Abstract
The purpose of this in vitro pilot study was to investigate the cleaning capacity of an air abrasive device with trehalose powder on implant surfaces compared to Er:YAG laser application in two different bone defect angulations. Forty ink stained dental implants were mounted in epoxy resin models representing standardized peri-implant defects with two different angulations (30° and 60°). An air powder abrasive device with a nozzle tip designed for subgingival usage and Er:YAG laser were used. A blinded and calibrated examiner evaluated the standardized photographs of instrumented implant surfaces with image-processing software. Student’s t test was used for the paired evaluation of the total cleaning effects according to defect angulations. The results showed that there were residual stained surfaces on all implants regardless of the cleaning methods and defect angulations. The percentage of total area cleaned by the air powder device in both defect angulations was significantly higher than that achieved by the Er:YAG laser application (p < 0.05). The differences between the two defect angulations within each cleaning method were statistically non-significant. Although a complete surface cleanliness was not provided with both cleaning methods, the air abrasive device revealed a better performance than the Er:YAG laser. The residual stained surfaces, especially at the deepest side of the defects, regardless of the cleaning method, suggest that special cleaning care is needed at the base of the peri-implant defects.
Introduction
Dental implant treatment is a multidisciplinary approach for achieving a restorative goal. With the advancements in implant dentistry over the years, implant-supported restorations are considered the most advanced approach to replace missing teeth in partially and fully edentulous patients [Citation1, Citation2].
Such an integrated approach requires a sophisticated treatment plan, especially regarding the specific periodontal, surgical and restorative aspects. As the understanding of implant treatment continues to improve, guidelines have been developed and continue to be developed for the long-term success [Citation3].
Despite the advances within treatments, there are some controversial aspects and issues that are still subject to debate. Failures requiring the implant removal can occur. One of the reasons is peri-implantitis. It is a plaque biofilm-associated disease that causes an inflammatory process in the soft and hard tissues surrounding an osseointegrated implant, leading to the loss of bone support [Citation4]. Reports indicate that it occurs in 1.4% to 53.5% of patients with dental implants [Citation5, Citation6]. Although poor oral hygiene, history of periodontitis and smoking is accepted as risk factors for peri-implant diseases, there is still no standard treatment protocol established in the literature [Citation7]. Peri-implant disease is a public health issue due to the huge increase in its prevalence and lack of a clearly agreed-on standard management. Similar to plaque-associated diseases, peri-implantitis-associated inflammatory reaction and tissue destruction occur when microbial biofilm accumulates on the implant surfaces and the superstructures [Citation8]. Host immune response to polymicrobial aggression plays a central role in the disease pathogenesis [Citation9]. Therefore, all cause-related therapeutic approaches aim, in the first place, to disrupt and remove the microbial biofilm from the implant surfaces [Citation10, Citation11]. To achieve this goal, studies have investigated several non-surgical mechanical treatment modalities including hand instrumentation, ultrasonic debridement, localized and/or systemic anti-microbial therapy and the use of dental lasers [Citation12]. In addition, there are surgical procedures with the adjunct use of mechanical cleaning methods and detoxifying agents, bone augmentation, polishing or removing implant threads, soft tissue augmentation and more [Citation13]. One of the key factors for the success of non-surgical and surgical peri-implantitis treatment is the decontamination of the implant surfaces by mechanical cleaning. However, many mechanical methods have been reported inadequate with mixed results, revealing that this is still an active and open research area [Citation14].
An approach that has received attention recently is the use of air power abrasive devices to improve the cleaning effectiveness on contaminated implant surfaces. An in vitro study suggested that air powder abrasion is the most efficient and less damaging cleaning modality in comparison to sonic scaler and curette in different defect morphologies [Citation15]. However, there is a paucity of information on the use of air powder abrasive devices to reach a definite conclusion and it is warranted to perform comparative studies evaluating the effectiveness. There is a new air-abrasive material called trehalose, a natural noncariogenic disaccharide with a good taste. It is thermostable and approved for use in food processing [Citation16]. It is highly water soluble (689 g/L) with a pH of 6.4. In one clinical study, subgingival air-polishing with trehalose powder has revealed encouraging clinical outcomes [Citation17].
Therefore, the aim of this study is to investigate the cleaning potential of an air powder abrasive device with trehalose powder on implant surfaces in comparison to Er:YAG laser application in an in vitro model of two different defect angulations.
