free filtrate after 72 h of incubation were freeze-dried and diluted with potassium bromide in the ratio of 1: 100. The FTIR spectrum of samples was recorded on FTIR instrument mode Nicolet (6700 FT-IR Spectrometer). All measurements were carried out in the range of 400– 4000 cm−1 at a resolution of 4 cm−1.
Extraction of fatty acid methyl ester and analysis
Fatty acid (FA) extraction and preparation of methyl esters were carried out according to a previously described method [Citation26]. FA methyl esters (FAMEs) were analysed by gas liquid chromatography using the Perkin-Elmer Model 910 Gas Chromatograph (Perkin Elmer, Shelton, CT, USA) equipped with a flame ionization detector. Identification (qualitative and quantitative) of FAMEs was based on comparison of sample retention times with those of an authentic methyl standard (Sigma Co., St. Louis, MO, USA).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Alternaria species producing and non-producing AgNPs were grown in potato dextrose broth medium in 250 mL conical flasks, at 23 °C for 4 days, then harvested by filtration through a sterile cloth. The protein profiles of Alternaria species were determined by the method described by Laemmli [Citation27] and modified by Studier [Citation28]. Briefly, 0.5 g of each freeze-dried fungal mat was mixed with 5 mL of extraction buffer (0.1 mol/L Tris-HCl, pH 6.8). The protein content was measured according to the method described by Bradford [Citation29]. From each sample, 25 µL of extract was loaded on a polyacrylamide gel. The separation gel (10%) and stacking gel (3.5%) were prepared from an acrylamide solution. The protein molecular marker used for the gel analysis was Cruz Marker™ molecular weight standards (Santa Cruz Biotechnology, USA) ranging between 97 and 20 kDa. After electrophoresis, the gels were stained by silver nitrate [Citation30]. The gel and protein pattern of each Alternaria species were photographed, scanned and analyzed using a UVP 8110 PhotoDoc Imaging System.
ISSR-PCR
Genomic DNA extraction was carried out using the method described above. Out of the 15 primers used, 10 primers that produced good PCR fragments were employed to specifically amplify Alternaria species genomic DNA (). PCR was carried out as described by [Citation31]. PCR products were detected with 1.5% agarose ethidium bromide gels in TAE 1x buffer (40 mmol/L Tris-acetate and 1.0 mmol/L ethylenediaminetetraacetic acid (EDTA)). A 100-bp DNA ladder (Intron Biotechnology, South Korea) was used as the molecular marker.
Table 1. ISSR primers used in the present study.
Silver nanoparticles as antifungal agents
Fungal sources
Three isolates of Aspergillus flavus were isolated from wheat and two isolates of Alternaria alternate were isolated from tomato plants on PDA supported by rose Bengal as an antibacterial agent.
Efficiency of silver nanoparticles as antifungal agents
The antifungal activity of the synthesized AgNPs was evaluated by the agar well diffusion assay. Three different concentrations (25, 50 and 100 mg/L) of silver nanoparticle were applied in autoclaved potato dextrose agar (PDA), retaining one as Control (PDA without silver nanoparticles). In the center of each Petri dish was mounted a disk (6 mm) of Aspergillus flavus (F) or Alternaria alternate (A) in PDA culture medium. Wells of 10 mm diameter were made in PDA agar using gel puncture. Different concentrations of AgNPs (50 and 100 mmol) were poured in each well. Petri dishes were incubated at 25 ± 2° C. The efficiency of treatment with silver nanoparticle was recorded by measuring the fungal colonies diameters after control completed, three replicated for each procedure. The following formula was used for calculation of the inhibition rate (%).
where R is the radial growth of the control and r is the radial growth of the cultures treated with AgNPs.
Statistical analysis
The data from three independent replicate trials were subjected to analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) 10.0 statistical software (Chicago, USA). The data are reported as the mean values with standard deviation (±SD).
