Abstract
Cancer stem cells are currently considered an important cause of tumoral heterogeneity, treatment failure and recurrence. SALL4 is a marker of stemness in many types of human cancers. In the present study, the expression of SALL4 was analysed in oral squamous cell carcinomas (OSCCs) using immunohistochemistry. This expression was linked to clinical and pathological characteristics of OSCC patients. Using tissue microarrays of OSCCs, a significant majority of these tumours were shown to positively express SALL4 protein in their cytoplasm and nuclei. This expression positively correlated with tumour grade and tumour location within the oral cavity. Furthermore, SALL4 expression was correlated with aberrant expression of beta-Catenin (AEB) in OSCCs. Morphologic evidence presents significant overlap between cells expressing the two proteins. Given the above, and in light of past evidence of SALL4 modulating the Wingless/Wnt/beta-Catenin pathway in other tumours, this study presents a possible role for the SALL4 protein in OSCC development and progression, and asserts the need for further studies into that role.
Introduction
Head and neck squamous cell cancer (HNSCC), of which oral squamous cell cancer is the most common, is one of the top ten most common malignancies in the world [Citation1,Citation2]. World-wide, this tumour has 5-year survival rates of less than 50%, with persistent treatment failures and frequent cases of relapse and recurrence [Citation1,Citation2]. This dismal prognosis is caused by locoregional recurrence at the primary site, late presentation, metastases in neck lymph nodes and low response to current treatment modalities [Citation3]. One of the causes of failures of conventional chemo-, radiation and biologic therapies is inter-tumoral heterogeneity resulting in treatment failure and tumour progression [Citation4]. Presence of cancer stem cells (CSCs) is an important cause for this intra-tumoral heterogeneity [Citation4]. While the existence of CSCs in the past was disputed, it has now been confirmed to be present in a number of solid tumours including breast, brain, lung, colon cancers and melanoma [Citation4,Citation5]. CSCs can renew themselves, allowing cancerous tumours an unlimited reserve pool of undifferentiated cells that are able to resist therapy, and mount a second wave of recurrent tumours and metastases [Citation6–9].
Another feature of CSCs is their ability to undergo epithelial-mesenchymal transition (EMT). Snail-induced EMT maintained the CSC-like phenotype in HNSCCs, and enhanced sphere formation capability, chemoresistance and invasive ability [Citation10–13].
Identifying these CSCs and charting their mechanisms of actions is of prime importance in the management of malignant tumours [Citation14]. Since stem cells are not present in most adult tissues, they could be ideal targets for cancer-specific diagnosis and treatment [Citation15].
SALL4 is a C2H2-zinc finger transcription factor, similar to other stem cell factors such as SOX2, OCT4 and NANOG [Citation16]. SALL4 is known to be a marker of cancer stem cells [Citation17]. It plays a vital role in protecting the properties of embryonic stem cells, and regulates cell growth, proliferation and apoptosis in embryonic stem cells and cancer stem cells [Citation17,Citation18]. SALL4 expression is silenced in differentiated tissues [Citation15]. There is evidence to suggest that a resurgence of SALL4 expression later in adult life could be associated with malignant transformation in different tissues [Citation19]. This protein is also recognized as a potential biomarker for assessing cancer prognosis. A high expression of SALL4 is significantly associated with increased overall cancer mortality and recurrence [Citation19,Citation20]. Knocking down SALL4 resulted in the inhibition of tumour formation in xenograft tumour models [Citation21]. The levels of expressions of this protein in the serum and tissues correlated with lymph node metastases, differentiation degree and tumour stage [Citation22]. Furthermore, SALL4 was shown to induce the acquisition of properties of the EMT phenomenon and to promote invasion and metastases in endometrial cancer cells [Citation23]. SALL4-knockdown restored chemotherapy sensitivity, reversed EMT and diminished cell metastases and suppressed the downregulation of E-cadherin and the upregulation of N-cadherin [Citation23].
