Abstract
The sodium channel NaV1.5, which is encoded by the SCN5A gene, underlies the fast upstroke of cardiac action potential and thus plays a crucial role in cardiac electrophysiology, but the mechanism governing the regulation of NaV1.5 has not been fully elucidated. The newly developed clustered regularly interspaced short palindromic repeats (CRSPR)/Cas9 transcription factors offer a powerful and precise approach for modulating gene expression. We investigated the potential of this new tool for activating stringently silenced SCN5A in human cells. We first selected the most efficient single guide RNA (sgRNA) targeting upstream transcription start sites to induce effective expression of SCN5A mRNA. We observed significant transcriptional activation of endogenous SCN5A, with the highly effective activity of sgRNA targeting the human SCN5A promoter. The optimized dCas-VP64/sgRNA enhanced the endogenous SCN5A transcription up to 20-fold in human HEK293T cells and ultimately generated the NaV1.5 protein. Interestingly, multiple transcript variants of SCN5A were generated by endogenous transcriptional activation. Functionally, the NaV1.5 current produced by endogenous activation exhibited a similar electrophysiological property to that produced by ectopic overexpression of NaV1.5. The results of our study suggest that Cas9-mediated transcriptional activation is a useful tool for modulating gene expression and conducting electrophysiological studies in human cells.
Introduction
Due to the hydrophobic barrier of the cell membrane, ions are not able to diffuse in and out of the cell [Citation1, Citation2]. The transport of these charged ions between phospholipid bilayers relies on ion channel proteins. Two characteristics of ion channels, selectivity and gating, control the passage and impediment for individual types of ions. This control is essential for the maintenance of physiological homeostasis [Citation3, Citation4]. For excitable cells, such as neurons and cardiac myocytes, ion channels are important to form the action potential [Citation5]. The orchestration of sodium channels, potassium channels and calcium channels, involving the influx of Na+ and Ca2+ currents and the efflux of K+ currents, constitute the fundamental basis of electrophysiology [Citation6].
A group of diseases or dysfunctions associated with ion channels are known as channelopathies [Citation7, Citation8]. Traditionally, channelopathies represent a variety of loss- or gain-of-function mutations in an ion channel subunit, resulting in the alteration of the biophysical properties of the channel, such as conductance and ionic selectivity. In recent years, the concept of channelopathy has broadened with the integration of human clinical and genetic data to include not only gene variants in voltage-gated sodium (Nav), potassium (Kv) and calcium (Cav) channels implicated in cardiac Brugada syndrome and long-QT syndrome (LQTS) but also defects related to ion channel membrane trafficking and posttranslational regulation [Citation9].
During the formation of the action potential, the sodium channel represents phase 0 of the action potential, allowing the influx of Na+ to initiate the depolarizing process. The human SCN5A gene, located on chromosome 3p21, encodes the major cardiac voltage-gated sodium channel α-subunit, NaV1.5. The human SCN5A gene has 28 exons, resulting in six transcript variants. The SCN5A gene is associated with many channelopathies, such as familial atrial fibrillation, type 3 long-QT syndrome and Brugada syndrome [Citation10, Citation11]. Interestingly, there is evidence that the splicing misregulation of SCN5A contributes to cardiac conduction delay and heart arrhythmia in myotonic dystrophy [Citation12–14].
Voltage-gated sodium channels underlie action potential firing in excitable cells, including cardiomyocytes. Thus, it is not surprising that this channel has been considered a potential target for treating cardiac arrhythmia [Citation15, Citation16]. Generally, the life cycle of NaV1.5 begins with transcription, RNA processing and protein synthesis followed by transport from the endoplasmic reticulum to the sarcolemma by the secretory pathway, and eventually, NaV1.5 is endocytosed from the cell membrane position and degraded [Citation17, Citation18]. However, at present, little is known about the details of these events. Recently, the clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 system has been demonstrated to modulate gene transcription without modifying the genomic sequence by fusing catalytically inactive Cas9 (dCas9) [Citation19–21]. CRISPR activation can be achieved by the recruitment of transcriptional factors or direct fusion of the transcriptional domain, offering a convenient approach to activate gene expression [Citation22, Citation23].
