Abstract
After air pollution, the most important environmental factor threatening human health is noise. Physiologic damage or psychological damage can occur because of noise. Increased oxidative stress, vascular changes and mechanical trauma may be responsible for the physiologic damage mechanism of noise. This study aimed to reveal the effect of noise on follicles in ovaries. In the study, a total of 30 female Wistar albino rats (12 weeks old) were randomly divided into three groups (n = 10): the control group, no treatment; the sham group, exposed to stress conditions (but not to any high waves) in a special room at the same time as the high sound wave group; and the high sound wave (HSW) group, exposed to HSW in a special room for 28 days. At the end of the 28th day, the rats were sacrificed and ovarian tissues were harvested. Stereologic and biochemical analyses were performed. All stereologic volumetric parameters and Gonadal Somatic Index (GSI) values, lipid peroxidation (LPO) and glutathione (GSH) levels in all groups were evaluated statistically, and significant differences were found between the control and HSW, sham and HSW groups, respectively. However, no statistical difference was found between the control and sham groups. A decrease in catalase (CAT) and superoxide dismutase (SOD) activities were found in the HSW group compared with the control and sham groups, suggesting that noise can cause oxidative stress and damage to the ovaries.
Introduction
Noise is considered as the second most important environmental factor threatening human health after air pollution. Many studies have reported that noise negatively affects the function of many tissues and organs by causing stress in living things [Citation1–5]. It can occur in the form of noise, physiologic damage or psychological damage. The physiologic damage mechanism of noise is not yet fully understood, but research has shown that factors such as increased oxidative stress, vascular changes and mechanical trauma may be responsible [Citation6–8].
The most important of the negative effects of noise on living things is the effect on hearing. Noise can cause hearing loss at various levels depending on the duration and level of the sound. It is reported that people with hearing loss are mostly those who live in noisy places or work in noisy workplaces [Citation4]. The hearing system is constantly active, even during sleep. Rapid and excessive stimuli caused by noise signals are subcortically linked to the hypothalamic-pituitary-adrenal axis (HPA-axis) via the amygdala. Therefore, noise causes the release of different stress hormones [e.g. corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH)], especially in people who are asleep [Citation9]. In this context, cytoplasmic steroid (cortisol) receptors acting with heat-shock proteins (HSP) in the most functional parts of the organism such as the heart, kidney and gonads change the behavior of functional tissues. These HSPs, which provide information about the biologic effect of environmental stress on organisms, are also increased by different stress factors. In the myocardial tissue of birds exposed to high noise, HPS70 was recently found to be increased [Citation10].
It has been shown that noise pollution has effects on cardiovascular diseases and hypertension in people living in noisy areas [Citation1]. Noise pollution increases the risk of cancer [Citation2]. It is known that noise decreases performance; causes sleep irregularities, an increase in noradrenaline and adrenaline and cortisol levels, anxiety, headache, and nausea; decreases children’s learning level, physical and cognitive abilities, and motivation; and causes psychological disorders [Citation5]. In experimental studies on rats, it has been found that noise has a reducing effect on neurotransmitters in the brain [Citation3].
The present study was conducted to determine whether noise can cause oxidative stress and investigate the effect of oxidative stress caused by noise on follicles in ovaries.
Materials and methods
Ethics statement
This study was approved with the consent of Ondokuz Mayıs University Experimental Animal Ethics Committee and all experimental procedures were performed in accordance with ethical rules.
Animals
A total of 30 twelve-week-old female Wistar albino rats (210–250 g) were obtained from Ondokuz Mayıs University Experimental Animal Application and Research Center. The rats were cared for and fed in standard sterile plastic cages at 55–60 °C humidity, 19–22 °C room temperature and 12 h light and dark cycles. All rats had unlimited access to feed and tap water ad libitum.
Experimental design
A total of 30 female Wistar albino rats were randomly divided into three groups as Control, Sham and HSW (n = 10). Each rat was placed in a separate cage. No procedure was applied to the rats in the control group. In the SHAM group, a loudspeaker was placed above the center of each cage, but the rats were not exposed to noise. The noise level inside the rooms of these two groups of rats did not exceed 60 db SPL.