Results and discussion
Molecular identification of fungal isolates
The four isolates were initially identified as belonging to Alternaria spp. based on morphology. This was further confirmed by sequence analysis of ITS1-5.8S-ITS2 regions. The amplified Alternaria species sequences ranged from 469 to 511 bp. The Alternaria spp. isolates were closely (98–99% similarity) related to Alternaria species in GenBank (). PCR-based assays have several advantages: specific, can be extremely sensitive, and results are obtained in a few hours. PCR amplification and sequence comparisons of ribosomal coding genes, such as the internal transcribed spacer 1 (ITS1) and ITS2 regions have offered a very good tool for the classification and identification of Alternaria species [Citation32]
Table 2. Identification of Alternaria species by sequencing of ITS1 and ITS 2 and the region of 5.8S rRNA gene compared with sequences listed in the GenBank.
Biosynthesis of AgNPs using Alternaria spp
The biosynthesis of AgNPs using three Alternaria spp. was investigated. The reaction of fungal cell filtrates of A. alternata PNU71 with AgNO3 solution () showed progressive change in color towards brown, which was in agreement with previous investigations [Citation23]. The presence of brown color was a sign of the development of AgNPs in the medium. The color change was captured within 72 h comparing with control (fungal biomass), which did not show any change of color. There was no change in color of the cell filtrate when the other isolate of A. alternata PNU75 was incubated with AgNO3 solution in similar lab conditions, meaning no formation of AgNPs ().
Figure 1. Representative vials containing the aqueous fungal biomass A. alternata PNU71 and AgNO3 solution at zero time of the reaction (a) and after 3 days of reaction (b). This isolate biosynthesiszes AgNPs (dark brown colour).

Figure 2. Representative vials containing the aqueous fungal biomass of A. alternata PNU75 and AgNO3 solution at zero time of the reaction (a) and after 3 days of reaction (b). This isolate is not competent for biosynthesizes of AgNPs.

Fungi are often considered powerful biological agents for the mycogenic synthesis of metal nanoparticles [Citation11]. The mycogenic synthesis of AgNPs depends on enzymes and/or proteins that act to reduce Ag+ to Ag0 in the fungal filtrate. Many biomolecules can react with silver ions and associated with the complex pathways involving electron transfer during the conversion of NADPH to NADP+ and NADH to NAD+ [Citation33,Citation34]. The fungal filtrate of most fungi contains reducing agents such as proteins, alkaloids and phenolic compounds which could induce the mycogenic synthesis of AgNPs [Citation35]. Not all the fungi are found to be responsible for the synthesis of AgNPs. Those fungi that contain the “silver resistance machinery” will synthesize AgNPs only if the concentration of the silver ions does not cross the “threshold limit” [Citation34].
Ultraviolet-visible spectrophotometry
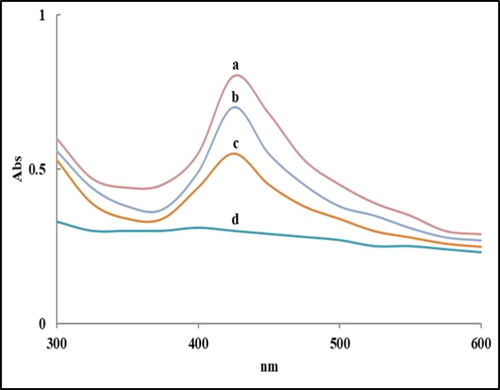
AgNPs produced by different Alternaria spp. were characterized by UV–Vis spectrophotometer. All isolates demonstrated absorption peaks at about 430 nm, which is explicit for AgNPs formation. Notably, there is a clear relationship between the UV–Vis light absorbance and the attributes of biosynthesized AgNPs (size and shape). A single peak was recorded for every species, showing the arrangement of spherical nanoparticles (). A. alternata isolate PNU75 (non-producing AgNPs) had not a single peak because this isolate did not produce AgNPs. The UV-visible spectra of fungal filtrates with silver nitrate showed a specific characteristic surface plasmon absorption band of AgNPs at 420 nm [Citation24]. The absorbance wavelengths range from 400 to 450 nm, with an absorbance peak (specific peak) indicating the presence of a larger number of nanoparticles [Citation12].