In the head and neck region, SALL4 has been shown to modulate the effect of radiation therapy on nasopharyngeal tumour cells, where inhibition of the SALL4 gene reduced proliferation, sensitized cells to radiation both in-vitro and in-vivo, increased radiation-induced DNA damage, increased apoptosis of tumour cells and resulted in tumour cell cycle arrest [Citation24]. While expression of SALL4 in oral squamous cell cancers has been reported previously [Citation25–27], quantifying that expression, correlating it with clinicopathologic characteristics and analysing its functional properties remain largely uncharted [Citation18].
Functional deregulation of the Wnt signalling pathway can promote oral cancer development and progression [Citation28]. Beta-Catenin, a known downstream target, and a key component of the canonical Wingless/Wnt signalling pathway [Citation29], may have a role in the stemness of cancer cells, when it interacts with reprogramming genes KIf4, Oct4 and Sox2 [Citation30]. This interaction will further enhance the expression of SALL4 during the induction of a somatic cell, and reprogramming it into a pluripotent stem cell [Citation30]. However, little is known about the expression of SALL4 and beta-Catenin, and their relationship in oral squamous cell cancer (OSCC).
Considering the above, this study analysed the expression of the SALL4 and beta-Catenin proteins and assessed any possible correlations and interactions in OSCC.
Materials and methods
Ethics statement
The study was approved by the Institutional Review Board (IRB) of the College of Medicine at Alfaisal University.
OSCC tissues
Following approval of the Institutional Review Board (IRB) of the College of Medicine at Alfaisal University, two commercial human oral microarrays (Catalogue no. OR601c, Biomaxc, US, Rockville, Maryland), with 50 cases of oral squamous cell carcinomas (OSCCs) and ten cases of non-neoplastic tissue, each, were used for this study. All cases were confirmed OSCCs. Major parameters of these tumours include age, sex, anatomic location within the oral cavity, pathologic diagnosis and grade.
Immunohistochemistry (IHC)
A rabbit polyclonal antibody against SALL4 (ab 29112; Abcam, UK), and a rabbit polyclonal antibody against beta-Catenin (ab 16051, Abcam, UK) were diluted in antibody diluent (Agilent, Santa Clara, California) per manufacturer’s instructions; and applied to 5-mm sections from formalin-fixed, paraffin-embedded tissue specimens, using the avidin-biotin peroxidase method (Vectastatin Elite ABC kit; Vector Laboratories, Burlingame, CA), following the manufacturer’s instructions. The immunohistochemical (IHC) stain was performed manually at room temperature. Negative controls were used with omission of primary antibody. Separate positive controls of mixed tumour (for SALL4) and appendix (for beta-Catenin) were used for test optimization and run validation.
Staining evaluation
Immunohistochemical staining of both markers, for all tumours, were evaluated by two pathologists (AO) and (ER), independently, and a consensus was reached for discordant cases. All tissues, normal controls, tissues adjacent to cancer or malignant tissue, were compared with the staining of the positive control. The degree of SALL4 positivity was scored by applying a semi-quantitative immunoreactivity scoring (IRS) system as described by Baccelli and co-workers [Citation31]. The staining intensity was categorized into four grades: 0, no immunostaining; 1, weak staining (light yellow); 2, moderate staining (buffy); and 3, strong staining (brown). The percentage of positive cells was categorized into five grades: 0 (0%); 1, (1–10%); 2 (11–50%); 3 (51–80%); and 4 (>80%). The staining intensity and percentage of positive cells were multiplied to obtain an IRS for SALL4 expression, in the range of 0–12 for each individual case [Citation31]. In line with studies done in other tissues [Citation16,Citation32], a case was scored as positive for SALL4 with an IRS of ≥3 defined as positive expression.
Beta-Catenin immunostaining was assessed using a previously described scoring method [Citation33,Citation34]. Staining was evaluated semi-quantitatively by comparing the intensity and cellular localization of the positive signals with those in the adjacent normal epithelium as an internal positive control. Staining was then recorded as normal or abnormal: Normal described uniform membranous staining, strong and similar to that in the internal controls. Partial loss (mixed areas of positive and negative cells with normal membranous staining) or complete loss (uniformly negative) of normal membranous staining, or altered cellular distribution in the cytoplasm and/or nucleus were defined as abnormal [Citation33,Citation34].