In the present study, we report a method that generated the SCN5A-encoded NaV1.5 protein by facilitating SCN5A transcription in HEK293 cells. Using this Cas9-mediated activation system, we successfully generated multiple transcript variants of SCN5A and subsequent INa current, which was functional in HEK293 cells.
Materials and methods
Cell culture and transfection
HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fisher Scientific Inc., Shanghai, China) with 10% foetal bovine serum and maintained at 37 °C and 5% CO2. Transfections were performed in six-well plates using 1 μg dCas9 expression vector and 0.3 μg of individual gRNA expression vector. The transfection of the plasmids was performed with Lipofectamine 3000 (L3000-015; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions.
Plasmid constructs
The dCas9-effector plasmid pLenti-dCAS-VP64_Blast was purchased from Addgene (Plasmid #61425). For the flexible insertion of sgRNA sequences and easy tracking of transfected cells, the sequence containing BsmBI enzyme sites and gRNA scaffold was cloned between the BamHI and EcoRI sites of pLVX-shRNA2 (632179; Clontech Laboratories, Takara, Japan) by using ClonExpress® II One Step Cloning Kit (C112-02, Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China). The resulting construct, pLVX-U6-BsmBI-Zsgreen1, was confirmed by Sanger sequencing. The gRNAs for endogenous human SCN5A activation were selected to bind between 1 and 1000 bp upstream of the transcriptional start site. To clone the guide sequence into the vector, two oligos harbouring the gRNA sequences were synthesized and ligated between the two BsmBI sites. The sequences for gRNAs are listed in the Supporting information.
Lentivirus production and stable cell lines
Lentiviral particles were generated by transfecting HEK293T cells with the pLVX-U6-sgRNA-Zsgreen1 or pLenti-dCAS-VP64_Blast plasmid and the psPAX2 and pMD2. G (Addgene #12260 and #12259) packaging vectors at a ratio of 4:3:1, respectively. HEK293T cells were cultured at ∼80–90% confluency for transfection. The expression vector and helper vectors were transfected using Lipofectamine 3000 (L3000-015; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. The virus supernatant was harvested 48–72 h posttransfection, filtered with a 0.45-µm filter (Millipore), concentrated using the PEG Virus Precipitation Kit (K904, BioVision), aliquoted, and stored at −80 °C.
Selection in media supplemented with 100 µg/mL blasticidin (461120, Invitrogen) began 72 h following lentivirus infection, and colonies were picked after 1 week of selection. Colonies were expanded to a 12-well plate and tested for the ability to express dCas9 and bsd by real-time polymerase chain reaction (RT-PCR). The clones that expressed the most abundant dCas9 were examined by Western blot analysis and selected for subsequent experiments.
Quantitative reverse-transcription polymerase chain reaction
Total RNA from transfected cells was isolated with TRIzol (Invitrogen, Shanghai, China) and reverse-transcribed into cDNA using oligo-dT and random primers (Takara, Kyoto, Japan). Then, cDNA was quantified by real-time quantitative PCR using SYBR Green (Qiagen, Shanghai, China). The primers used in this study were designed with Primer Express (Applied Biosystems), and the sequences are listed in . The results are expressed as the fold change above control mock-transfected cells after normalization to GAPDH expression using the △△Ct method, as previously described [Citation24].
Table 1. Primers for real-time PCR.
Western blotting
Cell harvests were lysed with RIPA buffer (Beyotime, Jiangsu, China) containing protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany), and the protein concentration was determined using the bicinchoninic acid method. Protein samples were then separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE; INP0316BOX, Invitrogen, Carlsbad, CA, USA) and transferred onto PVDF membranes. The membranes were incubated at room temperature for 1 h with 5% nonfat milk followed by incubation at 4 °C overnight with the appropriate primary antibodies. The following primary antibodies were used: NaV1.5 (ASC-005, Alomone Labs, Jerusalem, Israel), Cas9 (26758-1-AP, Proteintech Group, Wuhan, Hubei, China) and GAPDH (AF0006, Beyotime, Jiangsu, China). After that step, the blots were washed and probed with IRDye® 800CW secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) at room temperature for 1 h. Images were visualized using the Odyssey infrared imaging system and analyzed using the Odyssey Application Software v2 (LI-COR Biosciences).