Materials and methods
Defect models and implants
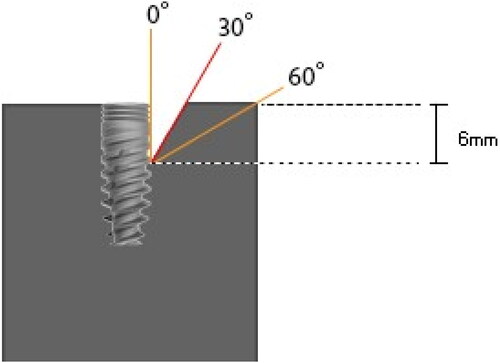
The set-up of the study followed the methodology described by Sahrmann et al. [Citation18] Two standardized study models representing two different defect morphologies with opening angulations of 30° and 60°, were custom-made from clear epoxy resin (Epoart, Polisan, Kocaeli, Turkey) (). The defect height in each model was 6 mm (down to the 3rd thread) and the defect width corresponded to the implant diameter (3.75 mm) simulating a semi-circumferential peri-implant defect.
Implants, 13 mm in length with a diameter of 3.75 mm (Rapid Implant, Mode Dental Implants, Istanbul, Turkey), were dipcoated with permanent ink [Citation18–20] (Staedtler permanent Lumocolor, Germany) to simulate an optically identifiable plaque accumulation over the complete rough titanium (65% hydroxyapatite + 35% calcium phosphate) surface. Implants were inserted into the study models in such a way that the rough surfaces leveled with the upper edge of the model [Citation18].
Two different cleaning methods were used on the implant surfaces as follows:
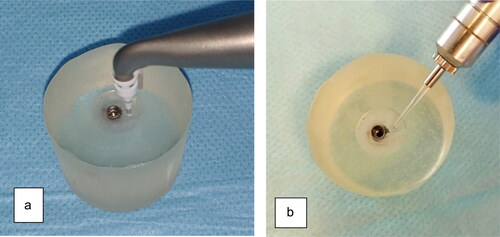
An air powder abrasive device (MyLunos®; Dürr Dental Group, Bietigheim-Bissigen, Germany) with trehalose powder (Perio Combi®; 30 µm grain size, Dürr Dental Group, Bietigheim-Bissigen, Germany) and a special hand piece for subgingival instrumentation were used with a nozzle tip parallel to the implant surface for 20 seconds. The nozzle tip was used for each implant defect surface in horizontal sweeping movements and then discarded ().
An Er:YAG laser (VersaWave, Delight; Hoya-Con Bio) with a wavelength of 2940 nm was used. The parameters were set at 20 Hz/120 mJ, with water irrigation according to the manufacturer’s instructions. The pulse width was 200 ms. The quartz chisel tip was selected (1.2–0.4 mm, rectangular shape) and applied in horizontal sweeping movements for 20 seconds ().
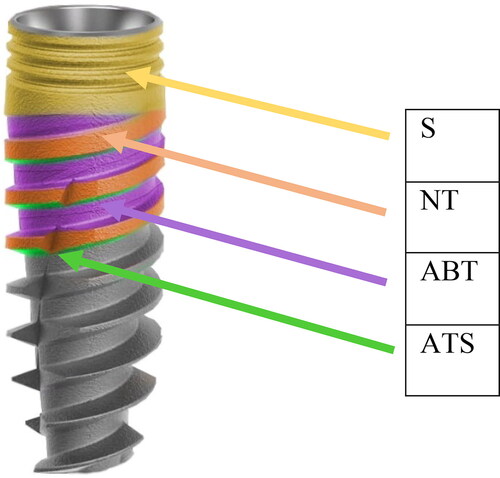
After both instrumentations, the implant surfaces were evaluated for residual stain using digital photographs at the following site locations: shoulder area (S), neck of the threads (NT), apically facing thread surface (ATS) and area between threads (ABT) (). One blinded and calibrated examiner (OLT) assessed the photographs twice by evaluating and grading the aforementioned implant sites for the residual stain. There was good agreement between the first and second measurements: the intraclass correlation coefficient (ICC) of reliability was close to 1.00, showing that the measurements can be repeated with a non-significant error.