Statistical analysis
The χ2 test was used to compare SALL4 expression and oral squamous cell cancer (N = 50). The χ2 test was also used to examine the relationship between SALL4 positive cases (N = 37) and various clinical, demographical and pathological criteria (gender, age, tumour grade and tumour location). Furthermore, the χ2 test was used to examine the relationship between beta-Catenin positive cases (N = 43) and tumour grade and location within the oral cavity; and to analyse the correlation between the two markers in cancer cells. All statistical analyses were performed with IBM SPSS Statistics software package, version 25.0. p < 0.05 was considered to indicate a statistically significant difference.
Results
Two copies of the human microarray (Catalouge no. OR601c, Biomax, US, Rockville, MD) with cases of oral squamous cell carcinomas were used for this study. The age of the cancer patients in this array was in the range of 35 to 80, with a median of 53 years. The OSCC grades were distributed as follows: 27 patients are grade I; 19 patients are Grade II and 4 patients are grade III. The group comprised of 17 females and 33 males. The tumours included SCCs from the tongue (n = 31), the bucca cavioris (n = 8), gum (n = 4), lip (n = 4), face (n = 1), jaw (n = 1) and oral cavity (n = 1). Non-malignant cases in the microarray (n = 10), were all from the oral cavity, and consisted of 7 from cancer-adjacent normal tongue tissue and 3 from non-malignant mucous membrane of the maxilla. The expression of SALL4 in normal epithelial mucosal oral cells was negative in all normal samples (IRS#0-2). In OSCC cases, on the other hand, 37/50 cases were positive for SALL4 (74% with an IRS of ≥3, χ2 = 11.52; p = 0.001, ). The rest of the OSCC cases (13 in total) had weak or negative SALL4 expression (IRS# 0-2). All of the OSCC cases included in this study (37/37, 100% of cases) expressed SALL4 in the cytoplasm and nuclei of the cells (Arrow heads, A–C, ). The expression of beta-Catenin in normal oral mucosa was membranous and weak in intensity (). The majority of OSCC cases had partial loss of membranous staining (thin arrows, ), with abnormal expression of beta-catenin in the cytoplasm and nuclei of those cells (Arrow heads, ).
Figure 1. SALL4 Expression in OSCC cases. (A, B) SALL4 Heavy cytoplasmic and nuclear staining in a grade 2 OSCC tumour arising in the bucca cavioris (Magnification: X20 (A), X10(B)). (C) Mild Membranous SALL4 staining with few cells exhibiting cytoplasmic staining in a grade 1 OSCC of the lower lip (Magnification X20)
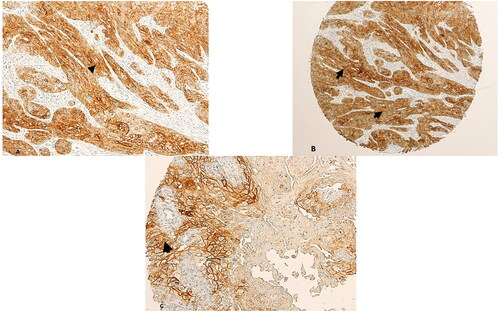
Figure 2. Expression of beta-Catenin in normal oral squamous mucosa. The expression is membranous and weak in intensity.