Electrophysiological recording
The standard whole-cell patch-clamp technique was used to measure INa currents (EPC-10; HEKA Elecktronik, Lambrecht, Germany), as previously reported [Citation25]. Borosilicate-glass electrode resistances ranging from 2 to 5 MΩ were filled with the internal solution and immersed in the external solution. The extracellular (bath) solution contained the following: 140 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L MgCl2, 11 mmol/L glucose and 10 mmol/L HEPES, pH adjusted to 7.4 with NaOH. The pipette solution contained the following: 130 mmol/L CsCl, 15 mmol/L NaCl, 10 mmol/L EGTA, 2 mmol/L MgCl2, 10 HEPES, pH adjusted to 7.4 with CsOH. The membrane capacitance was compensated by approximately 80–90% of their initial value. The INa current was expressed as the current density normalized to the cell capacitance. The INa current was recorded in HEK293 cells elicited by step depolarization of 40-ms duration to +60 mV from −100 mV in 10-mV increments, separated by a 1-s test interval at the holding potential of −120 mV.
Data analysis
All data are expressed as mean values with standard deviation (±SD). Differences were analyzed using an unpaired t test for the two groups and by analysis of variance (ANOVA) followed by Fisher’s least significant difference test for multiple groups. p values<.05 (two-tailed) were considered to be significant.
Results
Design of Cas9-mediated activation for endogenous SCN5A transcripts
To generate endogenous SCN5A, we adopted the CRISPR/Cas9 activator system for transcriptional activation of SCN5A in HEK293 cells. The activation system is composed of two vectors: one vector is the effector plasmid that encodes the dCas9-VP64 fusion protein, inserting the transactivation domain of VP64 at the N-terminus of dCas9, the catalytically inactive form of Cas9 for transcriptional enhancement; the other vector is the guide vector, providing the guide RNA for the specific binding of the effector protein to the targeting gene. For easy tracking of transfected cells, the sgRNA vector contains a GFP expression cassette for fluorescence-activated cell sorting and cell expansion ().
Figure 1. The Cas9-based two-vector system activates the transcription of endogenous SCN5A gene from proximal promoter regions. (A) Schematic illustration of two-vector system. Top, the construct of dCas9-VP64 fused protein (left) and guide RNA (right); bottom, Cas9-mediated transcriptional activation by using dCas9 and activation domain (VP64) at the transcriptional start site of SCN5A gene. (B) The mRNA level of SCN5A transfected with dCas9-VP64 and individual gRNA in HEK293 cells. The control group was transfected with indicated guide RNAs alone. Data are shown as the mean ± SD (n = 3 independent transfections). For *p < .05. Comparison of dCas9-VP64 versus Ctrl. (C) Quantitative PCR analysis of the immature mRNA levels of SCN5A gene in HEK293 cells. The HEK293 cells transfected with dCas9-VP64 were co-transfected with SCN5A-gRNA1 and control gRNA vector for 48 h, followed by the qPCR analysis of immature mRNA of SCN5A. Data are shown as the mean ± SD (n = 3 independent transfections). Imm, immature. For *p < .05. (D–E) The mRNA level of SCN5A after increasing the amounts of sgRNA and dCas9-VP64 expression plasmids in HEK293 cells. Data are shown as the mean ± SD (n = 3 independent transfections). *p < .05.