The rats in the HSW group were housed in a separate room at the animal laboratory facility, with one rat in each cage. The rats were exposed to octave band noise of 102 dB SPL (±1.5 dB) centered at 50 kHz for 4 h/day for 28 consecutive days. The noise exposure was delivered from a loudspeaker mounted above the center of each cage. Sound levels were measured at various locations within each cage at the height of the animals’ head using a sound level meter (Landtek SL5868P, China).
On the 28th day of the experiment, a vaginal smear was performed to check the estrus cycle of the animals in all groups and the data were recorded. At the end of the 28th day, all animals were weighed and the data compared with the initial results. After exposure to ketamine hydrochloride (40–50 mg/kg; Alfazyne®, Egevet, Turkey), all rats were sacrificed with 4% formaldehyde perfusion through the heart at room temperature. Ovarian tissues were removed and placed in 4% neutral buffered formalin fixation solution. Tissues were subjected to routine histologic procedures after fixation and then embedded in paraffin wax. Stereologic analyses were performed on the obtained tissue sections.
Calculation of gonadal somatic index (GSI)
The GSI was used as an indicator of the cyclic status of the animals. With this index, the maturation of Grafian follicles and a decrease in acyclic animals compared with their cyclic counterparts were shown. GSI was calculated using the formula below [Citation11]:
GSI= (gonadal weight/body weight) × 100
Stereologic analysis
The section thickness of the stereologic approach was estimated according to the pilot study. Therefore, 10-μm section thickness was used, then the sections were stained with hematoxylin-eosin for histologic examination and stereological analysis. Stereologic and histopathologic evaluations were performed using an Olympus BH 40 camera attachment on a light microscope. Ovarian volume, cortex/medulla ratio, and follicle volumes were estimated using the Cavalieri method, which is one of the unbiased stereologic approaches.
Cavalieri method
Regarding the volumetric estimations, the Cavalieri method, which is a branch of stereology, was used [Citation12]. Sections were collected according to systematic random sampling. After that, the surface areas of the relevant structures were estimated using pointed counting grids. Finally, volumetric data were obtained by multiplying the total surface area and average cross-sectional thickness. The pointed counting grid consists of systematic (+) signs representing points separated at equal intervals from each other were used. The area occupied by a point is known in the point counting grid and is called the area associated with the point and shown as [a (p)]. In this context, the points falling on the area of interest are counted and the total number (Σp) is obtained. The obtained numbers are then multiplied by a (p) to obtain surface area (A) measurements for ovarian volume, cortex/medulla ratio, and follicle volumes [Citation13].
The following formula was used during the calculations:
A = Σp · [a(p)]
Biochemical analysis
Tissue samples were harvested (200 mg) from each animal and homogenized in an appropriate buffer solution (4800 mL). Catalase (CAT), superoxide dismutase (SOD), lipid peroxidation (LPO) and glutathione (GSH) levels were assessed using tissue homogenates. The CAT test was performed using hydrogen peroxide (H2O2) at 240 nm. SOD measurements were performed using the method of Sun et al. [Citation14]. LPO levels were measured using thiobarbituric acid and GSH activity was measured according to the Sedlak and Lindsay method [Citation15].
Statistics
The data were analyzed using the SPSS 21.0 for Mac (IBM Corporation) software. Comparisons between the groups were performed using one-way analysis of variance (ANOVA) (Tukey-Post-Hoc). Differences were considered statistically significant at P-values <0.05.
Results
Gonadal somatic index
When the GSI values were evaluated statistically in all groups, it was found that the GSI values of the rats in the HSW group were significantly lower than the control and sham groups (p < 0.05). However, no statistical difference was found between the control and sham groups (p > 0.05) ()
Table 1. Body weight, ovary weight and GSI.
Histopathologic results
The germinal epithelium on the surface of the ovaries of the rats in the control group was observed to be properly located. No abnormal changes in the cortex and medulla were observed. Normal-appearing primordial, primary, secondary and mature follicles were observed in the cortex. The integrity of the corpus luteum was normal. Normally sized blood and lymph vessels were observed in the medullary stroma (C).
