Abstract
Bacteria that exhibit ionic-liquid (IL) tolerance are useful in chemical industries using renewable carbon sources pre-treated by ILs to produce biofuels and fine chemicals. An IL, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), has a remarkable ability to solubilize wood components, whereas [BMIM]Cl inhibits the growth of various bacterial hosts useful for bioconversion. We previously isolated a 10% [BMIM]Cl-tolerant bacterium Bacillus amyloliquefaciens CMW1. Here we report novel mechanisms of tolerance to [BMIM]Cl in strain CMW1 and a novel major facilitator superfamily transporter coded by an ionic-liquid tolerance (ILT) gene. First, using CMW1 cells grown in the presence or absence of 10% [BMIM]Cl, whole-transcriptome analysis and differentially expressed gene analysis were performed. Probable mechanisms of tolerance to [BMIM]Cl include the uptake of osmoprotectants from the culture medium toward CMW1 cells and the export of [BMIM] cations that accumulated in CMW1 cells. The finding represents a first step in elucidation of the mechanisms of IL resistance in Gram-positive bacteria. Second, we conferred tolerance to 5% [BMIM]Cl on [BMIM]Cl-susceptible Brevibacillus choshinensis using ILT gene. This finding provides a notable basis for engineering IL-tolerant bacterial hosts that are applicable for the effective and sustainable production of industrially important chemicals.
Introduction
Ionic liquids (ILs) are a great variety of organic-molten salts that are composed of a bulky asymmetric cation and a small anion. Cation and anion combinations can be modified, and hydrophilic and hydrophobic ILs can be prepared [Citation1,Citation2]. Unlike conventional organic solvents applied for biocatalytic reactions, ILs remain liquid over a wide range of temperatures and possess no vapour pressure [Citation3]. An IL, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), improved the digestibility of various biomasses (e.g. cellulose, chitin and keratin). But [BMIM]Cl was toxic to the bacteria that are used in the subsequent production step of valuable compounds (e.g. liquid fuels and fine chemicals) [Citation4–6]. Therefore, there exist demands for a bacterial host that is tolerant to [BMIM]Cl and elucidation of the resistance mechanisms to [BMIM]Cl for the development of an efficient production step.
The toxicity of ILs against microorganisms is considered to be due to the accumulation of ILs, which have hydrophobic and hydrophilic structures, toward the cell membrane and cytoplasm [Citation7,Citation8]. Nonetheless, several IL tolerant microorganisms have been identified recently. Fungi, such as Aspergillus sp. and Saccharomyces sp. [Citation9,Citation10], several Gram-positive bacteria, such as Nocardiopsis sp., Brevibacterium sanguinis, and Rhodococcus erythropolis [Citation11,Citation12], and a Gram-negative bacterium, such as Pluralibacter lignolyticus SCF1 [Citation8,Citation13], exhibit various levels of tolerance to various ILs. The fatty acid composition and transcriptional responses of the Gram-negative P. lignolyticus SCF1 were analyzed [Citation8]. It has been reported that the increase of cyclopropane fatty acids in the cell membrane and up-regulation of osmoprotectant transporters and drug efflux pumps are responsible for the resistance to 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl). The recombinant Escherichia coli expressing the multidrug efflux pump gene, eilA gene, of P. lignolyticus SCF1 grew in the presence of 410 mmol/L [EMIM]Cl [Citation14]. It was suggested that EilA transports [EMIM] cations out of the cells and confers resistance to [EMIM]Cl on the recombinant cells. EilA belongs to the major facilitator superfamily (MFS) transporter and plays a critical role in the tolerance to [EMIM]Cl.
MFS is composed of secondary transporters that use the electrochemical gradient of protons across the cell membrane to catalyze the export of substrates, such as drugs, toxic compounds and antibiotics [Citation15–17]. MFS is classified into 82 families according to the transporter classification system (http://www.tcdb.org). Drug: H+ antiporter families (DHA) 1, 2 and 3 are the largest drug exporter families in MFS. Although the properties of the efflux pump EilA of P. lignolyticus SCF1 were elucidated [Citation8,Citation14], those of the efflux pumps of other bacteria that exhibit IL tolerance have not yet been investigated. There are many unknowns in the effects of bacterial MFS against various ILs.
