Abstract
We explored the effects of subcutaneously injected Ecballium elaterium (L) A. Rich. (EE) extract on skin wound-healing in rats, as well as the effects on the liver and pancreas. Twenty-eight rats were divided into two groups of 14 each: a saline control group (S) and an EE group. Both groups were divided into two subgroups according to the day of sacrifice (S-7 and S-14, and EE-7 and EE-14). All animals received 2.5 cm skin incisions followed by subcutaneous injections of either saline or 2.5 mg/kg EE per margin (5 mg/kg in total). The S and EE groups were compared in terms of the severity and type of local and neighbouring inflammation, vascularization, fibrosis and effects on the liver and pancreas. In addition, apoptosis and vascularity between S and EE groups were compared immunohistochemically with caspase-3 and cd-34 antibodies. There was no significant difference between the staining rates of caspase-3 and cd-34 in the immunohistochemical assay between the S and E groups. Subcutaneous EE was not toxic to the pancreas or liver; the EE-14 group exhibited less fibrosis than the S-14 group. Therefore, it can contribute to the proper closure of the lesion by reducing fibrosis during wound healing.
Introduction
Wounds disrupt the anatomical structure and functional integrity, and activate mechanisms that maintain residual function and recover lost tissue function. Skin injury is followed by inflammatory cell infiltration, cell proliferation, new tissue formation and matrix and tissue remodelling; the injured area is thus regenerated in part. Repair is initiated by various growth factors, cytokines and low-molecular-weight materials released after injury. The search for treatments that accelerate healing and prevent excessive inflammation, infection and sequelae continues [Citation1,Citation2]. Healing, which is defined as complete closure of injured skin, is a dynamic process involving the interaction of parenchymal cells, the extracellular matrix, blood cells and various soluble mediators. Hemostasis, inflammation, cellular proliferation, matrix synthesis and remodelling, angiogenesis and granulation tissue formation are all involved in the healing process. Minimization of inflammation during wound-healing is important in terms of prognosis and healing outcome [Citation3].
The immune system plays a central role in tissue healing. Therefore, control of the immune system for tissue repair and regeneration seems to be important in planning the healing process [Citation4]. The immune system is important in the wound healing process and plays a role in restoring homeostasis after tissue damage through numerous mechanisms [Citation5]. The immune system’s response to tissue damage can activate stem/progenitor cells residing in the tissue, cause cell differentiation, stimulate growth factor release to promote extracellular matrix (ECM) accumulation and neoangiogenesis. As a result of disturbances in these processes, it can cause various wound pathologies, including chronic wounds and scar formation [Citation6]. Following the injury, an inflammatory response is normal and critical in restoring tissue homeostasis. Wound healing is divided into four stages: hemostasis, inflammation, proliferation and remodelling. These phases result from a series of events largely mediated by immune cells and signalling molecules [Citation3]. In response to stimuli from damaged ECM and tissue resident cells, platelets and immune cells accumulate at the wound site. Platelets, the first to arrive cells, help initiate the coagulation cascade to prevent blood loss and provide a temporary ECM for further cell infiltration. They play a critical role in activating fibroblasts and mesenchymal cells, recruitment and activation of neutrophils and macrophages by secreting TGF-b1 and platelet-derived growth factors (PDGFs) [Citation7–Citation9]. Neutrophils are the first immune cells to arrive in damaged tissue and remain for about 24 h before going through apoptosis [Citation10]. They contribute to both killing microbial agents and wound healing [Citation11]. Neutrophils control foreign pathogens by secreting various antimicrobial agents reactive oxygen species (ROS), antimicrobial peptides and antimicrobial proteases [Citation12,Citation13]. Neutrophils also secrete various cytokines and growth factors such as IL-17 and vascular endothelial growth factor (VEGF). Cytokines and growth factors are both chemotactic for inflammatory cells and contribute to the proliferation of fibroblasts, keratinocytes and endothelial cells [Citation11,Citation14]. Cytokines released by neutrophils during their apoptosis are chemotactic for monocytes that begin to reach the damaged area 5 to 6 h after injury. These monocytes differentiate into macrophages that can stay in the wound area for several weeks [Citation10]. Monocyte derived macrophages have been widely studied in the context of wound healing, and are often considered to be the most important immune cell type in this process [Citation15,Citation16]. In addition to macrophages derived from mobilized monocytes, most tissues have tissue-resident macrophages that can proliferate after injury [Citation3]. Neutrophils are the first immune cells recruited into injured tissue and remain for about 24 h before entering apoptosis [Citation10]. They both kill microorganisms and promote wound healing [Citation11]. Neutrophils control pathogen microorganisms by secreting various antimicrobial agents (reactive oxygen species (ROS), antimicrobial peptides and antimicrobial proteases) and phagocytosis with the help of neutrophil extracellular traps (NETs) [Citation12,Citation13]. Neutrophils also secrete a variety of cytokines and growth factors, including IL-17 and vascular endothelial growth factor (VEGF). Cytokines and growth factors are both chemotactic for inflammatory cells and induce the proliferation of fibroblasts, keratinocytes and endothelial cells [Citation11,Citation14].