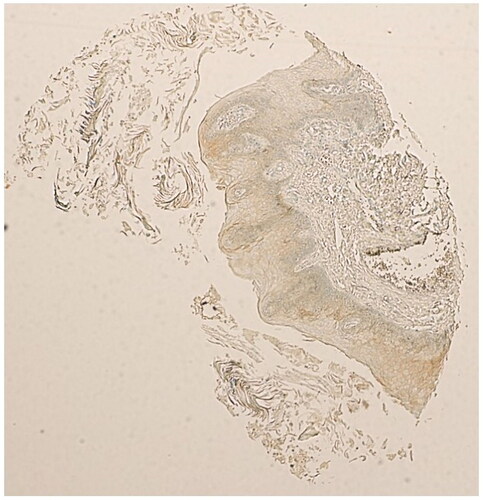
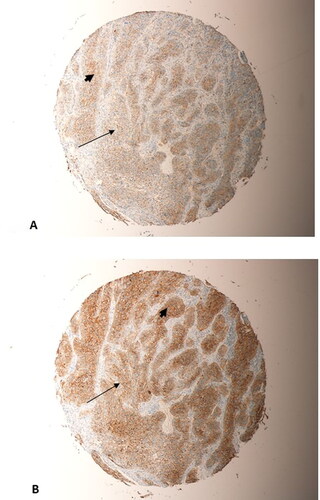
Figure 3. Beta-catenin expression in OSCC cases. (A) Partial loss of of membranous expression of beta-Catenin (thin arrow), in a grade 1 squamous cell carcinoma arising in the tongue (Magnification X10). (B) Partial loss of of membranous expression of beta-Catenin (thin arrow), in a grade 1 OSCC arising in the gum (Magnification X10). (C) Complete loss of membranous staining of beta-Catenin, replaced by heavy nuclear and cytoplsmic staining (arrow heads), in a grade 1 OSCC arising in the gum (Magnification X20). (D) Complete loss of membranous staining of beta-catenin (arrow heads), replaced by heavy nuclear and cytoplsmic staining (arrow heads), in a grade 1 OSCC arising from the gum (Magnification X20).
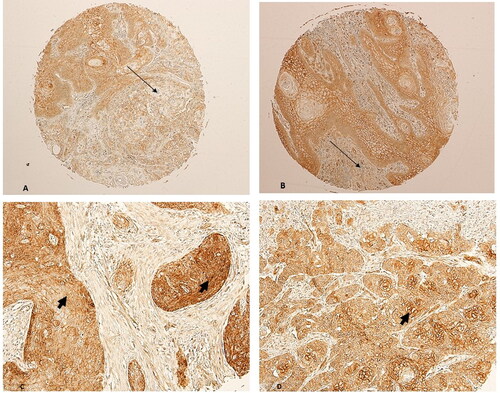
Table 1. SALL4 expression in OSCC and its significance.
Association between SALL4 immunohistochemical expression and clinic-pathological parameters of OSCCs
The association between SALL4 expression and clinical-pathological parameters was further analysed in the OSCC cases (). Positive expression for SALL4 was significantly correlated with OSCC grade (χ2 = 11.243; p = 0.004). SALL4 expression also correlated with the OSCC location within the mouth. (χ2 = 65.351; p < 001). There was no significant association between positive staining for SALL4 and other clinicopathological parameters, including age (p > 0.05) and sex (p > 0.05) ().
Table 2. SALL4 positive OSCC cases clinical-pathological characteristics.
Association between beta-Catenin immunohistochemical expression and OSCCs grade and location within the oral cavity
The association between beta-Catenin expression and clinical-pathological parameters was further analysed in the OSCC cases (). Positive staining for beta-Catenin was significantly correlated with tumour grade (χ2= 13.29; p = 0.001). Beta-Catenin expression also correlated with the OSCC location within the mouth (χ2= 68; p < 0001).
Table 3. Beta-Catenin positive OSCC cases clinical-pathological characteristics.
Association between SALL4 and beta-Catenin
OSCC cases with positive expression for SALL4 were significantly associated with aberrant expression of beta-Catenin (84%, χ2 = 16.892; p < 0.001, ). The aberrant expression of beta-Catenin in those cases was in the form of either loss of membranous expression, or a loss of membranous expression with a switch to cytoplasmic or nuclear expression (Arrow heads A–D).
Table 4. Correlation of SALL4 and beta-Catenin in OSCC cases.
In addition to the above, a strong morphological correlation could be clearly seen between the expression of SALL4 and beta-Catenin, when compared between tumours from the same positions on the array. Sections 4 A, 5 A, 6 A and 7 A were all stained with beta-Catenin, whereas their counterparts 4B, 5B, 6B and 7B were stained with SALL4. More cells expressed SALL4 (thick arrows, and ) than beta-Catenin, however, all the cells that expressed beta-Catenin also expressed SALL4 (thin arrows, ). This overlap is clearly evident, despite the higher intensity of SALL4 expression than beta-Catenin. The expression of both markers was predominantly in the cytoplasm and nuclei of the tumour cells (Arrow heads, and ).