We previously isolated a [BMIM]Cl-tolerant Gram-positive bacterium, Bacillus amyloliquefaciens CMW1 [Citation6]. Strain CMW1 is a moderately halotolerant bacterium that grows in the presence of >10% (v/v, 631 mmol/L) [BMIM]Cl, which is toxic to most bacteria [Citation11,Citation18]. Strain CMW1 produces an extracellular protease that exhibits tolerance to ILs, halo, alkaline and organic solvents and the bioactive peptide that exhibits antibacterial activity against Staphylococcus aureus [Citation18,Citation19]. Strain CMW1 was transformed by electroporation using a plasmid pHY300PLK, a shuttle vector for E. coli and Bacillus subtilis (Kurata, unpublished data). The transformation efficiency was 2.8 × 103 transformants per 1 µg of plasmid DNA. Although strain CMW1 has many utilities for industrial applications, the resistance mechanism of strain CMW1 against [BMIM]Cl has remained unclear. Therefore, to understand the molecular basis for the tolerance of strain CMW1 against [BMIM]Cl, we performed whole-genome gene expression analysis using RNA-sequencing and identified differentially regulated genes in the presence or absence of 10% [BMIM]Cl. Additionally, using the transporter gene that was up-regulated in the presence of 10% [BMIM]Cl, we showed the responsibility of the transporter gene for the tolerance of the bacterial cell to [BMIM]Cl using a heterologous gene expression system. The novel transporter gene was designated as the ionic-liquid tolerant (ILT) gene. Our finding represents an important advance in understanding the mechanisms of IL tolerance of bacteria and provides a foundation for engineering IL-tolerant strains.
Materials and methods
Bacterial strains, plasmids and propagation
B. amyloliquefaciens CMW1 (GenBank accession no. NZ_DF836085) was isolated from a Japanese fermented soybean paste [Citation6]. Strain CMW1 was grown aerobically at 37 °C in the medium (1% yeast extract (Becton Dickinson, MD), 0.25% tryptone (Becton Dickinson, MD), 0.1% MgSO4•7H2O, 0.01% CaCl2 and 0.5% KCl, pH 7.4) containing either 0% or 10% (v/v, 631 mmol/L) [BMIM]Cl (, Kanto Chemical, Tokyo, Japan). A pBIC2 plasmid (Takara Bio, Shiga, Japan) was used as the expression vector. The plasmid for expression of the ILT gene was transformed into Brevibacillus choshinensis (Takara Bio). Recombinant B. choshinensis harbouring the ILT expression vector pBIC-ILT and non-recombinant B. choshinensis were individually inoculated into 5% (v/v, 316 mmol/L) [BMIM]Cl-added and [BMIM]Cl-free media, respectively. The composition of the culture medium, except for 5% [BMIM]Cl, was described above. All bacterial growth was monitored by measurement of the optical density at 600 nm (OD600).