Transmission electron microscopy (TEM)
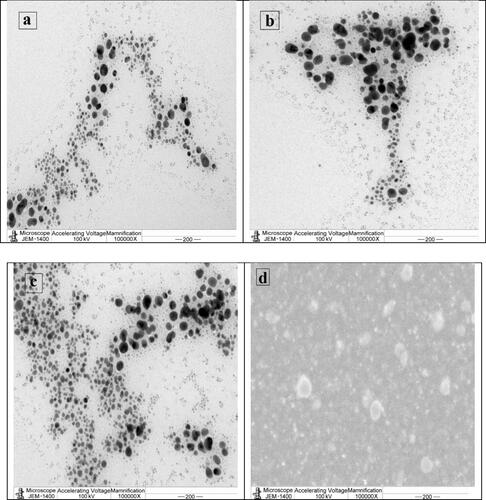
TEM analysis was also carried out to characterize the shape and size of the AgNPs produced by three selected Alternaria species. TEM confirmed the spherical shape without agglomeration of synthesized AgNPs (). The size of AgNPs synthesized by all species was in the range of 5–60 nm. The synthesized AgNPs demonstrated variation in the particle shape. The shape may be spherical or round in most fungi. Also, AgNPs had variation in the particle sizes with diameters ranging from 5 to 70 nm [Citation36,Citation37].
Scanning electron microscopy (SEM)
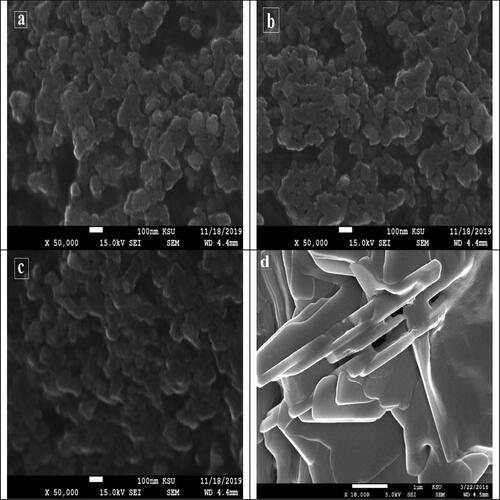
The SEM images of the synthesised AgNPs are presented in . At room temperature, the synthesised nanoparticles had a small size and spherical shape. The SEM micrograph of the AgNPs did not show a uniform surface. The AgNPs were irregularly shaped, like a coral reef. It was observed that there were clusters, which might be attributed to the aggregation of nanoparticles formed throughout sample preparation [Citation38,Citation39].
Energy dispersive spectroscopy (EDS)
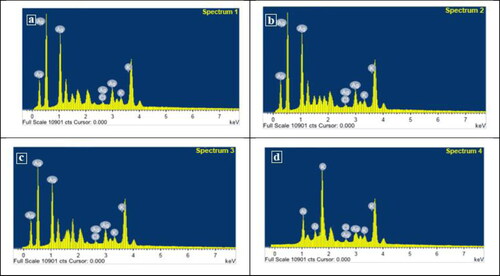
EDS spectroscopy confirmed the significant appearance of AgNPs based on three strong signals of AgNPs in the spectrum, whereas weak signals which correspond to other elements (). The EDS spectrum confirmed the production of AgNPs and showed a clear and strong optical absorption peak at approximately 3 keV, which was connected to the surface plasmon resonance of the AgNPs crystals [Citation40].