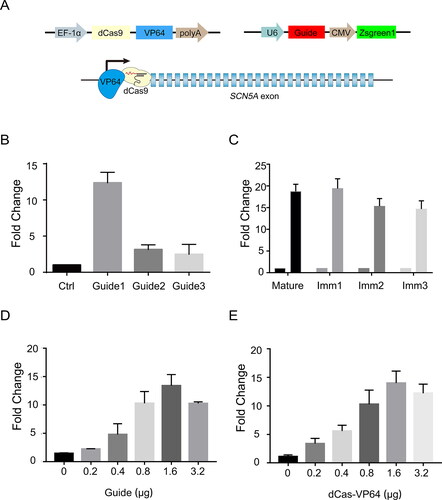
We first searched for the effective guide sequence in the promoter region of the SCN5A gene for transcriptional activation. The 20-bp sequences that precede NGG, the protospacer adjacent motif (PAM) required for sgRNA targeting, were selected as candidate sgRNA target sequences and inserted into the sgRNA expression vector [Citation26]. All three sgRNAs induced significant increases in SCN5A mRNA expression when coexpressed with dCas9-VP64 in human HEK293 cells (). The mean levels of activated expression varied over a fourfold range, and the highest expression was achieved as 20 times the control SCN5A expression, whereas the expression of a codon optimized dCas9-VP64 did not enhance the level of SCN5A mRNA.
Cas9-mediated activation generates endogenous SCN5A transcripts in HEK293 cells
To verify the activation of SCN5A in HEK293 cells, we designed primers that anneal to the introns of the SCN5A mRNA precursor to test the generation of immature mRNA. Our data showed similar increases in immature SCN5A mRNA to mature SCN5A mRNA, suggesting the generation of endogenous SCN5A transcripts in HEK293 cells (). In addition, increasing the amounts of sgRNA and dCas9-VP64 expression plasmids resulted in higher activation of the SCN5A gene (), demonstrating a dose-dependent effect.
Transcriptional activation of endogenous SCN5A specifically generates NaV1.5 protein
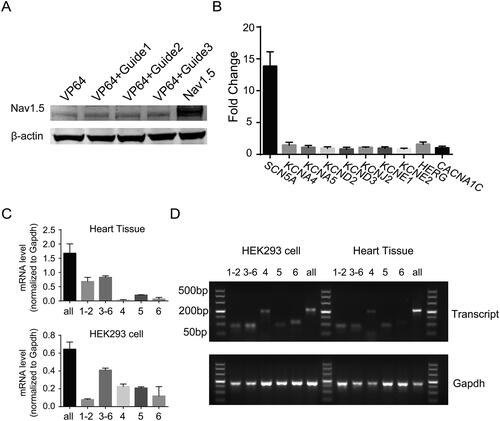
We next examined the expression of SCN5A-encoded NaV1.5 protein in HEK293 cells. As shows, the NaV1.5 protein was significantly increased in endogenously activated HEK293 cells transfected with dCas9-VP64 and gRNAs. The experiment with the highest efficacy of SCN5A-sgRNA demonstrated that the activation is specific for the SCN5A gene but not for other ion channel genes [Citation27], such as CACNA1C, KCNQ1 and HERG (). These results indicated that the Cas9-mediated endogenous activation of ion channel genes is effective and specific in human cells.
Figure 2. Targeting activation of endogenous SCN5A generates multiple transcript variances. (A) Western blot showing the expression levels of NaV1.5 in HEK293 cells transfected with dCas9-VP64 fusion protein and three gRNAs for 48 h. (B) Quantitative PCR analysis of the mRNA levels of ion channel genes rather than SCN5A. The specificity of SCN5A gRNA is evaluated by the measurement of mRNA expression level of KCNA4, KCNA5, KCND2, KCND3, KCNJ2, KCNE1, KCNE2, KCNJ2, HERG, and CACNA1C in co-transfected HEK293 cells. Data are shown as the mean ± SD (n = 3 independent transfections). *p < .05. (C) The qRT-PCR analysis of human SCN5A transcripts in cardiac tissues, Top. Bottom, the mRNA level of different SCN5A transcript variances in HEK293 cells transfected with Cas9-VP64 and SCN5A-gRNA1. Data are shown as the mean ± SD (n = 3 independent transfections). For *p < .05. (D) Agarose gel electrophoresis of PCR amplicon from different SCN5A transcript variances in human cardiac tissue and HEK293 cells.