Total RNA preparation from B. amyloliquefaciens CMW1
CMW1 cells were harvested from the 10% [BMIM]-containing medium (+10% [BMIM]Cl) or the [BMIM]Cl-free medium (− [BMIM]Cl) at late-exponential phases (OD600; 0.6 for +10% [BMIM]Cl and 0.4 for − [BMIM]Cl). The total RNA was purified using an RNeasy plus universal mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and quantified using a NanoDrop spectrophotometer. RNA integrities (RIN; 4.9 for +10% [BMIM]Cl and 7.7 for − [BMIM]Cl) were assessed by an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Library construction, sequencing and bioinformatic analysis
Using the total RNA samples (7.4 µg for +10% [BMIM]Cl and 9.2 µg for − [BMIM]Cl), each library was constructed and RNA-seq was carried out at Hokkaido System Science (Hokkaido, Japan). After the elimination of rRNA using a Ribo-zero rRNA removal kit (Illumina, San Diego, CA), each cDNA library was prepared using the TruSeq RNA sample preparation kit (Illumina). Each cDNA was sequenced using paired-end methods (100 bp) by a HiSeq System (Illumina) to obtain reads. The functional annotation of the strain CMW1 genome sequence was previously carried out [Citation6]. In , Annotation was performed by D-FAST (https://dfast.nig.ac.jp). The obtained reads were mapped to the reference CMW1 genome sequence (GenBank Accession No. DF836084 through DF836091) using the software tools Tophat (version 2.0.14) and Bowtie (version 1.1.1). The read abundances mapping to the reference CMW1 genome sequence was normalized by the FPKM calculation (fragments per kilobase of transcript per million RNA-seq reads) using the software tool Cufflinks (version 2.2.1). Subsequently, differentially expressed gene analysis was performed. Gene expression change due to the addition of 10% [BMIM]Cl is indicated by the value of log2 (fold change) that refers to the ratio of expression levels in the presence of 10% [BMIM]Cl against that in the absence of [BMIM]Cl. The false discovery rate is indicated by the p-value. The classification criteria for differentially expressed genes were p value < .05 and absolute value of |log2 (fold change)| > 1 [Citation20,Citation21]. In , ‘Locus’ indicates the location of the gene at genome sequence of B. amyloliquefaciens CMW1.
Table 1. Differential expression of genes related to uptake of osmoprotectants and genes of drug efflux pumps.
Phylogenetic analysis of ILT and homologous proteins
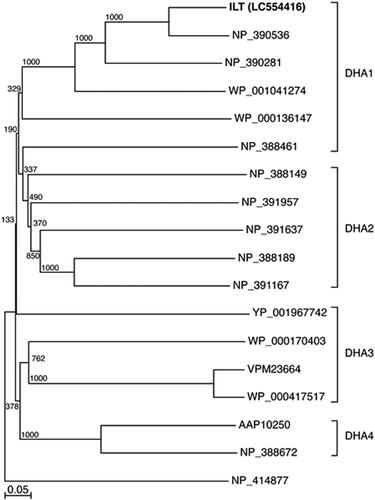
The phylogenetic tree was constructed using amino acid sequences of ILT (GenBank accession number LC554416) from B. amyloliquefaciens CMW1, homologous proteins from B. subtilis strain 168 (NP_388149, NP_388189, NP_388461, NP_388672, NP_390281, NP_390536, NP_391167, NP_391637, and NP_391957), Bacillus cereus ATCC 14579 (AAP10250), Staphylococcus aureus (WP_000170403 and WP_001041274), Streptococcus pneumoniae (VPM23664 and WP_000136147), Streptococcus pyogenes (WP_000417517) and Clostridium perfringens (YP_001967742), and lactose permease (NP_414877) from E. coli strain K-12 as an out group. Amino acid substitution rates were determined, and a distance matrix tree was constructed by using the neighbour-joining (NJ) method with the CLUSTALX program. Alignment gaps and unidentified base positions were not taken into consideration for the calculations. The topology of the phylogenetic tree was evaluated by performing a bootstrap analysis with 1000 replications. NJ plot software (http://pbil.univ-lyon1.fr/software/njplot. html) was used to prepare a graphical view of the phylogenetic tree.
Multiple alignment of ILT and homologous MFS transporters
Amino acid sequences of ILT (LC554416) from B. amyloliquefaciens CMW1 and homologous MFS transporters (EmrD; NP_418129, YdeA; P31122, and AraJ; P23910) from E. coli K-12 were aligned by the Clustal method using DNASTAR software (DNA Star, Madison, WI, USA).