FTIR analysis
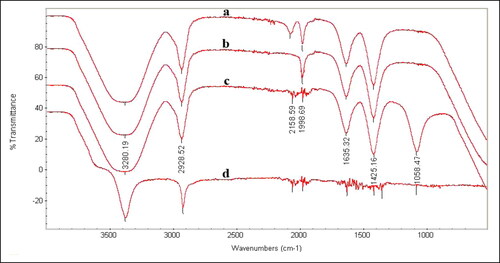
The FTIR spectrum helped to identify specific functional groups present, which may have functions as capping and reducing agents. As shown in (), the IR spectrum displayed intense bands at 3280, 2928, 2158, 1998, 1635, 1425 and 1058 cm−1. The broad band at 3280 cm−1 corresponds to the strong stretching vibrations of hydroxyl (–OH) group of phenolic compounds in the fungal cell-free filtrates and is ascribed to the N–H and H–O bonds. The peak at 2185 cm−1 is ascribed to the C–N bond, whereas the peak at 1998 cm−1 is related to the aromatic ring. The peak at 1635 cm−1 can be identified as the amide group. This amide band occurs due to C = O (carbonyl) stretch and N–H deformation vibrations in the amide linkage of proteins. The band at 1425 cm−1 corresponds to the C = N stretching vibration of aromatic amines. In this manner, taking all together, these results indicate that the AgNPs are encompassed by many proteins and secondary metabolites such as amine, alcohol, ketone and carboxylic acid specific functional groups contributing to the biosynthesis of AgNPs. The FTIR results revealed the importance of some proteins for the biosynthesis of AgNPs, possibly also important for the stabilization and prevention of agglomeration of AgNPs [Citation41].
Fatty acid profiles
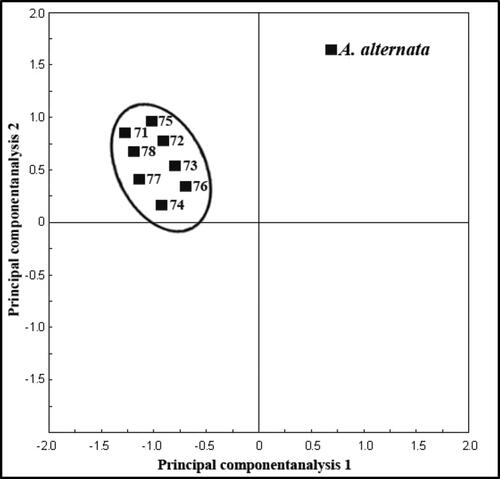
Fatty acid methyl ester (FAME) profiles were used for characterization and differentiation of eight isolates of A. alternata producing and non-producing AgNPs. FA profiles were obtained for each A. alternata isolate. The chain lengths of the FAs present in each isolate ranged from 14 to 18 carbons. Most isolates possess a similar FA composition, with varying concentrations of FAs. The most prevalent and abundant fatty acids extracted were palmitic acid (C16:0), stearic (C18:0), oleic acid (C18:1) and linoleic acid (C18:2), which comprised 90% or more of the total peak area for the eight isolates of A. alternata studied. Whole cellular fatty acid compositions of eight isolates tested were compared by conducting average linkage cluster analysis based on the ten FAME. The dendrogram produced from the detected fatty acids showed that the clustering was unrelated to producing and non-producing AgNPs (). A principal component analysis (PCA) plot of the FAME profiles generated from eight isolates of A. alternata is shown in . Variations in data and comparison of FAME profiles among eight isolates of A. alternata, were subjected to analysis of variance (ANOVA) and carried out using PCA.
Figure 8. Dendrogram showing relationships among 8 isolates of A. alternata producing (nano+) and non-producing (nano–) AgNPs based on the analysis of fatty acid methyl ester (FAME) profiles.
Figure 9. Relationship of fatty acid methyl ester (FAME) profiles among 8 isolates of A. alternata producing and non-producing AgNPs, as represented by plots of the first two principal component analyses.
Cellular FA composition is now routinely used for the identification and differentiation of numerous species of fungi such as Aspergillus spp. [Citation10] and Penicillium spp. [Citation42,Citation43]. Fatty acids such as stearic, palmitic and lauric acids are used as stabilizers, which adsorb on the surface (passivation) of the as-formed AgNPs and prevent aggregation. The passivated AgNPs are stable under normal conditions as a solution, films, and dry powders [Citation44].