Figure 1. Histological images of ovaries in the Control (A, B, C) and HSW (D, E, F) groups. M: medulla; GF: Graafian Follicle; CL: corpus luteum; P: Primary Follicle; S: Secondary Follicle; arrowhead: vascular congestion. Hematoxilin&eosin.
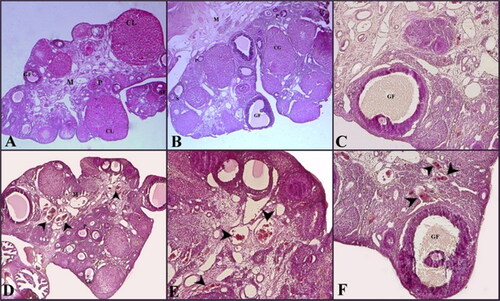
In the germinal epithelium limiting the ovaries of rats in the HSW group, spillages and detachments were observed. Degeneration was seen in the primordial and primary follicles. Congestion in areas with edema and blood vessels in the area associated with the medulla was detected (F).
Stereologic results
There were statistical differences between the control and HSW (p < 0.05), and sham and HSW (p < 0.05) groups, respectively. No statistical difference was found between the control and sham groups (p > 0.05) in terms of all the stereologic volumetric parameters listed below ().
Table 2. Stereological measurements of the relevant parameters.
Biochemical results
Antioxidant enzyme activities
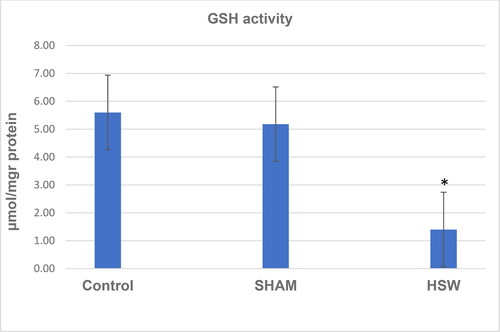
A decrease in CAT activity was found in the HSW group compared with the control (p < 0.05) and sham groups (p < 0.05) (). Although there was no significant difference between the control and sham groups (p > 0.05), SOD activity was found decreased in the HSW group compared with the control (p < 0.05) and sham groups (p < 0.05), respectively (). Similarly, statistical differences were found between the control and HSW (p < 0.05), and sham and HSW (p < 0.05) groups in terms of LPO levels, respectively. However, no statistical difference was found between the control and sham groups (p > 0.05) regarding the LPO levels (). There was also no statistical difference between the control and sham groups (p > 0.05) in terms of GSH levels. By contrast, there were significant differences between the control and HSW (p < 0.05), and sham and HSW (p < 0.05) groups, respectively ().
Figure 2. CAT activity values of rats in all groups. *indicates significant difference compared to the control group (p < 0.05). Note: Control, no procedure was applied to rats; Sham, a loudspeaker was placed in the center of each cage, but the rats were not exposed to noise; HSW, rats were exposed to 102 dB SPL (±1.5 dB) octave band noise centered at 50 kHz.
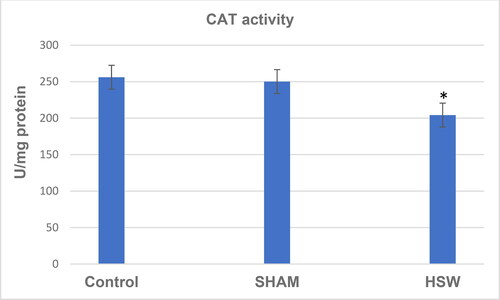
Figure 3. Superoxide dismutase activity (SOD) values of rats in all groups. *indicates significant difference compared to the Control group (p < 0.05). Note: Control, no procedure was applied to rats; Sham, a loudspeaker was placed in the center of each cage, but the rats were not exposed to noise; HSW, rats were exposed to 102 dB SPL (±1.5 dB) octave band noise centered at 50 kHz.