Figure 4. Two representative sections taken from the same tumour, a grade 2 OSCC from the tongue, on the microarray. (A) beta-Catenin staining; (B) SALL4 staining. More cells have stained with SALL4 than with beta-Catenin (thick arrows, (B)). However, all cells staining with beta-Catenin also stained with SALL4 (thin arrows, (A) and (B)). (Magnification X10).
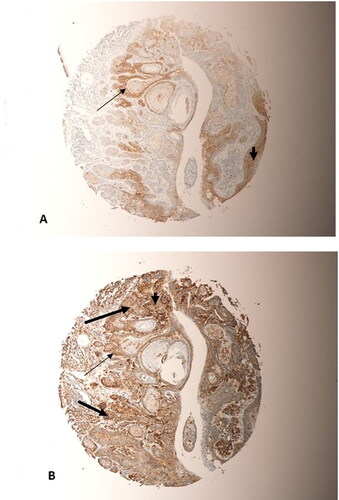
Figure 5. Two representative sections taken from the same tumour, a grade 2 OSCC from the Bucca cavioris, on the microarray. (A) beta-Catenin staining; (B) SALL4 staining. More cells have stained with SALL4 than with beta-Catenin (thick arrow, (B)). However, cells staining with beta-Catenin were also stained with SALL4 (thin arrows, (A) and (B)). Both markers has predominant nuclear and cytoplasmic staining of tumour cells (arrow heads, (A) and (B)). (Magnification X10).
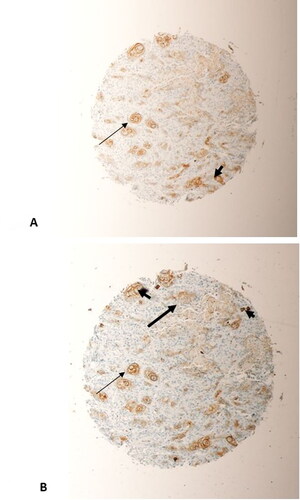
Figure 6. Represents two sections taken from the same tumour, a grade 2 OSCC from the jaw, on the microarray. (A) beta-Catenin staining; (B) SALL4 staining. Significant overlap between SALL4 and beta-Catenin in their staining of tumour cells (thin arrows, (A) and (B)) could be seen. Tumour cells predominantly express SALL4 and beta-Catenin in their nuclei and cytoplasm (arrow heads, (A) and (B)). (Magnification X10).
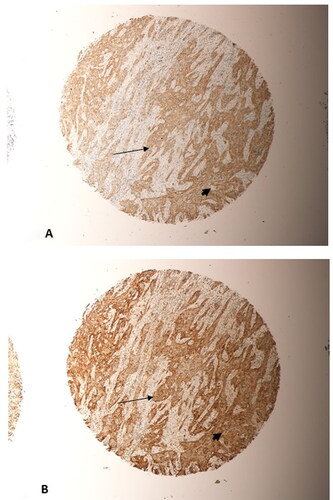
Figure 7. Represents two sections taken from the same tumour, a grade 2 OSCC from the tongue, on the microarray. (A) beta-Catenin staining; (B) SALL4 staining. Significant overlap in the expression of SALL4 and beta-Catenin in the same tumour cells could be seen (thin arrows, (A) and (B)). Predominanty nuclear and cytoplasmic expression in the tumour cells for both markers (arrow heads, (A) and (B)) could be seen. (Magnification X10).
Discussion
In this study, OSCC cells exhibited positive expression of Sal-like protein 4 (SALL4). SALL4 expression was significantly correlated with the grade of the OSCC cases and with location of these tumours within the oral cavity. While SALL4 expression was reported in oral cancer tissues in past work [Citation25–27], none of the above studies quantified the expression of this protein in oral cancer tissues, analysed it across the clinical and pathological characteristics of patients, or linked it with the Wingless/Wnt pathway, a major pathway in oral tumorigenesis.
