?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Personalized anatomic plate is used in the surgical treatment of femoral fracture for the good matching between individual femur and plate. However, the development process of personalized plate is time-consuming and laborious at present due to anatomic and fracture differences between individuals. To improve the efficiency and quality of plate design, this study proposes an integrated computer-aided approach for parametric investigation of anatomic plate design. Based on the constraint deformation of femur template, a reference model was constructed, and major fracture segments were quickly restored to each original anatomic region by rigid registration to the reference model. The experimental results indicate that personalized anatomic plates can be constructed and edited quickly and intuitively with semantic parameters in high level, and contribute to the improvement of design efficiency and quality of customized plates for individuals.
Introduction
In the past decades, open reduction with internal fixation by using orthopedic implants has become the standard treatment method in many clinical surgeries [Citation1]. Compared with other internal implants such as screws and intramedullary nails, bone plates have good resistance to compression and rotational forces [Citation2]. Therefore, plates are more commonly used for femoral fracture fixation and have become effective devices for treating fracture. Biological (BO) fixation [Citation3] advocates to protect the surrounding blood supply by reducing the contact between the implant and bone, so as to protect the soft tissue around the fracture, which is helpful to achieve the secondary healing of fracture. With the continuous evolution of plate technology, many types of plate products are introduced, such as limited contact dynamic compression plate (LC-DCP), point contact plate (PC-Fix), bridging plate (BP), locking compression plate (LCP) and minimally invasive fixation system (LISS). To solve the shortcomings of the above bone plates in the anatomical shape reduction of fracture treatment, anatomic plates which were pre-bent according to typical shape of femoral surface are widely used in the treatment of complex fractures, and significantly improve the quality of clinical surgical treatment [Citation4].
The ultimate goal of fracture fixation is to restore bone function. The key problem is to stabilize the fracture block according to the specific situation of patients (including fracture type, degree and bone quality). On the one hand, doctors’ clinical experience and surgical skills are really important. On the other hand, the reasonable selection and optimal design of bone plate are also the key factors to determine the effect of fracture treatment. Due to the large differences in fracture type, trauma degree, physiological load, etc. of injured femur, the biomechanical environment of the fracture site is complicated. After using an inappropriate bone plate to fix the fracture, high local strain and plant changes that change fatigue characteristics often occur. Complications are associated with implants, and lead to an increased risk of failure of surgical treatment of fractures [Citation5]. Therefore, the design of personalized plates has been receiving increasing attention from researchers in the fields of digital orthopedics and medical implant development during the past few years.
To improve the matching between the bone plate and individual bone anatomic shape is the primary problem to be solved in the design of personalized bone plate. The challenging issue is to represent the original anatomic shape and structure of the damaged bone. In previous studies [Citation6–9], the initial normal anatomic surfaces and structures of femur were restored by the manual reduction of fracture block with translation, rotation and other operations, and the NURBS surfaces which were constructed by hole repairing and surface smoothing of restored models were cut to create the undersurfaces of the femur plate. In the methods the shape designs of personalized anatomic plates were proposed, and bone plates can perfectly match the femur shape of individual patients. However, the manual reduction of fracture segments is time-consuming and laborious, and its accuracy largely depends on medical experience. Jun et al. [Citation10] proposed a semi-automatic method for personalized implant design based on the individual femur anatomic parameters of the patient’s femur model, and realized optimization of anatomic parameters and the maximum matching of plate bone shape, but the limitation is that the anatomic parameters cannot fully describe the shape of bone, especially the overall structure of bone.
There are two main challenges in the design of personalized anatomic plates. Firstly, the reduction efficiency of fracture segments is low. Manual splicing of broken bones is time-consuming and laborious, and the problem should be overcome by developing a full automation method. Secondly, the reusability of existing design methods is poor. Due to the difficulty in editing the complex shape of the anatomic femur plate, existing plate models are difficult to be reused in the design of new plates for different individuals.