Transcriptional activation of endogenous SCN5A generates multiple transcript variances
In general, the RNA of the SCN5A gene is derived from 28 different exons, in which exons 2–28 contain the protein-coding sequence, exon 1 and part of exon 2 encode the 5′-untranslated region (5′-UTR), and exon 28 contains the 3′-UTR [Citation28, Citation29]. Several SCN5A mRNA variants have been detected in the mammalian heart; most of these variants are generated by alternative splicing and apparently evolutionarily conserved mechanisms. In humans, there are seven transcripts encoding sodium channel protein type 5 subunit alpha isoform a–g. Several lines of evidence demonstrated that not all of these transcripts were expressed equally or translated into the NaV1.5 protein with equal efficiency. The splicing misregulation of SCN5A may lead to cardiac conduction delay and heart arrhythmia [Citation14]. This finding raises the questions of how these transcripts are switched and how the splicing of these transcripts is regulated, as well as what the contribution of these transcripts in foetal hearts and adult hearts is. To answer these questions, generating endogenous SCN5A transcripts in human cells may provide a convenient cell model for detailed study. Therefore, we next tested whether RNA-guided activators could also induce the expression of different transcripts of the human SCN5A gene in HEK293 cells. Using our transcriptional activation method, we obtained multiple SCN5A transcripts in HEK293 cells. We achieved similar expression across these transcripts, while in normal human heart tissues, the transcripts of SCN5A were differentially expressed ().
Generation of cell line stably expressing endogenous SCN5A
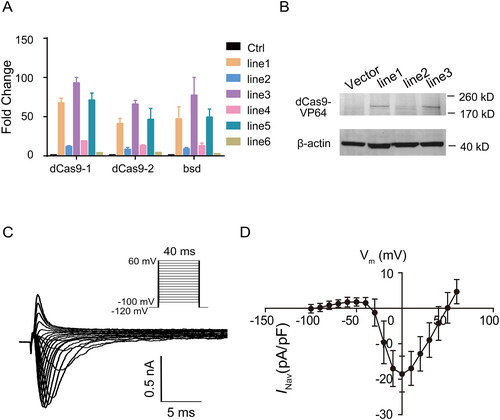
To decrease the variation between cells, we further packaged the lentivirus carrying dCas9-VP64 to build stably expressed cell lines. Six cell lines were picked and detected by qPCR and Western blotting. The mRNA and protein levels of dCas-VP64 were examined to identify the successful cell line with stable expression of the effector activator in HEK293 cells ().
Figure 3. The endogenous SCN5A transcriptional activation generates functional currents of NaV1.5 in HEK293 cells. (A) The mRNA levels of dCas9-VP64 in six cell lines with dCas9-VP64 stable expression. Data are shown as the mean ± SD (n = 3). For *p < .05. (B) Western blot showing the expression of Cas9 in different dCas9-VP64 stably transfected cell lines. (C) The representative Na + currents recorded from HEK293 cells transfected with dCas9-VP64 and SCN5A targeting gRNA. Currents were recorded with the whole-cell patch-clamp technique. Cells were held at −120 mV and INa was elicited by a family of voltage steps to potentials ranging from −100 to +60 mV with 10 mV increments. (D) Current-voltage relationship of INa in HEK293 cells transfected with dCas9-VP64 and SCN5A targeting gRNA control (n = 29). Currents were normalized to cell capacity.