Heterologous expression of ILT gene
A DNA fragment coding for ILT gene (1197 bp) was amplified by PCR with primers 5′-GAACACAAGGTCATGCAAAAACCGATTCGAGAAC-3′ and 5′-CATCCTGTTAAGCTTCTACTCCGAAACCTCACTAAC-3′, the genomic DNA of strain CMW1 as the template, and KOD DNA polymerase (Toyobo Life Science, Osaka, Japan). The program was as follows: denaturation at 98 °C for 30 s followed by 30 cycles of 98 °C for 10 s, 55 °C for 5 s, and 68 °C for 15 s. According to the manufacturer's instructions, the amplified PCR product was cloned into a pBIC2 vector (Takara Bio) to construct pBIC-ILT, and the constructed pBIC-ILT was transformed into B. choshinensis (Takara Bio). To confirm the expression of ILT gene, ILT was detected by SDS-PAGE. First, recombinant B. choshinensis harbouring pBIC-ILT and non-recombinant B. choshinensis were individually inoculated into 5% (v/v, 316 mmol/L) [BMIM]Cl-added and [BMIM]Cl-free media, respectively. Subsequently, each inoculated medium was shaken at 37 °C until the late-exponential phase. Each cell was harvested from the individual cell culture and disrupted by a sonicator to prepare the cell homogenate. Because expressed ILT should be localized in the cell membrane, cell debris were collected from the individual cell homogenates by centrifugation. Each cell debris was suspended in 10 mmol/L Tris-buffered saline (pH7.4), followed by SDS-PAGE analysis to detect ILT. Molecular weight (42,834.87) of ILT is predicted based on the amino acid sequence of the protein.
Strain CMW1 was transformed by electroporation (1000 V, 25μF, 200Ω, Gene Pulser, Bio-Rad) using a plasmid pHY300PLK (TaKaRa). The transformants were obtained on LB medium containing 50 μg/mL of tetracycline.
Evaluation of [BMIM]Cl tolerance with B. choshinensis harbouring pBIC-ILT
The growth of recombinant cells was compared with that of non-recombinant cells, and the [BMIM]Cl tolerance contributed by the ILT gene was investigated. Recombinant B. choshinensis harbouring pBIC-ILT and non-recombinant B. choshinensis were individually inoculated in the 5% (v/v, 316 mmol/L) [BMIM]Cl medium described above. Subsequently, each inoculated medium was shaken at 37 °C for 24 h, and each cell growth was confirmed by measuring OD600. The measurement of cell growth was carried out using three independent cell cultures with 5% [BMIM]Cl.
Results and discussion
RNA sequencing and differentially expressed gene analysis
We previously reported that the [BMIM] cations are not degraded by strain CMW1 and the [BMIM] cations do not accumulate in CMW1 cells [Citation18]. Therefore, we investigated the molecular mechanisms of tolerance of B. amyloliquefaciens CMW1. B. amyloliquefaciens CMW1 grew until the late-exponential phase in the presence of 10% [BMIM]Cl (+10% [BMIM]Cl) or in the absence of [BMIM]Cl (− [BMIM]Cl). Each RNA was prepared and sequenced. The RNA sequencing generated 46,797,520 reads covering a total of 4508 Mbp (Phred > Q30 = 95.4%) for the +10% [BMIM]Cl condition, and 44,637,150 reads covering a total of 4727 Mbp (Phred > Q30 = 95.3%) for the − [BMIM]Cl condition. Trimmed reads (46,503,904 reads for +10% [BMIM]Cl and 44,402,576 reads for − [BMIM]Cl) were individually mapped to the B. amyloliquefaciens CMW1 genome DNA sequence (3,908,571 bp, DF836084 through DF836091). The genome covering rates of the trimmed reads were 99.2% for +10% [BMIM]Cl and 99.1% for − [BMIM]Cl. A total of 9175 protein-coding genes in the CMW1 genome were predicted [Citation6]. The gene expression levels of CMW1 cells grown in the +10% [BMIM]Cl condition were compared with those of CMW1 cells grown in the − [BMIM]Cl condition (Supplemental Table S1). Transcriptional analysis revealed that gene expression levels of 359 genes were changed in the presence of 10% [BMIM]Cl using the parameters p value < .05 and |log2 fold-change| > 1. Of these, 277 genes were up-regulated (>two-fold increase) and 82 genes were down-regulated (>two-fold decrease) in the presence of 10% [BMIM]Cl.