Macrophages are essential for tissue repair and regeneration, but can also contribute to tissue damage and fibrosis. Macrophages have a variety of functional phenotypes against different stimuli. There are two types of in vitro that are best characterized. Of these, the proinflammatory ‘M1’ phenotype is produced by exposure to IFN-γ and TNF-α. The anti-inflammatory ‘M2a’ phenotype is produced by IL-4 or IL-13. The M2a subset is often referred to as ‘wound healing’ macrophages, as it refers to factors important for tissue repair [Citation17].
Ecballium elaterium (EE) is a plant of the Cucurbitaceae family traditionally used to treat fever, liver cirrhosis, sinusitis, hypertension, rheumatic disease and cancer [Citation18,Citation19]. EE inhibits the expression of chemotactic factors including TNF-α, IL-1 [Citation19] and IL-6 [Citation20]. Cucurbitacin B is a potent anti-inflammatory component of fruit juice, as shown by its effects on vascular permeability [Citation21] and carrageenan-, serotonin- and bradykinin-induced hind paw edema in mice [Citation22]. EE suppresses the growth of pancreatic, laryngeal, breast and lung cancers; it also inhibits microvascular endothelial cell angiogenesis and exerts an anti-integrin effect [Citation23]. The aim of this study was to explore the effects of subcutaneously injected Ecballium elaterium (L.) A.Rich. (EE) extract on skin wound-healing in rats, as well as the effects on the liver and pancreas.
Materials and methods
Ethics statement
The study was approved by the Local Ethics Committee for Experimental Animals of Dicle University (decision no. 5, annotated 10.12.2014-2014/60).
Animals
We used 28 Wistar Albino rats (mean weight, 250–300 g). The rats were kept in cages under controlled conditions of temperature (21 ± 1 °C), light (12 h light/12 h dark cycle) and relative humidity (%40–70). They were fed a balanced pellet diet and water, and acclimatized to conditions for one week before use. All animals were anesthetized via intramuscular administration of 50 mg/kg ketamine hydrochloride (Ketalar; Pfizer, Istanbul, Turkey) and 5 mg/kg xylazine hydrochloride (Rompun; Bayer Sisli, Istanbul, Turkey) under aseptic conditions. The dorsal regions were shaved and povidone iodine was used as the antiseptic. A 2.5-cm-long full-thickness skin incision was created on the dorsum of each rat; the site was then disinfected with povidone iodine and sutured with 5/0 vicryl.
The rats were randomly divided into saline control (S) and EE groups. Half of the animals in both groups were sacrificed after 7 days, and the other half after 14 days. EE (2.5 mg/kg per wound side; 5 mg/kg in total) was subcutaneously injected in the test group and the wound condition was monitored daily. At the time of sacrifice, 1 mg/100 mg ketamine/xylazine was administered intramuscularly and intracardiac blood was removed. The skin and subcutaneous adipose tissue were excised from each incisional area. All 28 rats used in the study were incised on the same day and at the same location. EE extract was applied to 14 of the rats the same day after the incision and this group was named the EE group. Saline was applied to the remaining 14 rats on the same day after the incision and this group was named S group. On the 7th day after the incision, a total of 14 rats, 7 from the S group and 7 from the EE group, were sacrificed. These groups were named S-7 and EE-7 groups. On the 14th day after the incision, a total of 14 rats, including the remaining 7 rats from the S group and the remaining 7 rats from the EE group, were sacrificed. The rats in these groups were named S-14 and EE-14. EE extract was applied to rats only once on the day of incision.