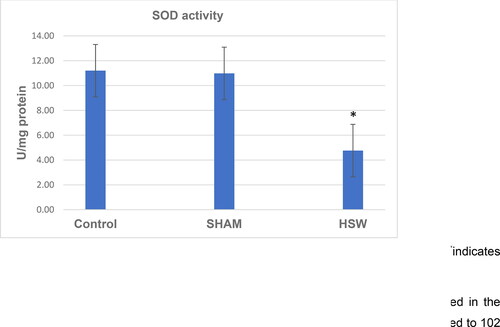
Figure 4. LPO levels in ovarian tissues of rats in all groups. *indicates significant difference compared to the Control group (p < 0.05). Note: Control, no procedure was applied to rats; Sham, a loudspeaker was placed in the center of each cage, but the rats were not exposed to noise; HSW, rats were exposed to 102 dB SPL (±1.5 dB) octave band noise centered at 50 kHz.
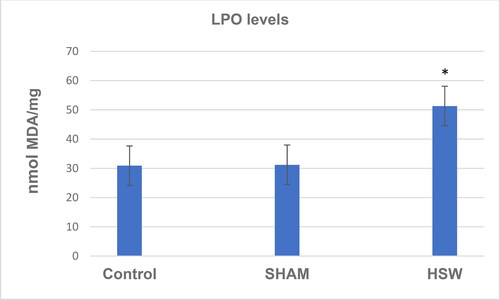
Figure 5. GSH levels in ovarian tissues of rats in all groups. *indicates significant difference compared to the Control group (p < 0.05). Note: Control, no procedure was applied to rats; Sham, a loudspeaker was placed in the center of each cage, but the rats were not exposed to noise; HSW, rats were exposed to 102 dB SPL (±1.5 dB) octave band noise centered at 50 kHz.
Discussion
In this study, we aimed to determine the impact of HSW on gonadal function by comparing gonadal somatic index and oxidative stress markers including multiple parameters such as CAT, SOD, LPO, and GSH levels. Our data analysis revealed significantly lower GSI, CAT, SOD activity and GSH levels, but significantly higher LPO levels in the HSW group. These results led us to conclude that high noise waves resulted in higher oxidative stress, and this increased oxidative stress caused a lower ovarian turnover, which was proved by the lower gonadal index. This result has importance for reproductive function showing a significant impact of noise on reproductive function.
Noise is considered one of the environmental stress factors. Noise exposure leads to the generation of free oxygen radicals [Citation16, Citation17]. Reactive oxygen species (ROS) cause lipid peroxidation by interacting with unsaturated fatty acids, especially in the cell membrane. Lipid peroxidation changes the membrane lipid structure by removing a hydrogen atom from the chain of fatty acids in the membrane structure and indirectly produces reactive aldehydes, and a very harmful chemical chain reaction occurs that damages the structure and functions of other cell components [Citation18, Citation19]. Once this reaction is initiated autocatalytically, it continues in a chain and, if not inhibited, destroys the cell membrane and then breaks down organelles, causing lysosomal enzymes to be released and cellular autolysis.
There are many studies in the literature investigating the effects of noise on various organs such as the brain, liver and spleen [Citation20–23]. However, to our knowledge, there are no studies on the effects of noise on the ovaries of rats. In this study, using biochemical and stereologic methods, we investigated whether noise caused oxidative stress in female rats and thus toxic effects on ovaries.
As noise levels increase and exposure to noise increases, it becomes possible to observe adverse effects on the cardiovascular, gastrointestinal, endocrine and nervous systems [Citation24, Citation25]. The loss of balance between ROS and the antioxidant defense system as a result of the increase in ROS or the decrease in antioxidants leads to oxidative stress and oxidative damage, which have been proven to play a role in the pathogenesis and progression of many diseases [Citation26].
In this study, it was observed that the activities of CAT and SOD antioxidant enzymes were decreased in rats exposed to noise. CAT is an important antioxidant enzyme in the decomposition of hydrogen peroxide into water and oxygen [Citation27]. Many studies on noise have shown that chronic exposure to noise reduces the activities of these enzymes below normal [Citation28–31]. In addition, in the present study, LPO and GSH levels in the ovarian tissues were examined and it was found that the LPO level increased significantly in the HSW group compared with the control group, and the GSH level decreased significantly.