Molecular characterization
Protein electrophoresis
Proteins associated with synthesized AgNPs were separated by SDS-PAGE for visualization of the protein bands (). Here, we found 6 proteins related to the biosynthesis of AgNPs and summarized in . shows proteins associated with the biosynthesis of AgNPs, i.e. proteins that appear in isolates producing AgNPs but not in the isolate that did not produce AgNPs.
Figure 10. Electrophoretic banding profiles of Alternaria species proteins: M, molecular size marker; Lane 1, A. alternata PNU71; Lane 2, A. chlamydospora PNU09; Lane 3, A. solani PNU11; Lane 4, A. alternata PNU75 non-producing AgNPs.
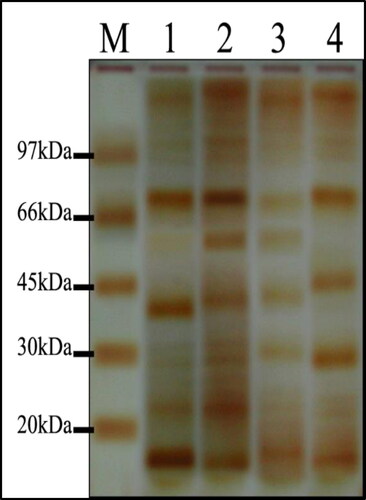
Table 3. Proteins associated with the biosynthesized SNPs were processed by SDS-PAGE.
Nearly 18 proteins were reportedly associated with the biosynthesis of AgNPs in available literature. The cellular enzyme system shows unique redox properties and it can act as an electron shuttle in metal reduction and biosynthesis of AgNPs. It was evident that microorganisms are capable of reducing ions to nanoparticles. This cellular enzyme system includes various proteins such as ATPase, ferredoxin–NADP reductase and nuclear histone (H4) [Citation45,Citation46].
ISSR-PCR
Ten ISSR primers were screened and ten were selected for genetic analysis of eight isolates (two isolates PNU71, PNU75 and six additional isolates) of A. alternata producing and non-producing AgNPs. A total of 74 clear amplicons ranging from 150 to 2200 bp were obtained. Among these, 57 fragments were polymorphic, with an average of 6 polymorphic bands per primer. Most markers produced polymorphisms. Percentage polymorphism was assessed by considering the number of polymorphisms produced per fragment (). Some primers had high polymorphism such as (AG)8YC, which showed 87.5%, (AG)8T, (CA)8G and (GT)8YA 85.7%, whereas (AG)8G showed the lowest polymorphism 66.6%. The dendrogram generated using ISSR markers grouped the eight isolates of A. alternata producing and non-producing AgNPs into three major clusters () and two main clusters (). Every cluster indicated different genetic characterization and variability within and among each cluster. No specific pattern or model was observed for producing and non-producing AgNPs of isolates.
Figure 11. Bar graph showing various fragments produced by ISSR primers and total polymorphisms produced by them.
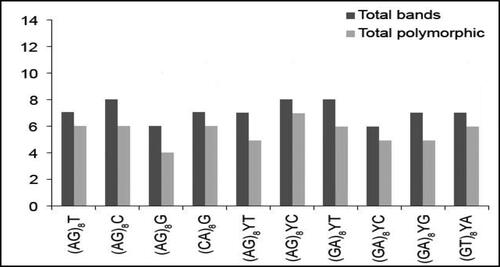
Figure 12. UPGMA dendrogram based on Jaccard’s coefficient illustrating the genetic similarities among eight isolates of A. alternata producing (nano+) and non-producing (nano–) AgNPs with primers (AG)8T (a) and (AG)8G (b).