Functional NaV1.5 currents can be recorded in HEK293 cells with endogenous SCN5A activation
To examine whether the endogenous activation of SCN5A was functional, we performed electrophysiological analysis of the INa current. The sgRNA vector with the highest SCN5A mRNA induction and control vector were individually transfected into HEK293 cells with stable dCas9-VP64 expression followed by whole-cell patch-clamp analysis at 48 h after transfection. Our data showed that when the transfected HEK293 cells were depolarized to test potentials between −100 and +60 mV by 10-mV steps applied once every 40 ms from the holding potential of −120 mV, the SCN5A-encoded INa current could be induced by transcriptional activation of endogenous human SCN5A (). The plot of current density versus voltage revealed that the peak amplitude occurred at 0 mV for dCas9-VP64/SCN5A-gRNA-overexpressing HEK293 cells, similar to ectopic NaV1.5 expression in HEK293 cells.
Discussion
Previously, gain-of-function studies were primarily limited to cDNA overexpression, which has some drawbacks: (1) overexpression is often beyond physiological levels and endogenous regulation; (2) transcriptional isoforms are lacking in cDNA overexpression systems; (3) large cDNA sequences are often difficult to clone and deliver, especially for cardiac myocytes or neurons; and (4) the construction of vectors is laborious.
In this report, we describe a Cas9-based method that can achieve flexible delivery and easy tracking. The lentivirus-based expression vectors are easy to overexpress in HEK293 cells in transient expression and can easily obtain stable expression. Green fluorescent proteins are helpful for the estimation of guide RNA delivery [Citation30]. Furthermore, this method can maintain the diversity of transcriptional isoform variants. The six isoforms of the SCN5A gene were all detected in transfected HEK293 cells, consistent with the expression of SCN5A in heart tissues. Specifically, the predominant types of isoforms of SCN5A in activated HEK293 cells are similar to those observed in cardiac myocytes. Given that different variant sequences for SCN5A are commonly present in human myocardium and they exhibit functional differences among themselves [Citation31, Citation32], it is possible that the function of each transcript can be clarified in these SCN5A-activated HEK293 cells. Activation of the endogenous SCN5A gene in HEK293 cells generates a functional INa current, as revealed by our electrophysiological experiment. However, as the SCN5A transcript variances are produced in an unbiased manner, the immature transcripts are equally generated [Citation33]. Further study should be done to increase the specific type of isoforms [Citation34, Citation35].
Conclusions
We found that a Cas9-based method can activate the endogenous SCN5A gene in HEK293 cells and then generate a functional INa current. Our data and analysis may establish the foundation for further research examining the relationship between the different SCN5A transcripts and INa current. These results may be helpful for analyzing the functions of the sodium channel NaV1.5 in excitable cells. Beyond the utility of the two-component system as a technological improvement of sodium channels, we can envision that targeting multiple ion channel genes with one vector drastically improves the activation of a number of currents, thereby establishing cardiomyocyte-like HEK293 cells to dissect the function of ion channels in regulation and coordination.
Authors’ contributions
Rui Shi and Liang Xu designed the study. Liang Xu designed, performed the experiments, and Rui Shi analyzed the results. Rui Shi and Liang Xu drafted the manuscript.
Supplemental Material
Download TIFF Image (1.2 MB)Acknowledgements
We are thankful to Jiahe Xu for supporting us for draft and figure preparation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Credi A. A molecular cable car for transmembrane ion transport. Angew Chem Int Ed Engl. 2019;58(13):4108–4110.
- Cubero-Font P, De Angeli A. Connecting vacuolar and plasma membrane transport networks. New Phytol. 2021;229(2):755–762.
- Zheng SP, Huang LB, Sun Z, et al. Self-assembled artificial ion-channels toward natural selection of functions. Angew Chem Int Ed Engl. 2020;60(2):566–597.
- Harvey JRM, Plante AE, Meredith AL. Ion channels controlling circadian rhythms in suprachiasmatic nucleus excitability. Physiol Rev. 2020;100(4):1415–1454.
- Nguyen HX, Bursac N. Ion channel engineering for modulation and de novo generation of electrical excitability. Curr Opin Biotechnol. 2019;58:100–107.
- Abriel H, Rougier JS, Jalife J. Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death. Circ Res. 2015;116(12):1971–1988.