Evaluation of surface cleanliness
After instrumentations, implants were removed carefully from the models. Loosened ink particles were cleaned by gentle water and air spray. Digital color photos were taken in standardized conditions by a digital camera (dark chamber, ISO 125, aperture f/7.1, shutter speed 1/60 s, distance 31.4 cm with a Nikon D5300 camera, Tokyo, Japan), positioned vertically to the implant axis. A Twin flash R1C1 wireless close-up Speedlight system (Tokyo, Japan) was used with power settings of ½ on one side. The evaluations on the surface ink remnants were performed with an image processing software (ImageJ 1.46r, National Institutes of Health, Bethesda, Maryland, USA).
Data analysis
For statistical analysis, IBM SPSS Statistics 22 (IBM SPSS, Turkey) was used. The normal distribution of the data was assessed by Kolmogorov-Smirnov and Shapiro Wilks tests, and the parameters followed the normal distribution. Student’s t test was used for the paired evaluation of the total cleaning effects of the methods according to two different defect angulations. Differences were considered statistically significant at the p < 0.05 level.
Results
In this study, following treatment, there was residual staining on all implants regardless of the cleaning methods and defect angulations. The percentage of total area cleaned by the air powder device in both defect angulations was significantly higher (p < 0.05) than that after the Er: YAG laser application (). The differences between the two defect angulations within each surface cleaning method were not statistically significant ().
Table 1. Total implant surface area cleaned by two different cleaning applications in two defect angulations.
The air powder abrasive device produced significantly better results (p < 0.05) than the Er:YAG laser for all defect angulations (30° and 60°) at different site locations of the implant surfaces except the S (the most coronal part of the defect) at 30° angulation in which the stain-removing performance was similar for both types of treatment. Both applications also showed non-significant differences at the third thread level of ATS (the deepest side of the defects) at 60° angulation. However, the cleaning performance of both devices towards the base of the defect was found inadequate at both angulations. Furthermore, the cleanability decreased as we move down to the base of the defect ().
Table 2. Percentage of the cleaned surface area at different implant site locations in two defect angulations.
Next, we compared the different angulations within each of the methods at different site locations. There was statistically significant difference only between 30° and 60° angulations at ABT in the first thread of the implant for the Er:YAG laser method (p < 0.01)
Discussion
The recreation of biologically acceptable implant surface is essential and the keystone for the success of the treatment in peri-implant diseases. Besides non-surgical treatments, this is also particularly important during surgical procedures aimed at preventing the progression of peri-implant infections with either regenerative approaches, during which the defect is filled, or resective procedures, during which peri-implant pocket is eliminated [Citation21]. This study evaluated two mechanical cleaning devices and a new cleaning powder on the implant surfaces following their exposure to a staining ink. The results could reflect the clinical aspects of peri-implant treatment. Peri-implantitis infections contaminate the implant surfaces; therefore, in order to achieve a successful treatment, decontamination of the implant surfaces by mechanical and/or chemical means is a “must” [Citation22–24]. Mechanical or chemical interventions for decontamination may alter the surfaces. The reason for in vitro studies is to find the least detrimental approach for decontamination and cleaning in order to provide and keep the implant health in the long term. Without proper surface cleaning systems, there is no way to treat peri-implant infection or prevent further bone destruction [Citation24–27].
Power-driven subgingival air-abrasive powder devices, Er-YAG lasers, metal curettes or ultrasonic curettes with or without plastic sleeves can be used as part of the peri-implantitis treatments [Citation28–30]. Such treatments usually provide clinical improvements such as reduced bleeding tendency, and in some cases a pocket-depth reduction of ≤1 mm because of the process removing the biofilm and the related disease modifying factors on the implant surface [Citation31]. However, there is still no consensus and a standard treatment protocol for peri-implant infections and there is also a paucity of information for a standard surface decontamination method, leading researchers to investigate this open and critical subject matter [Citation32]. Peri-implant infections with supporting bone loss have become highly prevalent untreatable disease forms and are the main reasons for implant failures [Citation33].
Therefore, the present study investigated the cleaning capacity of a power-driven subgingival air abrasive device with trehalose powder on ink stained implant surfaces in comparison to Er:YAG laser in an in vitro model of two different defect morphologies presenting 30° and 60° angulations. After both types of instrumentations, residual stain deposits were detected on the implant surfaces in all study models. However, the air abrasive powder device provided better cleaning results than the Er:YAG laser in both defect angulations (p < 0.05) regarding the comparison of the cleaning potential for different defect angulations. The total cleaning performance was similar within each method ().