SALL4 protein is a master regulator which contributes to cell stemness in biological development and tumour growth [Citation17]. A cancer stem cell is currently an accepted explanation for the poor survival rates seen in cancer in general, and in oral squamous cell carcinomas in particular. The 5-year survival rates of oral cancer range between 15 and 50% [Citation1,Citation2]. Following initial treatment and cancer cell death, cancer stem cells provide a reserve pool, which may give rise to newly formed cancer cells, thus resurrecting the tumour (Satpute et al. 2013). The role of this sturdy group of cells goes beyond recurrence, and they are possibly involved in resisting conventional therapies, and metastases [Citation35–39]. Identifying these cancer stem cells and analysing their properties are proving to be promising tools in the management of cancer [Citation4].
SALL4 plays, initially, a central role in embryo-fetal development. SALL4 expression is then silenced in differentiated tissues. Evidence suggests that resurgence of SALL4 expression later in adult life is seen in several cancers; worsening the prognosis [Citation19,Citation20]. Levels of expressions in serum and tissues of the SALL4 protein highlighted a correlation with lymph node metastases, differentiation and tumour stage [Citation22]. SALL4 has been shown to be over-expressed in many other tumours, including breast lung cancer (74.5% of cancer tissue at the mRNA level) [Citation40], gastric cancer (two-fold or higher increase in 79.2% cancer tissue at the mRNA level) [Citation41] and oesophageal squamous cell cancer (three-folds increased mRNA expression in 62% of cancer tissue when compared to normal) [Citation42].
This study also found correlation between the expression of SALL4 and beta-Catenin. Beta-Catenin is a critical element of the Epithelial-Mesenchymal Transition (EMT) Phenomenon, and a key player in the canonical Wnt molecular pathway; an important process in oral carcinogenesis. Aberrant beta-Catenin expression (ABE) in the cytoplasm and nuclei, as seen in the OSCC cells of this study, represents the activated state of beta-Catenin [Citation43,Citation44].
The dual expression of the two proteins by OSCC cells, shows the close relationship between stemness, of which SALL4 is a representative, and the EMT process represented by beta-Catenin. Interestingly, knockdown of SALL4 resulted in reversal of the EMT phenomenon and reduced cellular invasion and metastases elsewhere in the body [Citation23]. Other aspects of the interaction of the two proteins exist. In a normal state, SALL4 is directly regulated by the canonical Wnt signalling pathway, because within a minimal SALL4 promoter region of 31 bp exists a consensus TCF/LEF-binding site [Citation45]. Targeting SALL4 in oesophageal squamous cell cancer cells resulted in decreased tumorigenicity involving EMT, through modulation of the Wnt/beta-Catenin pathway [Citation46]. Furthermore, knockdown of SALL4 decreased the protein expression of Wnt3 and beta-Catenin in osteosarcoma cells [Citation47]. And finally Chen and co-workers have shown that SALL4 may promote cell growth and cervical cancer development by upregulating the activity of the Wnt/beta-Catenin signalling pathway by directly binding to the CTNNB1 and trans-activating it [Citation48]. The above, taken collectively, with evidence presented in this current study, may provide a platform to further explore the nature of the relationship between the two proteins in OSCC.
The versatility of SALL4, a master regulator of many downstream targets such as the SALL4/OCT4 loop [Citation49], BMI-1 [Citation50], HOXA9 [Citation51], PTEN [Citation52], genes regulating apoptosis [Citation53], MYC [Citation54], Sonic Hedgehog (SHH) pathway [Citation53], EpCAM [Citation55] and EMT related factors in cancers [Citation23], adds more emphasis on the need to explore further the possible role of this protein in OSCC development and progression.
Conclusions
In summary, this study provided evidence of SALL4 positive expression in OSCC and correlated this expression with the tumour grade and location within the oral cavity. Furthermore, SALL4 expression correlated with that of beta-Catenin. This positive expression and the correlations presented, may shed some light on a possible role of this protein in oral carcinogenesis, and the possibility that it may be used in the diagnosis, prognosis and treatment of oral cancer.