Expression changes of genes related to uptake of osmoprotectants and genes related to drug efflux pumps
It was reported that P. lignolyticus SCF1 grows in the presence of 8.2% (v/v, 562 mmol/L) [EMIM]Cl [Citation8]. P. lignolyticus SCF1 probably resists the toxic effect of [EMIM]Cl by taking up osmoprotectants (sugars and amino acids) from the environment and pumping the [EMIM] cations out of the cells. We reported that B. amyloliquefaciens CMW1 has genes associated with the uptake of osmoprotectants [Citation6]. Under the 10% [BMIM]Cl-added condition, genes for uptake of osmoprotectants, such as glycine betaine, L-carnitine and choline, from the culture medium were up-regulated in strain CMW1 (). In Bacillus sp. bacteria, proline and analogous compounds are considered to be effective osmoprotectants [Citation22,Citation23]. In the metabolism of proline and analogous compounds in strain CMW1 under the 10% [BMIM]Cl-added condition, the gene expression levels of amino acid permease in the 4-hydroxyproline catabolic gene cluster (Gene No. 60) and proline dehydrogenase (Gene No. 93) were up-regulated with 6.04036- and 2.987-fold increases, respectively (Supplemental Table S1). The permease and dehydrogenase probably aid the intercellular accumulation of osmoprotectants or their precursors to offset the osmotic pressure generated by exposure to [BMIM]Cl. The result shows that the osmotic-stress-resistance genes contributed to the IL tolerance in strain CMW1 as in P. lignolyticus SCF1.
We showed the expression levels for the genes of drug efflux pumps in strain CMW1(). Although the drug resistance transporter gene (Gene No. 95) was down-regulated with a − 2.17571-fold decrease in the presence of 10% [BMIM]Cl, the multidrug resistance protein B gene (Gene No. 175) was up-regulated with a 2.80335-fold increase. Amino acid sequence analysis predicted that multidrug resistance protein B (Gene No. 175) belongs to a membrane transporter. Although we identified at least 29 membrane transporter genes in the genome sequence of strain CMW1 [Citation6], significant changes of expression levels of other genes, except for the multidrug resistance protein B (Gene No. 175), were not detected by classification using the parameters p value < .05 and |log2 fold-change| > 1. Therefore, it was suggested that the membrane transporter coded by the gene (Gene No. 175) functions as a specific efflux pump against [BMIM] cations in strain CMW1.
Conservation of ILT gene in gram-positive bacteria
We found the gene (Gene No. 175) that probably catalyzes the transport of [BMIM] cations out of the cells and designated it the ionic-liquid tolerant (ILT) gene. Amino acid sequence analysis using the BlastP program in the transporter classification system (http://www.tcdb.org) classified ILT as a multidrug efflux transporter of MFS. We show the phylogenetic relationship of the amino acid sequence of ILT to those of representative MFS transporters (namely, Drug: H+ antiporter family (DHA) 1 through 4) of Gram-positive bacteria (, Bar, 0.05 amino acid substitutions per site). ILT clearly belongs to DHA1. It was reported that transporter EilA of P. lignolyticus SCF1 is involved in the tolerance to [EMIM] cations and is an MFS transporter by amino acid sequence analysis [Citation14]. BlastP analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) found no high homology between the amino acid sequence of ILT and that of EilA, indicating that ILT is different from EilA. Therefore, we found that ILT is a novel transporter belonging to DHA1 of MFS and potentially transports [BMIM] cations.