The liver and pancreas were sampled for histopathological examination. Tissues were fixed in 10% (v/v) formalin and embedded in paraffin blocks; 5-µm-thick sections were stained with hematoxylin and eosin and examined under a light microscope. The extent and nature of inflammation, and the extent of vascularization, fibrosis and inflammation in surrounding tissues were evaluated as follows: 1 point, low; 2 points, moderate and 3 points, extensive. The type of inflammation was graded as follows: 0 points, chronic; 1 point, mixed (acute and chronic) and 3 points, acute. Vascularization was also evaluated by staining for CD34 using a monoclonal antibody, and apoptosis was explored using anti-caspase-3 antibody (both antibodies from Leica Biosystems, Newcastle, UK). Tissue sections were stained using standard techniques with a Ventana BenchMark Ultra Automated Immunostainer (Ventana Medical Systems, Tucson, AZ, USA). Evaluations were performed under a light microscope (BX53; Olympus, Tokyo, Japan). Liver and pancreatic tissues were prepared for histopathological examination using routine procedures.
Histopathological examination
A single pathologist evaluated the samples for necroinflammatory activity. Pancreatic features were scored as follows:
Edema: none, 0 points; focal expansion of the interlobular septae, 1 point; diffuse expansion of the interlobular septae, 2 points; diffuse expansion of the interlobular septae with focal expansion of intercellular spaces, 3 points; diffuse expansion of the interlobular septae with diffuse expansion of intercellular spaces, 4 points and enlargement of the distance between cells, 5 points.
Acinar cell necrosis: none, 0 points; 1–4 necrotic cells, 1 point; 5–10 necrotic cells, 2 points; 11–16 necrotic cells, 3 points and ≥ 16 necrotic cells, 4 points.
Hemorrhage: none, 0 points; one area, 1 point; two areas, 2 points; three areas, 3 points and four areas, 4 points.
Inflammation and perivascular infiltration: 0–1 intralobular or perivascular leukocytes, 1 point; 2–5 intralobular or perivascular leukocytes, 2 points; 6–11 intralobular or perivascular leukocytes, 3 points; 12–20 intralobular or perivascular leukocytes, 4 points and > 20 intralobular or perivascular leukocytes or a micro-abscess, 5 points.
Preparation of the extract
The fruits of the EE plant were collected from open land in Antalya. The material was homogenized and mixed. Approximately 50 g of chopped material was mixed in 200 mL of 80% ethanol. Then it was rested for 24 h at room temperature. Parts of solid material were separated by filtration with Whatman paper. The ethanol in the extract was evaporated off and freeze-dried at −80 °C. The extract was dissolved in sterile isotonic solution at a concentration of 100 mg/mL. It was stored in a refrigerator at 8 °C until use.
Statistical analysis
Pearson’s chi-square test was used to analyse wound-healing. Differences were considered statistically significant at a p ≤ 0.05. All data were processed using SPSS for Windows software (ver. 20.0; SPSS Inc., Chicago, IL, USA).