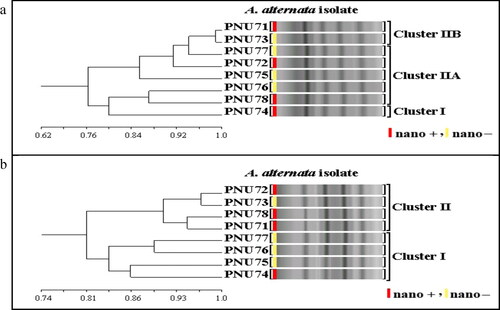
ISSR markers were used with the aim to genetically characterize 8 isolates of Alternaria alternata and discriminate between AgNPs producing and non-producing AgNPs isolates. In general, there was no clear pattern between the clustering of AgNPs-producing and non-producing isolates. Only two papers concentrated on this point when using ISSR markers with AgNPs-producing and non-producing Aspergillus spp. in agreement with the results of our research. ISSR markers have been attributed to the exploration of genetic variation of isolates and are considered very useful as an auxiliary tool for genetic characterization of AgNPs-producing and non-producing Aspergillus spp. [Citation47,Citation48].
Silver nanoparticles as antifungal agents
Inhibititory effect of various concentrations of AgNPs on the growth of fungal cultures like Aspergillus flavus (AF) and Alternaria alternata (AA) is shown in . The inhibitory effect of AgNPs was evaluated in PDA at three various concentrations of AgNPs (25, 50 and 100 mg/L) as shown in . Generality, higher inhibition of fungal growth was observed at 100 mg/L. The lowest level of inhibition was observed on PDA treated with 25 mg/L against AF-1 and AA-1. Furthermore, the inhibition percentages reached 87.6% and 77.1% in the case of treatment with a 100 mg/L of AgNPs against AA-2 and 77.1% against AF-3 compared with the control.
Figure 13. Antifungal activity of biosynthesized AgNPs evaluated by agar well diffusion assay. Representative images of A. alternata (a) and A. flavus (b) control and treatment agar plates.
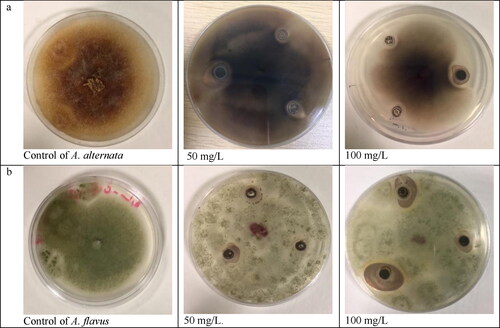
Table 4. Effect of different concentrations of silver nanoparticles on percentage inhibition of mycelia growth.
Many efforts have been given to the improvement of methods which less danger to humans and animals. In this study, we analysed in vitro the inhibitory effect of AgNPs against two species of plant pathogenic fungi. The results suggest that AgNPs are capable of inhibiting these pathogens. Most of the tested fungi displayed a strong inhibitory effect at 100 mg/L concentration of silver nanoparticles. The inhibition increased as the concentration of ANPs increased. The mechanism of silver ions' inhibitory effect on microorganisms has shown that DNA lacks its capacity to replicate after Ag+ treatment [Citation49], the major fungicidal function of the silver ion lies in its interaction with the ribosome and the ensuing suppression of some enzymes and proteins required for ATP production, which makes the cell unable to support the membrane structures and results in cell disruption [Citation50]. Additionally, the treatment with AgNPs affects the role of membrane-bound enzymes, such as those in the respiratory chain [Citation51].
Conclusions
The profile of three Alternaria species (four isolates) by a polyphasic approach, combining physical, chemical, and molecular methods, allowed the reliable identification of AgNPs producing and non-producing isolates. The identification showed differentiation between four Alternaria isolates and related this differentiation to AgNP-producing (three isolates) and non-producing (one isolate). Physical approaches like UV-Vis spectroscopy, TEM, SEM and FTIR provided evidence that supported the biosynthesis of AgNPs by three isolates. The chemical characterization included the survey of four isolates of Alternaria for fatty acids (FAs) with a similar FA structure, although the relative concentration varied. Molecularly, the findings of the fungal protein pattern of three isolates of Alternaria producing AgNPs had significant variation as compared to the isolate not producing AgNPs with up to 6 proteins.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University, through the Research Groups Program Grant No. (RGP-1441-0031).
Data availability statement
All data that support the findings from this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Anonymous. Nanobiotechnology report of national nanotechnology initiative workshop 9–11 October 2013. by subcommittee of nanoscale science, engineering and technology (NSET). 106p.