*Funding
No funding was received for this project.
Acknowledgements
*The author would like to thank Professor Emad Raddaoui, consultant and senior pathologist at King Khaled University Hospital, King Saud University, Riyadh, Saudi Arabia, for his help in the immunohistochemical scoring of this research work. He also would like to thank Dr Ko Jin Quek, Faculty of Biomedical Sciences, Macquarie University, Sydney NSW, Australia; for her help with the statistical analysis of this work.
*Disclosure statement
The author wishes to report no conflict of interest.
*This is to acknowledge that no financial interest or benefit that has arisen from the direct applications of this research.
Data availability statement
The data that support the findings of this study are available from the corresponding author, (AO), upon reasonable request.
References
- Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816.
- McCullough MJ, Prasad G, Farah CS. Oral mucosal malignancy and potentially malignant lesions: an update on the epidemiology, risk factors, diagnosis and management. Aust Dent J. 2010;55:61–65.
- Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:9.
- Dawood S, Austin L, Cristofanilli M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park). 2014;28(12):1101–1107. 1110
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134.
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337.
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835.
- O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110.
- Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–765.
- Grünert S, Jechlinger M, Beug H, et al. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–665.
- Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321.
- Ota I, Masui T, Kurihara M, et al. Snail-induced emt promotes cancer stem cell-like properties in head and neck cancer cells. Oncol Rep. 2016;35(1):261–266.
- Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6(2):103–115.
- Barbato L, Bocchetti M, Di Biase A, et al. Cancer stem cells and targeting strategies cells. Cells. 2019;8(8):926.
- Tatetsu H, Kong NR, Chong G, et al. SALL4, the missing link between stem cells, development and cancer. Gene. 2016;584(2):111–119.
- Hao L, Y, Zhao Y, Wang Z, et al. Expression and clinical significance of SALL4 and b-catenin in colorectal cancer. J Mol Hist. 2016;47(2):117–128.
- Xiong J. SALL4: engine of cell stemness. Curr Gene Ther. 2014;14(5):400–411.
- Dirican E, Akkiprik M. Functional and clinical significance of SALL4 in breast cancer. Tumour Biol. 2016;37(9):11701–11709.
- Nicolè L, Sanavia T, Veronese N, et al. A systematic review with meta-analysis. Oncotarget. 2017;8(14):22968–22979.
- Shen H, Li L, Wang D, et al. Higher expression of SALL4 predicts poor cancer prognosis: a meta-analysis. Cancer Biomark. 2017;19(4):365–373.
- Yong KJ, Gao C, Lim JSJ, et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013;368(24):2266–2276.
- Wu H, Liu C, Fan X, et al. Spalt-like transcription factor 4 as a potential diagnostic and prognostic marker of colorectal cancer. CBM. 2017;20(2):191–198.
- Liu L, Zhang J, Yang X, et al. SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS One. 2015;10(9):e0138515.
- Nie X, Guo E, Wu C, et al. SALL4 induces radioresistance in nasopharyngeal carcinoma via the ATM/Chk2/p53 pathway. Cancer Med. 2019;8(4):1779–1792.
- Featherston T, Yu HH, Dunne JC, et al. Cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma express components of the renin-angiotensin system. Front Surg. 2016;3:52.
- Ram R, Brasch HD, Dunne JC, et al. The identification of three cancer stem cell subpopulations within moderately differentiated lip squamous cell carcinoma. Front Surg. 2017;4:12.
- Yu HH, Featherston T, Tan ST, et al. Characterization of cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma. Front Surg. 2016;3:46.
- Gonzalez-Moles MA, Ruiz-Avila I, Gil-Montoya JA, et al. β-catenin in oral cancer: an update on current knowledge . Oral Oncol. 2014;50(9):818–824.
- Doucas H, Garcea G, Neal CP, et al. Changes in the Wnt signalling pathway in gastrointestinal cancers and their prognostic significance. Eur J Cancer. 2005;41(3):365–379.