Results
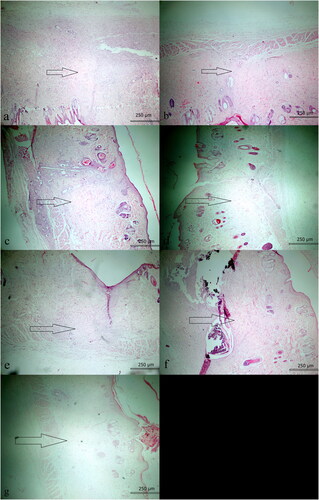
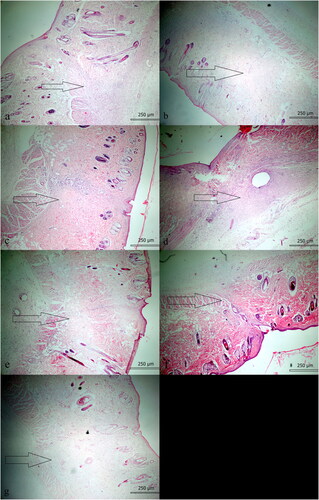
No animal died due to infection or a reaction to treatment. The severity of inflammation (chronic, mixed and acute) did not differ significantly between the S-7 and EE-7 (p = 0.310) or S-14 and EE-14 groups (p = 0.094). Histopathologically, the extent of fibrosis did not differ significantly between the S-7 and EE-7 groups (p = 0.515), but decreased significantly in the EE-14 compared to the S-14 group (p = 0.031) ( and ). Histopathologically, the extent of vascularization did not differ between the S-7 and EE-7 groups (p = 0.135), or the S-14 and EE-14 groups (p = 0.094). Although the severity of inflammation and nature of inflammation in the surrounding tissue were more prominent in the EE-14 group, no significant difference was observed. Fibrosis change between the S-14 and EE-14 groups was also noted in Masson Trichrome stain (). Tissue vascularization during wound-healing was evaluated via CD34 immunohistochemistry. We found no difference between the EE-7 (p = 0.237) and EE-14 groups (p = 0.212) compared to the S-7 and S-14 groups; the CD34 levels did not differ on either day 7 (p = 0.280) or day 14 (p = 0.094). The effect of EE on apoptosis during wound-healing was also evaluated. We found no significant difference in the caspase-3 level between the S-7 and EE-7 groups (p = 0.809), or the SF-14 and EE-14 groups (p = 0.212). The extent of inflammation, vascularization, fibrosis and immunohistochemical staining for caspase-3 and CD34 was compared between the S-7 and EE-7 groups, and between the S-14 and EE-14 groups. With the exception of fibrosis, which was less in the EE-14 group, there was no difference between the groups (all p > 0.05; ).
Figure 3. Significant fibrosis (left) was observed in the S-14 group, while mild fibrosis (right) was noted in the EE-14 group. (Masson Trichrome; ×40).
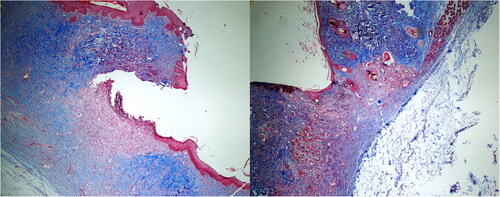
Table 1. Histopathological and immunohistochemical data.
Discussion
EE has been found to exhibit anticancer activity in various human cancer cell lines and tumour xenografts (Tannin-Spitz et al. 2007; Wakimoto et al. 2008). The mechanism by which cucurbitacin B affects tumour growth remains unknown. In a model of squamous cell carcinoma, cucurbitacin B partially inhibited the laryngeal STAT3 signalling pathway [Citation24], and also exerted effects on the leukemia cell line K562 [Citation25]. The antioxidant and anti-inflammatory activity of EE has been demonstrated in many studies [Citation26]. It has been reported that E. elaterium fruits have potential to be used in food applications and pharmaceutical industry as antimicrobial, antioxidant and anti-inflammatory agents [Citation27]. It has been suggested that the hydrophobic components of EE increase ATP production and increase insulin secretion from β-cells and consequently the lower blood glucose levels [Citation28]. Cucurbitasin B is an ingredient derived from EE.TanninSpitz et al. have demonstrated by ESR spectroscopy that cucurbitasin B + E glycosides exhibit antioxidant properties, possibly with the incorporation of a direct scavenging effect on a few free radicals [Citation29].
In this study, the effect of EE, previously known to have antimicrobial, anti-inflammatory and anti-tumoura properties, on the wound healing process was evaluated [19,20,23,Citation31,Citation30]. We created open skin wounds with tissue loss, and histologically evaluated the healing effect of EE in terms of fibrosis, vascularization, anti-inflammatory effects and immunohistochemical apoptosis.