- Moradi R, Sebt SA, Karimi-Maleh H, et al. Synthesis and application of FePt/CNTs nanocomposite as a sensor and novel amide ligand as a mediator for simultaneous determination of glutathione, nicotinamide adenine dinucleotide and tryptophan. Phys Chem Chem Phys. 2013;15(16):5888–5897.
- Emeka EE, Ojiefoh OC, Aleruchi C, et al. Evaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus). Micron. 2014;57:1–5., ),”
- Elyasi M, Khalilzadeh MA, Karimi-Maleh H. High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples. Food Chem. 2013;141(4):4311–4317.
- Sadeghi R, Karimi-Maleh H, Khalilzadeh MA, et al. A new strategy for determination of hydroxylamine and phenol in water and waste water samples using modified nanosensor. Environ Sci Pollut Res Int. 2013;20(9):6584–6593.
- Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156(1-2):1–3.
- Das VL, Thomas R, Varghese RT, et al. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. 2014;4(2):121–126.
- Kagithoju S, Godishala V, Nanna RS. Eco-friendly and green synthesis of silver nanoparticles using leaf extract of Strychnos potatorum Linn.F. and their bactericidal activities. 3 Biotech. 2015;5(5):709–714.
- Netala VR, Kotakadi VS, Bobbu P, et al. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti-microbial studies. 3 Biotech. 2016;6(2):132–139.
- Al-Zaban MI, Abdel Azim NS, Abd El-Aziz ARM. Antifungal and anti-aflatoxin efficacy of biogenic silver nanoparticles produced by Aspergillus species: Molecular study. Pak. J. Pharm. Sci. 2019;32:2509–2526.
- Guilger-Casagrande M, de Lima R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:287–Published 2019 Oct 22.
- Elamawi RM, Al-Harbi RE, Hendi AA. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biol Pest Control. 2018;28(1):1–11.
- Birla SS, Gaikwad SC, Gade AK, et al. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Scient World J. 2013;2013:796018ID 796018.
- Metuku RP, Pabba S, Burra S, et al. Biosynthesis of silver nanoparticles from Schizophyllum radiatum HE 863742.1: their characterization and antimicrobial activity. 3 Biotech. 2014;4(3):227–234.
- Samson R, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173.
- Versalovic J, Swanson DS, Musser JM. Nucleic acid sequencing studies of microbial pathogens: insight into epidemiology, virulence, drug resistance, and diversity. In: Persing DH, editor. PCR protocols for emerging infectious diseases. Washington (DC): ASM Press; 1996. Chapter 4, p. 59–88.
- Mahmoud MA, Ali HM, El-Aziz ARM, et al. Molecular characterization of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolates collected from corn grains. Genet Mol Res. 2014;13(4):9352–9370.
- Kim MS, Klopfenstein NB, Hanna JW, et al. Characterization of North American Armillaria species: genetic relationships determined by ribosomal DNA sequences and AFLP markers. Forest Pathol. 2006;36(3):145–164.
- Varady ES, Bodaghi S, Vidalakis G, et al. Microsatellite characterization and marker development for the fungus Penicillium digitatum, causal agent of green mold of citrus. Microbiologyopen. 2019;8(7):788–198.
- Amer OE, Mahmoud MA, El-Samawaty AM, et al. Non liquid nitrogen-based-method for isolation of DNA from filamentous fungi. Afr J Biotechnol. 2011;10:14337–14341.
- Pavon MA, Gonzalez I, Rojas M, et al. PCR Detection of Alternaria spp. in Processed Foods, Based on the Internal Transcribed Spacer Genetic Marker. J Food Prot. 2011;74(2):240–247.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729.,
- Abd El-Aziz ARM, Al-Othman MR, Eifan SA, et al. Green synthesis of silver nanoparticles using Aspergillus terreus (kc462061). Dig J Nanomater Biostruct. 2013;8(3):1215–1225.