Multiple sequence alignment of ILT and its homologous transporters
Sequence analysis using BlastP showed that ILT exhibits an amino acid sequence similarity to multidrug efflux pump EmrD (NP_418129), MFS transporter AraJ (P23910), and sugar efflux transporter YdeA (P31122) from E. coli K-12 with 21.84%, 19.85% and 19.00% amino acid identities, respectively. In , we indicated multiple alignment of amino acid sequence of ILT with those of MFS transporters. Conserved amino acid residues in the transporters are indicated by black boxes.
Transmembrane (TM) helices 1 through 12 of ILT and those of EmrD are indicated by dashed and solid lines, respectively. The TM helices of EmrD were determined by X-ray structure analysis [Citation24]. The TM helices of ILT were predicted using the HMMTOP program (http://www.enzim.hu/hmmtop/). The sequence analysis indicated that ILT is a homologue of proton antiporters, possessing 12 TM helices that span the inner membrane. The predicted TM 1-8 of ILT overlapped well with TM1-8 of EmrD. The predicted TM 9-12 of ILT partially overlapped with TM 9–12 of EmrD.
Sequence alignment revealed that amino acid sequence motifs characteristic for MFS transporters, namely motif B (L-x-x-x-R-x-x-x-G at TM4, x indicating any amino acid) and motif C (G-x-x-x-G-P-x-x-G-G at TM5), were conserved in ILT. Motifs B and C are indicated under the aligned amino acid sequences. Motif B contains a basic amino acid residue (Arg105 of ILT) that plays a role in proton transfer, and motif C is conserved only in exporters and not importers [Citation25,Citation26]. The functional and structural characterizations of motifs B and C support the probable efflux function of ILT.
In the case of EmrD, the hydrophobicity of Val17, Ile28, Tyr52 and Ile217 play a critical role in recognition of the hydrophobic structure of substrates [Citation24], and the hydrophobicity was conserved by Phe20, Ile30, Phe54 and Phe225 in ILT. It suggests that these amino acids recognize the hydrophobic domain of the [BMIM] cation. In , the amino acids are indicated by closed triangles. Glu227 of EmrD, which is involved in import of protons, is conserved in ILT as Glu235. In , the amino acid is indicated by an open circle. Asp123, which is involved in the topology of EmrD, is conserved in ILT as Asp125. In , the amino acid is indicated by an open triangle. Consequently, amino acid sequence analysis indicated that ILT would probably function as a novel proton antiporter that localizes at the cell membrane and exports the [BMIM] cation that accumulated in the cytosol.
Expression of ILT in B. choshinensis and evaluation of [BMIM]Cl tolerance
It was reported that recombinant E. coli expressing EilA transporter grows in the presence of 410 mmol/L [EMIM]Cl and exhibits [EMIM]Cl tolerance by pumping [EMIM] cations out of the cells [Citation14]. Therefore, we investigated whether the ILT gene confers resistance to 5% (v/v, 316 mmol/L) [BMIM]Cl on [BMIM]-susceptible B. choshinensis. In , comparison of the recombinant strain (+pBIC-ILT) with the non-recombinant strain (−pBIC-ILT) indicates that ILT protein (M.W.: 42834.87) is detected in + pBIC-ILT, but not in − pBIC-ILT, on the Coomassie blue-stained SDS-PAGE gel. Arrowheads indicate recombinant ILT. The size of the recombinant ILT on the gel is in agreement with the molecular mass of ILT (M.W.: 42834.87). Additionally, as shown in , the growth of recombinant B. choshinensis expressing ILT was promoted in the medium containing 5% [BMIM]Cl, compared with that of non-recombinant B. choshinensis. The results suggested that ILT of B. amyloliquefaciens CMW1 contributes to the bacterial resistance against [BMIM]Cl by pumping [BMIM] cations as in EilA of P. lignolyticus SCF1.
Figure 4. Detection of recombinant ILT and growth of B. choshinensis harbouring ILT gene in the presence of 5% (v/v, 315 mol/L) [BMIM]Cl. (A) Detection of ILT in the cell membrane of recombinant B. choshinensis. (B) Comparison of recombinant B. choshinensis expressing ILT (+pBIC-ILT) with non-recombinant B. choshinensis (−pBIC-ILT). The data are expressed as means ± standard deviations of three samples (*p value; .00627, Student’s t-test).