Therefore, with the consideration of the advantages of feature technology in complex model representation and editing, an integrated computer-aided approach for parametric investigation of anatomic plate design was proposed in this study. Based on feature templates, personalized femur plate models well-matching individual patients can be rapidly generated and modified in high level according to specific requirements, and then it can contribute to the fracture stabilization and successful surgical treatment for individuals.
Subjects and methods
Subjects
In this study, 50 right femurs of healthy male volunteers from South Jiangsu of China with no previous trauma or disease were used, and the mean age was 30.1 years (ranging from 23 to 41 years). Each femur was scanned by using a CT scanner (Sensation Open 128-slice CT scanner, Siemens) and reconstructed with three-dimensional (3D) mesh models.
Ethics statement
The institution’s internal review board approved the protocol and each patient provided an informed consent form before this study.
Methods
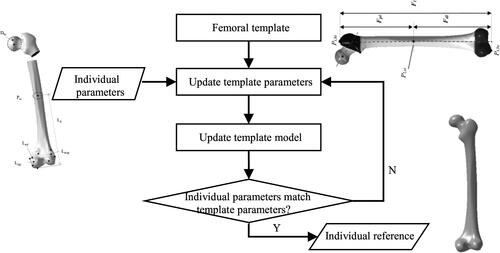
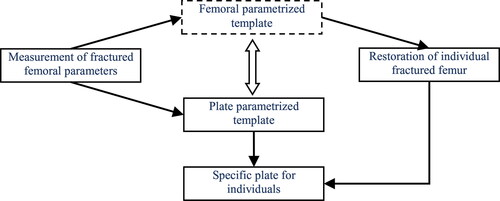
The proposed method comprises of three main stages: construction of femoral parameterized template, restoration of individual fractured femur and feature parameterized design of anatomic plate (as shown in ). In the first stage, a femoral mesh representing typical anatomies was divided into five feature regions, and parameterized design was deployed to describe and control the global and local feature shapes with restrained deformations. In the second stage, a reference femoral model was created based on the deformation of parameterized template to adjust the fracture segments to each original part by rigid registration. In the third stage, features of anatomic plate namely outline and solid thickness are designed to describe and control the whole shape and local structure of the region corresponding to the fracture site, and then the personalized plate for individual bone is created from the instantiation of selected template with hierarchical features.
Figure 1. Framework of the integrated computer-aided approach for parametric investigation of anatomic plate design.
Anatomical representation of the femoral model
Model construction of average shape
Compared to a specific femur model, an average model constructed based on statistical shape method can retain typical femur shapes and shield individual differences, and has better generality in the representation of typical anatomic features for a specific population group. In this study, the average femur surface model is built from 50 femur samples based on typical feature points. First, we define a sample as the reference femur model Fr, and select an individual model Fi randomly from the sample set defined from the library, and then set default value to each weight coefficient ti. Then, we implement a coast registration between Fr and Fi based on geometric feature points (GFPs) by using Iterative Closet Points (ICP) algorithm [Citation11]. Next, we perform a fine rigid registration to achieve anatomic alignment based on the anatomic feature points [Citation12]. Afterward, we compute the temporary average model Fta from corresponding 3D points according to EquationEq. (1) (1)
(1) , then add 1 to the coefficient of Fr. Finally, we select another model Fi randomly, then go to the first step until the set is null.
(1)
(1)
Template creation based on parameterized features
Either point cloud or mesh model consists of a large number of discrete vertices, and has a significant advantage in the description of the overall and local shape of the femur. However, the processing of large scale low-level objects results in more computation resource consumption and low efficiency in model deformation. In view of the advantage of surface features, the obtained average model is reconstructed by surfaces. The creation of a femur parameterized template of femur has been reported described in a previous study [Citation13].
Fracture reduction of individual femur
Model creation of individual reference
The individual reference model is created by automatic modification of the feature template model according to various parameters measured from the individual bone [Citation13]. The detailed processes are shown in . Firstly, we measure a small quantity of individual parameters [Citation14] from the fractured bone sample. Secondly, we update the template parameters and the feature template from constraint relations. Finally, we edit the template parameters recursively to obtain the individual reference model.