In general, although there is insufficient evidence to recommend adjunctive application of lasers to nonsurgical/surgical therapy of peri-implant diseases [Citation34], Er:YAG lasers are considered one of the options for implant surface decontamination in terms of working mechanism and safety [Citation26, Citation35–37]. A meta-analysis [Citation34] presented the clinical outcomes of laser applications for peri-implantitis surface decontamination. It concluded that laser treated implant surfaces were not different from the implant surfaces decontaminated by other common surface cleaning methods. The comparison between Er:YAG laser and plastic curettes again revealed statistically insignificant differences in periodontal parameters at 12 and 24 months. In this study, for both defect angulations, the Er:YAG laser showed lower cleaning potential than the air-abrasive powder device. The laser-based technique failed to remove the ink staining, especially towards the base of the defects, revealing the insufficient access capacity of the device and the tip. Similar to our study, Hakkı et al. [Citation38], evaluated the cleaning potential of glycine based air powder versus Er:YAG laser in an in vitro model. They observed residual stain deposits on the threads of the apical part of the implants in their laser group. However, since statistical analysis was not reported in their study, it was not possible to make a direct comparison with the results of the present study. A review by Kamel et al. [Citation35] stated that an optimal irradiation protocol could not be reached for the lasers since researchers employed different test specimens, different decontamination techniques, different irradiation settings, different outcome measures and evaluation methods. However, Er:YAG laser irradiation at 30 mJ/pulse and 30 Hz with water spray was also reported in this review as having no negative effects on the color or microstructure of implant surfaces [Citation35]. The present study used 20 Hz/120 mJ irradiation with water irrigation.
Air abrasive devices, especially using small particle-size powders are encouraging and reveal better or equal capacity of cleaning when compared to the other methods in in vitro studies [Citation20, Citation39–41]. This study used trehalose powder, a brand new small particle-size natural noncariogenic disaccharide, and the results supported the use of air abrasive device revealing superior mechanical cleaning potential over Er:YAG lasers.
In vitro studies with different air powder materials reported that large-sized particles have more surface cleaning capacity besides their harmful effects on the microstructure [Citation40, Citation41]. Small particle-size powders excluded the harmful effects; however, there is limited data on the cleaning effects of different small particle-size air abrasive powders on implant surfaces [Citation41]. The abrasive effect of trehalose powder is as low as that of glycine [Citation17] and trehalose powder has very small particle size diameters of 25–35 µm. The use of this powder in this study with a special subgingival plastic tip also enabled an easy access to the most of the surfaces of the implants in the study models and indicated significantly better results compared to the Er:YAG laser application used with a rigid fragile quartz chisel tip. The trehalose air-powder was not compared with other powder materials of different particle size or hand instrumentation. However, this is the first step of further planned studies, and as a pilot study trehalose powder was compared with Er:YAG laser irradiation, representing an “easy to use”, “handy” and “safe” mechanical cleaning approach on the implant surfaces.
Most previous research on power driven air abrasive devices for implant surface cleaning was performed on discs and only evaluated the flat surface cleanliness excluding the screw components and the defect morphology [Citation42–47]. Furthermore, only a limited number of studies attempted to mimic open flap cleaning using air abrasive device in a simulation model of peri-implantitis with different defect morphologies [Citation15, Citation18, Citation20, Citation40]. In one of these studies, Sahrmann et al. [Citation20], compared the cleaning capacity of glycine air abrasive powder, ultrasonic device and curettes in three different defect angulations (30°, 60° and 90°). Powder abrasion provided the least ink remnants (11.3 ± 5.4%), followed by ultrasonic (18.5 ± 3.8%) and manual (24.1 ± 4.8%) instrumentations. In their study, the performance of the powder-driven air abrasive powder expressed in percentages was similar to our findings (total surface area cleaned at 30° angulation, 89.22 ± 17.01%; 60° angulation, 84.28 ± 9.28%). Matsubara et al. [Citation41] indicated less cleaning capacity for glycine (33.01 ± 1.2%) and erythritol ((25.01 ± 0.7%), two small-sized particles, in comparison to large particle-size sodium bicarbonate (49.3 ± 3.6%). Another study by Wei et al. [Citation40] compared different particle-size air abrasive powders (glycine, sodium bicarbonate, calcium carbonate) in a 60° defect angulation peri-implantitis defect model. The results revealed that the best cleaning evaluated in terms of the lowest residual ink area, was obtained with calcium carbonate powder (5.5%) followed by sodium bicarbonate (13%). Both calcium and sodium bicarbonate powders were found more effective in cleaning than the small-sized glycine powder (39.7%) [Citation40]. The results obtained in this study with the air abrasive device were better than the ones with the Er:YAG laser; however both applications did not provide complete accessibility to the base of the defects. Although the implant and the defect type (circumferential) as well as the assessment methods were different in the aforementioned studies [Citation18, Citation20, Citation40], the same model of surface ink staining representing the microbial plaque biofilm was used [Citation15, Citation18–20, Citation40]. Ink staining may be more recalcitrant in removal or vice versa; however, the residual staining has the advantage of being easily detected in the direct photographic evaluation compared to biofilm assessment methods that require many area specific steps [Citation48].