![Figure 4. Detection of recombinant ILT and growth of B. choshinensis harbouring ILT gene in the presence of 5% (v/v, 315 mol/L) [BMIM]Cl. (A) Detection of ILT in the cell membrane of recombinant B. choshinensis. (B) Comparison of recombinant B. choshinensis expressing ILT (+pBIC-ILT) with non-recombinant B. choshinensis (−pBIC-ILT). The data are expressed as means ± standard deviations of three samples (*p value; .00627, Student’s t-test).](/cms/asset/326a7318-f950-4f18-8dd4-ce2a38480bc8/tbeq_a_1885995_f0004_b.jpg)
Generally, it is investigated that various biomasses are dissolved in ILs and subsequently converted to valuable compounds by microorganisms [Citation27–29]. There is a potential economic incentive for unifying the process by using an IL-tolerant bacterium that would carry out degradation of pre-treated biomass and production of valuable compounds. We conferred tolerance to 5% [BMIM]Cl on [BMIM]Cl-susceptible B. choshinensis using ILT gene. We plan to examine what concentration of [BMIM]Cl the transformant can grow at.
Conclusions
We reported the first phylogenetic and functional characterization of ILT as a member of the DHA1 in MFS. When [BMIM] cations accumulate in the CMW1 cell, ILT would use the electrochemical gradient of protons across the cell membrane to export the accumulated [BMIM] cations out of cells. Thus, ILT potentially functions as a specific transporter for an imidazoliumic cation of IL. In future studies, we will purify ILT from B. amyloliquefaciens CMW1 and investigate the characteristics of ILT, such as, transporter activity, substrate specificity and so on. Transcriptional analysis of IL tolerance microorganisms that are identified recently and elucidation of the properties of IL efflux pumps are urgently required for development of useful compounds production systems using ILs and biocatalysts.
Supplemental Material
Download MS Excel (40.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The data that support the findings reported in this study are available at https://datadryad.org/stash/dataset/doi:10.5061/dryad.fj6q573st.
Additional information
Funding
References
- Hejazifar M, Lanaridi O, Bica-Schröder K. Ionic liquid based microemulsions: A review. J Mol Liq. 2020;303:1–19.
- Yoo CG, Pu Y, Ragauskas AJ. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr Opin Green Sustain Chem. 2017;5:5–11.
- Kragl U, Eckstein M, Kaftzik N. Enzyme catalysis in ionic liquids. Curr Opin Biotechnol. 2002;13(6):565–571.
- Alayoubi R, Mehmood N, Husson E, et al. Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew Energy. 2020;145:1808–1816.
- Brandt-Talbot A, Gschwend FJ, Fennell PS, et al. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017;19(13):3078–3102.
- Kurata A, Hirose Y, Misawa N, et al. Draft genome sequence of the ionic liquid-tolerant bacterium Bacillus amyloliquefaciens CMW1. Genome Announc. 2014;2(5):e01051-14.
- Abramenko N, Kustov L, Metelytsia L, et al. A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. J Hazard Mater. 2020;384:1–14.
- Khudyakov JI, D'haeseleer P, Borglin SE, et al. Global transcriptome response to ionic liquid by a tropical rain forest soil bacterium, Enterobacter lignolyticus. Proc Natl Acad Sci U S A. 2012;109(32):E2173–E2182.
- Singer SW, Reddy AP, Gladden JM, et al. Enrichment, isolation and characterization of fungi tolerant to 1-ethyl-3-methylimidazolium acetate . J Appl Microbiol. 2011;110(4):1023–1031.
- Sitepu IR, Shi S, Simmons BA, et al. Yeast tolerance to the ionic liquid 1-ethyl-3-methylimidazolium acetate. FEMS Yeast Res. 2014;14(8):1286–1294.