GSH is a powerful reductant and antioxidant molecule, protecting cells against the harmful effects of endogenous and exogenous oxidants [Citation32]. GSH directly reacts with free radicals, preventing oxidative damage by reducing disulfides [Citation33]. In a study investigating the effects of noise on brain tissue, it was determined that the GSH activity in brain tissue decreased [Citation34]. Studies reported that in animals treated with noise, malondialdehyde (MDA) levels, which are accepted as an indicator of free radical damage and result from lipid peroxidation, significantly increased [Citation35, Citation36]. Studies show that noise plays an important role in tissue damage due to the generation of free radical molecules and ultimately oxidative stress [Citation29, Citation37, Citation38]. In line with our study results and the available literature, we can say that noise causes oxidative stress in the ovaries.
In addition, in this study, we estimated the volumes of the ovaries of rats and the volumes of various follicles in the ovaries using a stereologic method. According to the results, the ovary and medulla volumes of rats in the HSW group increased compared with the control group. When the volumes of primary follicles in the HSW and control groups were compared, there was no statistically significant change. The volume of secondary follicles in the ovaries in the HSW group increased compared with the control group, whereas the antrums of the secondary follicles were smaller. In addition, although there was no significant difference in the volumes of Graffian follicles in the ovaries in the HSW group compared with the control group, the volumes of the Graffian follicles’ antrums were significantly decreased. Our stereologic findings suggest that oxidative stress caused by noise in ovarian tissues may be associated with damage to the ovaries.
Several studies have shown that exposure to noise causes changes in hormone levels. For example, increases in norepinephrine, cholesterol, and corticosterone levels have been reported. The increase in stress hormone levels suggests an activation of the HPA axis caused by noise, and this can cause various problems with abnormally high circulating levels of stress hormones [Citation39, Citation40].
Conclusions
The vast majority of HSW studies in the literature indicated that it had harmful effects on human health. However, despite molecular and epidemiologic studies on both experimental animals and humans, the effects of HSW on ovarian tissues related to the female reproductive system is still a topic of discussion. Based on our study results, it may be concluded that noise can cause oxidative stress and damage the ovaries.
Authors’ contributions
Study Design: Savaş Kanbur; Data Collection: Savaş Kanbur; Performing Experimental Steps: Dilek Sağır; Obtaining Data: Dilek Sağır, Savaş Kanbur; Interpretation of Results: Savaş Kanbur; Manuscript Writing: Savaş Kanbur, Dilek Sağır; Final Approval: Savaş Kanbur, Dilek Sağır.
Ethical consideration
This experimental study was performed following approval the Animal Experiments Local Ethics Committee of the Ondokuz Mayis University with the number of 2018-07.
Data availability
All data supporting the findings from this study are available from the corresponding author upon reasonable request
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jarup L, Dudley ML, Babisch W, HYENA Consortium, et al. Hypertension and exposure to noise near airports (HYENA): study design and noise exposure assessment. Environ Health Perspect. 2005;113(11):1473–1478.
- Visser O, Wijnen JHV, Leeuwen FEV. Incidence of cancer in the area around Amsterdam Airport Schiphol in 1988–2003: a population-based ecological study. BMC Public Health. 2005;5:127.
- Ravindran R, Devi RS, Samson J, et al. Noise-stress-induced brain neurotransmitter changes and the effect of ocimum sanctum (Linn) treatment in albino rats. J Pharmacol Sci. 2005;98(4):354–360.
- Abbate C, Concetto G, Fortunato MO, et al. Influence of environmental factors on the evolution of industrial noise-induced hearing loss. Environ Monit Assess. 2005;107(1–3):351–361.
- Stansfeld SA, Matheson MP. Noise pollution: non-auditory effects on health. Br Med Bull. 2003;68:243–257.
- Mason JO, McGinnis JM. Overview of national health promotion and disease prevention goals. Public Health Reports. 1990;105(5):441.
- Evans G, Bullinger M, Hygge S. Chronic noise exposure and physiological response: a prospective study of children living under environmental stress. Psychol Sci. 1998;9(1):75–77.
- Seidman MD, Standring RT. Noise and quality of life. Int J Environ Res Public Health. 2010;7(10):3730–3738.
- Spreng M. Possible health effects of noise induced cortisol increase. Noise Health. 2000;2(7):59–64.
- Hoekstra KA, Iwama GK, Nichols CR, et al. Increased heat shock protein expression after stress in Japanese quail. Stress. 1998;2(4):265–272.