- Li MCH, O'Brien TJ, Todaro M, et al. Acquired cardiac channelopathies in epilepsy: evidence, mechanisms, and clinical significance. Epilepsia. 2019;60(9):1753–1767.
- Demirbilek H, Galcheva S, Vuralli D, et al. Ion transporters, channelopathies, and glucose disorders. IJMS. 2019;20(10):2590.
- Curran J, Mohler PJ. Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annu Rev Physiol. 2015;77:505–524.
- Kapoor A, Lee D, Zhu L, et al. Multiple SCN5A variant enhancers modulate its cardiac gene expression and the QT interval. Proc Natl Acad Sci U S A. 2019;116(22):10636–10645.
- Peltenburg PJ, Blom NA, Vink AS, et al. In children and adolescents from Brugada Syndrome-Families, only SCN5A mutation carriers develop a type-1 ECG pattern induced by fever. Circulation. 2020;142(1):89–91.
- Remme CA. Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J Physiol. 2013;591(17):4099–4116.
- Li W, Yin L, Shen C, et al. SCN5A variants: association with cardiac disorders. Front Physiol. 2018;9:1372.
- Freyermuth F, Rau F, Kokunai Y, et al. Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy. Nat Commun. 2016;7:11067
- Remme CA, Bezzina CR. Sodium channel (dys)function and cardiac arrhythmias. Cardiovasc Ther. 2010;28(5):287–294.
- Marionneau C, Abriel H. Regulation of the cardiac Na + channel NaV1.5 by post-translational modifications. J Mol Cell Cardiol. 2015;82:36–47.
- Delisle BP, Anson BD, Rajamani S, et al. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004;94(11):1418–1428.
- Rook MB, Evers MM, Vos MA, et al. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res. 2012;93(1):12–23.
- Joung J, Konermann S, Gootenberg JS, et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12(4):828–863.
- Haldeman JM, Conway AE, Arlotto ME, et al. Creation of versatile cloning platforms for transgene expression and dCas9-based epigenome editing. Nucleic Acids Res. 2019;47(4):e23.
- Liu Q, Zhao K, Wang C, et al. Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Adv Sci (Weinh)). 2019;6(1):1801423.
- Chavez A, Scheiman J, Vora S, et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12(4):326–328.
- Konermann S, Brigham MD, Trevino AE, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108.
- Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113 Suppl 1:i39–i48.
- Xu H, Xiao T, Chen CH, et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015;25(8):1147–1157.
- Anderson KR, Haeussler M, Watanabe C, et al. CRISPR off-target analysis in genetically engineered rats and mice. Nat Methods. 2018;15(7):512–514.
- Shang LL, Pfahnl AE, Sanyal S, et al. Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101(11):1146–1154.
- Jagu B, Charpentier F, Toumaniantz G. Identifying potential functional impact of mutations and polymorphisms: linking heart failure, increased risk of arrhythmias and sudden cardiac death. Front Physiol. 2013;4:254
- Romei MG, Boxer SG. Split green fluorescent proteins: scope, limitations, and outlook. Annu Rev Biophys. 2019;48:19–44.
- Hoagland DT, Santos W, Poelzing S, et al. The role of the gap junction perinexus in cardiac conduction: potential as a novel anti-arrhythmic drug target. Prog Biophys Mol Biol. 2019;144:41–50.
- Zegkos T, Panagiotidis T, Parcharidou D, et al. Emerging concepts in arrhythmogenic dilated cardiomyopathy. Heart Fail Rev. 2020;online ahead of print
- Onkal R, Mattis JH, Fraser SP, et al. Alternative splicing of Nav1.5: an electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms and critical involvement of a lysine residue. J Cell Physiol. 2008;216(3):716–726.
- Glazer AM, Wada Y, Li B, et al. High-throughput reclassification of SCN5A variants. Am J Hum Genet. 2020;107(1):111–123.
- Zhang Z, Huang X, Qian Y, et al. Engineering smart nanofluidic systems for artificial ion channels and ion pumps: from single-pore to multichannel membranes. Adv Mater. 2020;32(4):e1904351.