- Kurata A, Shimizu S, Shiraishi Y, et al. Degradation of ionic liquids by a UV/H2O2 process and CMCase from novel ionic liquid-tolerant alkaliphilic Nocardiopsis sp. SSC4. Biotechnol Biotechnol Equip. 2017;31:749–755.
- Megaw J, Busetti A, Gilmore BF. Isolation and characterisation of 1-alkyl-3-methylimidazolium chloride ionic liquid-tolerant and biodegrading marine bacteria. PloS One. 2013;8(4):e60806–e60809.
- Deangelis KM, D'Haeseleer P, Chivian D, et al. Complete genome sequence of “Enterobacter lignolyticus” SCF1. Stand Genomic Sci. 2011;5(1):69–85.
- Ruegg TL, Kim E-M, Simmons BA, et al. An auto-inducible mechanism for ionic liquid resistance in microbial biofuel production. Nat Commun. 2014;5(1):1–7.
- Ahmad I, Nawaz N, Dermani FK, et al. Bacterial multidrug efflux proteins: a major mechanism of antimicrobial resistance. Curr Drug Targets. 2018;19:1–13.
- Perland E, Fredriksson R. Classification systems of secondary active transporters. Trends Pharmacol Sci. 2017;38(3):305–315.
- Kumar S, Lekshmi M, Parvathi A, et al. Functional and structural roles of the major facilitator superfamily bacterial multidrug efflux pumps. Microorganisms. 2020;8(2):266.
- Kurata A, Senoo H, Ikeda Y, et al. Properties of an ionic liquid-tolerant Bacillus amyloliquefaciens CMW1 and its extracellular protease. Extremophiles. 2016;20(4):415–424.
- Kurata A, Yamaguchi T, Kira M, et al. Characterization and heterologous expression of an antimicrobial peptide from Bacillus amyloliquefaciens CMW1. Biotechnol Biotechnol Equip. 2019;33(1):886–893.
- Zhang J, Pan H, Gao Z, et al. Transcriptome analysis of colouration-related genes in two white-fleshed nectarine varieties and their yellow-fleshed mutants. Biotechnol Biotechnol Equip. 2018;32(4):899–907.
- Hajrah NH, Abdul WM, Al-Garni SM, et al. Gene expression profiling to elucidate the pharmacological and toxicological effects of Ricinus communis L. leaf extract in mammalian cells. Biotechnol Biotechnol Equip. 2019;33(1):397–407.
- Mahipant G, Paemanee A, Roytrakul S, et al. The significance of proline and glutamate on butanol chaotropic stress in Bacillus subtilis 168. Biotechnol Bioeng. 2017;10:1–14.
- Moses S, Sinner T, Zaprasis A, et al. Proline utilization by Bacillus subtilis: Uptake and catabolism. J Bacteriol. 2012;194(4):745–758.
- Yin Y, He X, Szewczyk P, et al. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312(5774):741–744.
- Kroeger JK, Hassan K, Vörös A, et al. Bacillus cereus efflux protein BC3310 – A multidrug transporter of the unknown major facilitator family, UMF-2. Front Microbiol. 2015;6:1–12.
- Paulsen IT, Skurray RA. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes – An analysis. Gene. 1993;124(1):1–11.
- Sun N, Rahman M, Qin Y, et al. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009;11(5):646–655.
- Yokoo T, Miyafuji H. Reaction behavior of wood in an ionic liquid, 1-ethylpyridinium bromide. J Wood Sci. 2014;60(5):339–345.
- Hart WE, Harper JB, Aldous L. The effect of changing the components of an ionic liquid upon the solubility of lignin. Green Chem. 2015;17(1):214–218.

![Figure 1. Cationic and anionic structure of 1-butyl-3-methylimidazolium chloride ([BMIM]Cl).](/cms/asset/e1532a66-978b-4b47-ac20-8f500928e885/tbeq_a_1885995_f0001_b.jpg)