- Chan YS, Don MM. Biosynthesis of silver nanoparticles from Schizophyllum commune and in-vitro antibacterial and antifungal activity studies. J Phys Sci. 2013;24:83–96.
- Elamawi RM, Re A-H, Hendi AH. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biol Pest Control. 2018;28(1):28–39.
- Lepage G, C. C. Roy Direct transesterifcation of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27(1):114–119.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685.
- Studier FW. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973;15(2):237–248.
- Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-Dye Binding. Anal Biochem. 1976;72(1/2):248–254.
- Rabilloud TG, Carpentier A, Tarroux P. Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis. 1988;9(6):288–291.
- Nasehi A, Kadir JB, Ashtiani FA, et al. Alternaria capsicicola sp. nov., a new species causing leaf spot of pepper (Capsicum annuum) in Malaysia. Mycol Prog. 2014;13(4):991.
- Chou H, Wu W. Phylogenetic analysis of internal transcribed spacer regions of the genus Alternaria, and the significance of filament-beaked conidia. Mycol Res. 2002;106(2):164–169.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262.
- Gudikandula K, Vadapally P, Charya MAS. Biogenic synthesis of silver nanoparticles from white rot fungi: their characterization and antibacterial studies. Open Nano. 2017;2:64–78.
- Abdel-Hafez SI, Nafady NA, Ir A-R, et al. Biogenesis and optimisation of silver nanoparticles by the endophytic fungus cladosporium sphaerospermum. Int J Nano Chem. 2016;2(1):11–19.
- Krishna G, Kumar SS, Pranitha V, et al. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against salmonella SP. Int J Pharm Pharm Sci. 2015;7:84–88.,
- Rajasab AH. Silver nanoparticles synthesis from different fungal species and their antifungal effect. Int J Pharm Pharm Sci. 2015;7:165–170.
- Puchalski M, Dabrowski P, Olejniczak W, et al. The study of silver nanoparticles by scanning electron microscopy, energy dispersive X-ray analysis and scanning tunnelling microscopy. Mater Sci – Poland. 2007;25:473–478.
- Selvi KV, Sivakumar T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int J Curr Microbiol Appl Sci. 2012;1:56–62. –
- Khan NT, Khan MJ, Jameel J, et al. An overview: biological organisms that serves as nanofactories for metallic nanoparticles synthesis and fungi being the most appropriate. Bioceram Dev Appl. 2017;7(101):1–4.
- Sanghi R, Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol. 2009;100(1):501–504.
- Shareef JU, Navya RM, Anand S, et al. Synthesis and characterization of silver nanoparticles from Penicillium sps. Mater Today Proc. 2017;4(11):11923–11932.
- Mahmoud MA, Abd El-Aziz ARM, Al-Othman MR. Molecular and biochemical taxonomic tools for the identification and classification of plant-pathogenic Penicillium species. J Biotechnol Biotechnol Equip. 2016;30(6):1090–1096.
- Rao CRK, Trivedi DC. Biphasic synthesis of fatty acids stabilized silver nanoparticles: Role of experimental conditions on particle size. Mater Chem Phys. 2006;99(2-3):354–360.
- Gade AK, Bonde P, Ingle AP, et al. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mat Bioenergy. 2008;2(3):243–247.
- Barwal I, Ranjan P, Kateriya S, Yadav S. Cellular oxido-reductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles. J Nanobiotechnology. 2011; 9:56
- Mahmoud MA, Abd-El-Aziz ARM, Al-Othman MR. Genetic characterization of Aspergillus flavus and A. niger producing and non producing silver nanoparticles using DNA markers. Res J Biotechnol. 2015;10:73–81.
- Abd El-Aziz ARM, Al-Othman MR, Mahmoud MA, et al. Molecular characterization of Aspergillus parasiticus and A. Terreus producing and non producing silver nanoparticles using DNA markers. Digest J Nanomater Biostruct. 2015;10(1):31–41.
- Feng QL, Wu J, Chen GQ, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668.,
- Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol. 2005;71(11):7589–7593.
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179.