Further research is needed to compare the cleaning effects and implant surface alterations with trehalose powder versus conventional or other small-size air powders under standardized conditions. Moreover, clinical investigations are needed to investigate the microbial changes and recolonization as well as to clarify the relationship of implant surface alterations with regenerative treatment outcomes.
Conclusions
Although complete surface cleanliness was not achieved with both cleaning methods, the air abrasive device demonstrated better cleaning capacity than the Er:YAG laser. The residual ink stained surfaces regardless of the cleaning method, especially towards the deepest side of the defects, suggest that special instrumentation care is necessary at the base of the peri-implant bone defects.
Acknowledgements
The authors would like to express their sincere gratitude to Prof. Dr. Bahar KURU for her guidance and contributions to their research.
Data availability
The dataset is available at: https://data.mendeley.com/datasets/g4d6wkjghk/1.
Disclosure statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- Tomasi C, Wennström J, Berglundh T. Longevity of teeth and implants – a systematic review. J Oral Rehabil. 2008;35(s1):23–32.
- Trullenque-Eriksson A, Guisado M. B. Retrospective long-term evaluation of dental implants in totally and partially edentulous patients: part II: periimplant disease. Implant Dent. 2015;24:217–221.
- Drago C, Carpentieri J. Treatment of maxillary jaws with dental implants: guidelines for treatment. J Prosthodont. 2011;20(5):336–347.
- Renvert S, Persson GR, Pirih FQ, et al. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89(Suppl 1):S304–S312.
- Buser D, Janner SF, Wittneben JG, et al. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. 2012;14(6):839–851.
- Matuliene G, Studer R, Lang N, et al. Significance of periodontal risk assessment in the recurrence of periodontitis and tooth loss. J Clin Periodontol. 2010;37(2):191–199.
- Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304.
- Carcuac O, Berglundh T. Composition of human peri-implantitis and periodontitis lesions. J Dent Res. 2014;93(11):1083–1088.
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11.
- Mombelli A, Lang NP. Microbial aspects of implant dentistry. Periodontol 2000. 1994;4:74–80.
- Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167–181.
- Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(8 Suppl):305–315.
- Renvert S, Polyzois IN. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol 2000. 2015;68(1):369–404.
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282–285.
- Keim D, Nickles K, Dannewitz B, et al. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin Oral Impl Res. 2019;30:550–558.
- Neta T, Takada K, Hirasawa M. Low-cariogenicity of trehalose as a substrate. J Dent. 2000;28(8):571–576.
- Kruse AB, Akakpo DL, Maamar R, et al. Trehalose powder for subgingival air-polishing during periodontal maintenance therapy: a randomized controlled trial. J Periodontol. 2019;90(3):263–270.
- Sahrmann P, Ronay V, Sener B, Jung RE, et al. Cleaning potential of glycine air-flow application in an in vitro peri-implantitis model. Clin Oral Impl Res. 2013;24(6):666–670.
- Ronay V, Merlini A, Attin T, et al. In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach. Clin Oral Impl Res. 2017;28(2):151–155.
- Sahrmann P, Ronay V, Hofer D, Attin T, et al. In vitro cleaning potential of three different implant debridement methods. Clin Oral Impl Res. 2015;26(3):314–319.
- Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):325–345.
- Koo K-T, Khoury F, Keeve PL, et al. Implant surface decontamination by surgical treatment of periimplantitis: a literature review. Implant Dent. 2019;28(2):173–176.