- Walker DM, Kermath BA, Woller MJ, et al. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154(6):2129–2143.
- Onger ME, Kaplan S, Deniz OG, et al. Possible promoting effects of melatonin, leptin and alcar on regeneration of the sciatic nerve. J Chem Neuroanat. 2017;81:34–41.
- Onger ME, Gocer H, Cirakli A, et al. Effect of electromagnetic wave on bone healing in fixed and unfixed conditions. J Craniofac Surg. 2016;27(6):1606–1608.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and non-protein sulfhydryls groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25(1):192–205.
- Trimarchi JR, Liu L, Porterfield DM, et al. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62(6):1866–1874.
- Pınar T, Atlı K, Alacam H, et al. The effects of nois on oxidative and antioxidative balance in human erythrocytes. UHOD. 2011;21(1).
- Basaga HS. Biochemical aspects of free radicals. Biochem Cell Biol. 1990;68(7–8):989–998.
- Cighetti G, Duca L, Bortone L, et al. Oxidative status and malondialdehyde in β-thalassaemia patients. Eır J Clin İnvestig. 2002;32:55–601.
- Escabi CD, Frye MD, Trevino M, et al. The rat animal model for noise-induced hearing loss. J Acoust Soc Am. 2019;146(5):3692–3709.
- Meng X, Zhang X, Su Y, et al. Hyperbaric oxygen treatment mitigates liver damage in mice with noise exposure. Clin Exp Pharmacol Physiol. 2020;47(9):1564–1574.
- Hayes SH, Manohar S, Majumdar A, et al. Noise-induced hearing loss alters hippocampal glucocorticoid receptor expression in rats. Hear Res. 2019;379:43–51.
- Xue L, Zhang D, Wang T, Xiaokaiti·Yibulayin, et al. Effects of high frequency noise on female rat’s multi-organ histology. Noise Health. 2014;16(71):213–217.
- N A. Ear diseases and microsurgery. Vol. 1. Ankara; Ongun Printing House; 1980. p. 587–598.
- Ersoy A, Sahin ER, Duzgun S, et al. Possible effects of rosuvastatin on noise-induced oxidative stress in rat brain. Noise Health. 2014;16(68):18–25.
- Kargın F, Fidancı UR. Antioxidative metabolism in dogs with kidney disease Turk. J Vet Anim Sci. 2001;25:607–613.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed.New York: Oxford University Press; 1999. p. 246–351.
- Samson J, Sheeladevi R, Ravindran R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology. 2007;28(3):679–685.
- Manikandan S, Devi RS. Antioxidant property of alpha-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res. 2005;52(6):467–474.
- Manikandan S, Srikumar R, Parthasarathy N, et al. Protective effect of Acorus calamus LINN on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol Pharm Bull. 2005;28(12):2327–2330.
- Demirel R, Mollaoğlu H, Yeşilyurt H, et al. Noise induces oxidative stress in rat. Eur J Gen Med. 2009;6:2024.
- Ashok BT, Ali R. Free radical theory of aging. Exp Gerontol. 1999;34(3):293–303.
- Meister A. Glutathione ascorbate and cell cycle regulation. FEBBS Lett. 1994;47:1–4.
- Samson J, Devi RS, Ravindran R, Senthilvelan M. Effect of noise stress on free radical scavenging enzymes in brain. Environ Toxicol Pharmacol. 2005;20(1):142–148.
- Dehghani A, Ranjbarian M, Khavanin A, et al. Exposure to noise pollution and its effect on oxidant and antioxidant parameters in blood and liver tissue of rat. Zahedan J Res Med Sci. 2013;15(5):13–17.
- Yeşilyurt H. Investigation of oxidative changes induced by noise stress, 2008.
- Van Campen LE, Murphy WJ, Franks JR, et al. Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res. 2002;164(1–2):29–38.
- Srikumar R, Parthasarathy NJ, Manikandan S, et al. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem. 2006;283(1–2):67–74.
- Turner JG, Parrish JL, Hughes LF, et al. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med. 2005;55(1):12–23.
- Castelhano-Carlos MJ, Baumans V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress, 2009.