EE is a herbaceous plant of the Cucurbitaceae family. Ointments prepared from fruits and roots are used to treat tumours, chronic skin wounds such as eczema and rheumatic pain. In Anatolia, the aroma of compressed fresh pulp is inhaled to treat sinusitis, although uvular edema may develop with excessive use. A lot of clinical studies have found that EE exhibited anti-inflammatory effects by reducing the synthesis of chemotactic factors including TNF-α, IL-1 and IL-6 [Citation22,Citation32]. Uslu et al. showed that EE exerted an anti-inflammatory effect by inhibiting nitric oxide synthase [Citation20]. Arslan et al. found that intraperitoneally injected EE (total of 2.5 mg/kg delivered as two equal doses at 30 min and 12 h) triggered pancreatitis [Citation33]. Unlike Arslan et al., we found no such effect, probably because we injected EE subcutaneously. Okur et al. found that rat collagen synthesis, and by extension intraperitoneal adhesion, were significantly reduced by EE compared to controls [Citation34]. Demir et al. [Citation35] showed that EE reduced rat alveolar wall thickness, whereas Eti et al. [Citation36] found that EE reduced rat nasal passage fibrosis. We observed a significant decrease in fibrosis by day 14 in the EE group. Both vascularization and inflammation were somewhat, but not significantly, reduced relative to the control group. The Okur and Demir groups injected EE at twice the dose used in this study, intraperitoneally and subcutaneously, respectively. The Eti group did not publish dose data. All three of these groups evaluated mucosal samples histopathologically, whereas we evaluated the skin.
Touihri-Barakati et al. [Citation23] showed that cucurbitacin B, an EE derivative, effectively inhibited angiogenesis in vitro by exerting an anti-α5β1 integrin effect. We found that angiogenesis increased somewhat, but not significantly, in the EE groups by days 7 and 14 (p > 0.05). Arslan et al. [Citation37] showed that the severity of rat brain inflammation was reduced by EE. The number of inflammatory cells in our EE group was somewhat less at both 7 and 14 days compared to the S groups, but the differences were not significant (p > 0.05).
Especially in the EE-14 group, the increase in vascularity made us think that it increased the number of neutrophil leukocytes by increasing vascular permeability and, in parallel, it caused more severe inflammation in the treatment group. Otherwise, it is known that macrophages undergo phenotypic changes during the wound healing process. It is known that it transforms from the proinflammatory stage to pro-resolution state at the injury site and causes fibrosis due to transition from M1 macrophages to M2 macrophages. In this study, the more pronounced inflammatory activity observed in the EE-14 group and the milder fibrosis suggested that EE might have reduced the transition from M1 to M2 macrophages.
EE can play an important role in the immune system in the skin. Therefore, the mechanisms by which the EE extract increases neutrophil leukocytes and decreases fibrosis and by which mechanism it affects macrophages can be demonstrated.
Conclusions
Subcutaneous EE injection into skin at 5 mg/kg has not been studied previously. In this study, EE reduced fibrosis and was not toxic to the rat pancreas or liver. The mechanism by which EE reduces fibrosis should be further studied in vivo and in vitro.
Acknowledgements
We thank İsmail Yıldız (Dicle University Medical Faculty Department of Biostatistics, Diyarbakır) for his endeavours.
Data availability
Data supporting the findings from this study can be obtained from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mutsaers SE, Bishop JE, McGrouther G, et al. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29(1):5–17.
- Theoret CL. The pathophysiology of wound repair. Vet Clin NA Eq Pract. 2005;21(1):1–13.
- Delavary BM, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762.
- Larouche J, Sheoran S, Maruyama K, et al. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209–231.
- Julier Z, Park AJ, Briquez PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signalling, and translation. Wound Healing. 2014;6:265–266.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289.
- Szpaderska AM, Egozi EI, Gamelli RL, et al. The effect of thrombocytopenia on dermal wound healing. J Invest Dermatol. 2003;120:1130–1137.
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607.
- Temenoff JS, Mikos AG. Biomaterials: the intersection of biology and materials science. 1st ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2008.
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175.
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376.
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535.
- Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (New Rochelle). 2013;2:379–388.
- Willenborg S, Eming SA. Macrophages—sensors and effectors coordinating skin damage and repair. J Ger Soc Dermatol. 2014;12:214–221.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature. 2011;11:723–737.
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–881.
- Sezik E, Yeşilada E, Tabata M, et al. Traditional medicine in Turkey VIII. Folk medicine in east anatolia; Erzurum, Erzíncan, Ağri, Kars, Iğdir provinces. Econ Bot. 1997;51(3):195–211.