- Renvert S, Hirooka H, Polyzois I, et al. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants–Consensus report of working group 3. Int Dent J. 2019;69:12–17.
- Figuero E, Graziani F, Sanz I, et al. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014;66(1):255–273.
- Mellado-Valero A, Buitrago-Vera P, Solá-Ruiz MF, et al. Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal. 2013;18(6):e869–e876.
- Subramani K, Wismeijer D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implant. 2012;27:1043–1954.
- Suárez-López Del Amo F, Yu S-H, Wang H-L. Non-surgical therapy for peri-implant diseases: a systematic review. J Oral Maxillofac Res. 2016;9:e13.
- John G, Sahm N, Becker J, et al. Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study. Clin Oral Invest. 2015;19(8):1807–1814.
- Lupi S, Granati M, Butera A, et al. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: a six-month randomized clinical trial. Int J Dent Hygiene. 2017;15(4):287–294.
- Ji YJ, Tang ZH, Wang R, et al. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: a pilot clinical trial. Clin Oral Impl Res. 2014;25(6):683–689.
- Renvert S, Lindahl C, Roos Jansåker AM, et al. Treatment of peri-implantitis using an Er: YAG laser or an air‐abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38(1):65–73.
- Vandana K, Dalvi P, Nagpal D. Management of peri-implant infections. J Int Clin Dent Res Organ. 2015;7(3):160.
- Shankar T, Gowd MS, Ranjan R, et al. Peri-implantitis: a concern for implant failure. IJOCRWEB. 2016;4(3):204–206.,
- Mailoa J, Lin GH, Chan HL, et al. Clinical outcomes of using lasers for peri-implantitis surface detoxification: a systematic review and meta-analysis. J Periodontol. 2014;85(9):1194–1202.
- Kamel MS, Khosa A, Tawse-Smith A, et al. The use of laser therapy for dental implant surface decontamination: a narrative review of in vitro studies. Lasers Med Sci. 2014;29(6):1977–1985.
- Schwarz F, Sculean A, Rothamel D, et al. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implant Res. 2004;16(1):44–52.
- Gholami GA, Karamlou M, Fekrazad R, et al. Comparison of the effects of Er, Cr: YSGG laser and super-saturated citric acid on the debridement of contaminated implant surfaces. J Lasers Med Sci. 2018;9(4):254–260.
- Hakki SS, Tatar G, Dundar N, et al. The effect of different cleaning methods on the surface and temperature of failed titanium implants: an in vitro study. Lasers Med Sci. 2017;32(3):563–571.
- Moharrami M, Perrotti V, Iaculli F, et al. Effects of air abrasive decontamination on titanium surfaces: a systematic review of in vitro studies. Clin Implant Dent Relat Res. 2019;21(2):398–421.
- Wei MC, Tran C, Meredith N, et al. Effectiveness of implant surface debridement using particle beams at differing air pressures. Clin Exp Dent Res. 2017;3(4):148–153.
- Matsubara VH, Leong BW, Leong MJ, et al. Cleaning potential of different air abrasive powders and their impact on implant surface roughness. Clin Implant Dent Relat Res. 2020;22(1):96–104.
- Cochis A, Fini M, Carrassi A, et al. Effect of air polishing with glycine powder on titanium abutment surfaces. Clin Oral Impl Res. 2013;24(8):904–909.
- Schwarz F, Ferrari D, Popovski K, et al. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009;88(1):83–91.
- Tastepe CS, Liu Y, Visscher CM, et al. Cleaning and modification of intraorally contaminated titanium discs with calcium phosphate powder abrasive treatment. Clin Oral Implant Res. 2013;24:1238–1246.
- Schmage P, Kahili F, Nergiz I, et al. Cleaning effectiveness of implant prophylaxis instruments. Int J Oral Maxillofac Implants. 2014;29(2):331–337.
- Gehrke SA, Boligon J, Shibli JA. Evaluation of the cleaning and alterations in titanium surfaces with different mechanical instruments using an artificial calculus. Infection. 2014;2:4.
- Schmage P, Thielemann J, Nergiz I, et al. Effects of 10 cleaning instruments on four different implant surfaces. Int J Oral Maxillofac Implant. 2012;27:308–317.
- Ntrouka VI, Slot DE, Louropoulou A, et al. The effect of chemotherapeutic agents on contaminated titanium surfaces: a systematic review. Clin Oral Implants Res. 2011;22(7):681–690.