- Zhang L, Xu Z, Xu X, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33(48):5491–5500.
- Baccelli I, Stenzinger A, Vogel V, et al. Co‑expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget. 2014;5(18):8147–8160.
- Di C, Sun J, Zhang H, et al. High expression of SALL4 is associated with poor prognosis in squamous cell carcinoma of the uterine cervix. Int J Clin Exp Pathol. 2018;11(3):1391–1398.
- Papadavid E, Pignatelli M, Zakynthinos S, et al. Abnormal immunoreactivity of the E-cadherin/catenin (alpha-, beta- and gamma-) complex in premalignant and malignant nonmelanocytic skin tumours. J Pathol. 2002;196(2):154–162.
- Yun X, Wang L, Cao L, et al. Immunohistochemical study of β-catenin and functionally related molecular markers in tongue squamous cell carcinoma and its correlation with cellular proliferation. Oncol Lett. 2010;1(3):437–443.
- Brabletz T, Jung A, Spaderna S, et al. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749.
- Davis SJ, Divi V, Owen JH, et al. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136(12):1260–1266.
- Granit RZ, Slyper M, Ben-Porath I. Axes of differentiation in breast cancer: untangling stemness, lineage identity, and the epithelial to mesenchymal transition. Wiley Interdiscip Rev Syst Biol Med. 2014;6(1):93–106.
- Grosse-Wilde A, Fouquier d’Hérouël A, McIntosh E, et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One. 2015;10(5):e0126522.
- Sun S, Wang Z. Head neck squamous cell carcinoma c-Met cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129(10):2337–2348.
- Kobayashi D, Kuribayashi K, Tanaka M, et al. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncol Rep. 2011;26(4):965–970.
- Zhang P, Chang W-H, Fong B, et al. Regulation of induced pluripotent stem (iPS) cell induction by Wnt/β-catenin signaling . J Biol Chem. 2014;289(13):9221–9232.
- Forghanifard MM, Khales SA, Javdani-Mallak A, et al. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol. 2014;31(4):922.
- Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97(8):4262–4266.
- Lim SC, Lee MS. Significance of E-cadherin/beta-catenin complex and cyclin D1 in breast cancer. Oncol Rep. 2002;9(5):915–928.
- Böhm J, Sustmann C, Wilhelm C, et al. SALL4 is directly activated by TCF/LEF in the canonical wnt signaling pathway. Biochem Biophys Res Commun. 2006;348(3):898–907.
- He J, Zhou M, Chen X, et al. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via Wnt/β-catenin pathway in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35(1):98.
- Zhang D, Jiang F, Wang X, et al. Knockdown of SALL4 inhibits proliferation, migration, and invasion in osteosarcoma cells. Oncol Res. 2017;25(5):763–771.
- Chen M, Li L, Zheng P. SALL4 promotes the tumorigenicity of cervical cancer cells through activation of the Wnt/β-catenin pathway via CTNNB1. Cancer Sci. 2019;110(9):2794–2805.
- Yang J, Gao C, Chai L, et al. A novel SALL4/OCT4 transcriptional feedback network for pluripotency of embryonic stem cells. PLoS One. 2010;5(5):e10766.
- Wang F, Guo Y, Chen Q, et al. Stem cell factor SALL4, a potential prognostic marker for myelodysplastic syndromes. J Hematol Oncol. 2013;6(1):73.
- Gao C, Kong N, Li A, et al. SALL4 is a key transcription regulator in normal human hematopoiesis. Transfusion. 2013;53(5):1037–1049.
- Lu J, Jeong HW, Kong N, et al. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS One. 2009;4:e5577.
- Yang J, Chai L, Gao C, et al. SALL4 is a key regulator of survival and apoptosis in human leukemic cells. Blood. 2008;112(3):805–813.
- Li A, Jiao Y, Yong KJ, et al. SALL4 is a new target in endometrial cancer. Oncogene. 2015;34(1):63–72.
- Oikawa T, Kamiya A, Zeniya M, et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology. 2013;57(4):1469–1483.