- Yeşilada E, Ustün O, Sezik E, et al. Inhibitory effects of Turkish folk remedies on inflammatory cytokines: Interleukin-1alpha, interleukin-1beta and tumor necrosis factor alpha. J Ethnopharmacol. 1997;58(1):59–73. doi:10.1016/s0378-8741(97)00076-7
- Uslu C, Karasen RM, Sahin F, et al. Effect of aqueous extracts of Ecballium elaterium rich, in the rabbit model of rhinosinusitis. Int J Pediat Otorhinolaryngol. 2006;70(3):515–518.
- Yesilada E, Tanaka S, Sezik E, et al. Isolation of an anti-inflammatory principle from the fruit juice of Ecballium elaterium. J Nat Prod. 1988;51(3):504–508.
- Yesilada E, Tanaka S, Tabata M, et al. Antiinflammatory effects of the fruit juice of Ecballium elaterium on edemas in mice. Phytother Res. 1989;3(2):75–76.
- Touihri-Barakati I, Kallech-Ziri O, Ayadi W, et al. Cucurbitacin B purified from Ecballium elaterium (L.) A. Rich from Tunisia inhibits α5β1 integrin-mediated adhesion, migration, proliferation of human glioblastoma cell line and angiogenesis. Eur J Pharmacol. 2017;797:153–161.
- Liu T, Peng H, Zhang M, et al. Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling pathway, enhances the chemosensitivity of laryngeal squamous cell carcinoma cells to cisplatin. Eur J Pharmacol. 2010;641(1):15–22.
- Chan KT, Li K, Liu SL, et al. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010;289(1):46–52.
- Bourebaba L, Gilbert-López B, Oukil N, et al. Phytochemical composition of Ecballium elaterium extracts with antioxidant and anti-inflammatory activities: comparison among leaves, flowers and fruits extracts. Arab J Chem. 2020;13(1):3286–3300.
- Bohlooli S, Jafari N, Jahed S. Cytotoxic effect of freeze-dried extract of Ecballium elaterium fruit on gastric adenocarcinoma (AGS) and esophageal squamous cell carcinoma (KYSE30) cell lines. J Gastrointestinal Cancer. 2012;43:579–583.
- Shimada T, Kato F, Dwijanti DR, et al. Bitter melon fruit extract enhances intracellular ATP production and insulin secretion from rat pancreatic β-cells. Br J Nutrition. 2021;13:57–54.
- Tannin-Spitz T, Bergman M, Grossman S. Cucurbitacin glucosides: antioxidant and free-radical scavenging activities. Biochem Biophys Res Commun. 2007;364(1):181–186. doi:10.1016/j.bbrc.2007.09.075. 17942079
- Wakimoto N, Yin D, O’Kelly J, et al. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99(9):1793–1797. doi:10.1111/j.1349-7006.2008.00899.x. 18627377
- Adwan G, Salameh Y, Adwan K. Effect of ethanolic extract of Ecballium elaterium against Staphylococcus aureus and Candida albicans. Asian Pacific J Trop Biomed. 2011;1(6):456–460.
- Hassan OA, Paulis MG. Anti-inflammatory and antioxidant properties of Ecballium elaterium fruit juice against cyclophpsphamide induced hepatotoxicity in rats. Egypt J Forensic Sci Appl. Toxicol. 2020;20(1):51–62.
- Arslan S, Okur MH, Zeytun H, et al. A new experimental rat model of pancreatitis using Ecballium elaterium. Int J Surg. 2015;23:160–164.
- Okur MH, Aydogdu B, Arslan MS, et al. Intra-peritoneal administration of Ecballium elaterium diminishes postoperative adhesions. Acta Cir Bras. 2014;29(10):639–643.
- Demir M, Taylan M, Kaya H, et al. Histopathological and biochemical effects of Ecballium elaterium on sepsis-induced lung injury. J Invest Surg. 2016;29(5):302–308.
- Eti C, Vayisoglu Y, Kardas B, et al. Ecballium elaterium applied to nasal mucosa in a rat rhinosinusitis model. Ear Nose Throat J. 2018;97(6):E14–E17.
- Arslan D, Ekinci A, Arici A, et al. Effects of Ecballium elaterium on brain in a rat model of sepsis-associated encephalopathy. Libyan J Med. 2017;12(1):1369834.