Fracture reduction of individual
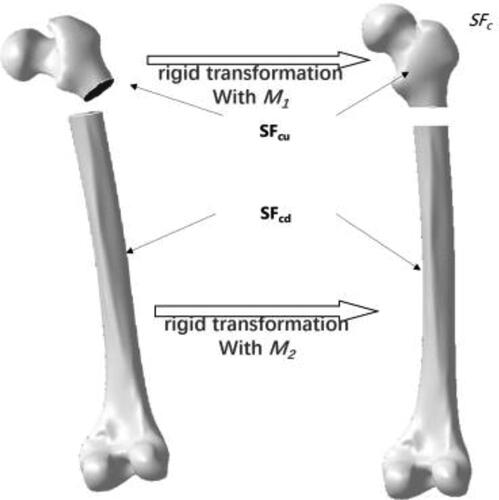
Due to the accurate description of the overall surface shape, especially the anatomic structure of the feature parameterization template, the individual reference model constructed above is used to restore individual fracture segments with feature-based rigid registration [Citation15]. First, we define and extract typical feature points in the reference model according to prior medical knowledge. Then, we extract valid feature points of the fractured bone and corresponding ones of the reference. Next, we apply translation and selection of the upper segment (SFcu) and the lower one (SFcd) based on matrices respectively as shown in .
Feature parameterization of plate template
Creation and parameterization of outline sketch
In consideration of the fact that anatomic plates are generally fixed in the lateral side of fractured femurs during surgical treatment, the outline sketch is created in the sagittal plane of the femur. Based on the fracture fixation requirements and profiles of existing commercial plate products, the outline sketch Splt is drawn in sagittal plane Pv of the fractured bone as shown in .
Figure 4. Creation and parameterization of outline of anatomic plate. Outline sketch creation (a) and outline parameterization (b).
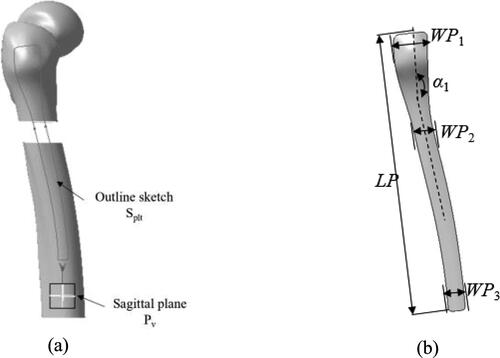
The contour features directly affect the whole shape of the bone plate. For the quick creation, modification and reusing of plate model, the contour is parameterized by a few high-level parameters with significant semantics including plate length (LP), there widths of various parts (WP1–WP3) and bending angle (α1) as shown in . Therefore, the contour feature Fct is defined with the following form described in EquationEq. (2) (2)
(2) .
(2)
(2)
Generation and parameterization of solid model
The features of the solid model of the plate are usually defined as two parts, namely the features of undersurface and the ones of thickness. In this study, the features of undersurface are represented by the ones of outline mentioned above. Various thickness values of different regions in the existing bone plate products have been proved to be helpful for the stable fixation of fracture blocks in clinical orthopedic surgery [Citation16]. Therefore, the features of the solid model Fsm are only parameterized with a few of thickness values as shown in .(3)
(3)
After the hierarchical feature parameterization mentioned above, the features in four levels are imported to a feature library, and are grouped to represent the feature of a typical anatomic plate Fplate.(4)
(4)
Generation of personalized anatomic plate
Generational of undersurface of plate
The undersurface of an anatomic plate consists of some free-form surfaces, and the shape is very complex. Undersurface design is one of the important issues in the design of a personalized anatomic bone plate. The closer the base surface of the bone plate is to the bone shape, the more the bone plate can promote the anatomic reduction and functional recovery of the bone. Here, the undersurface is generated from the surfaces of restored bones to match individual specific anatomy. The detailed steps are as follows:
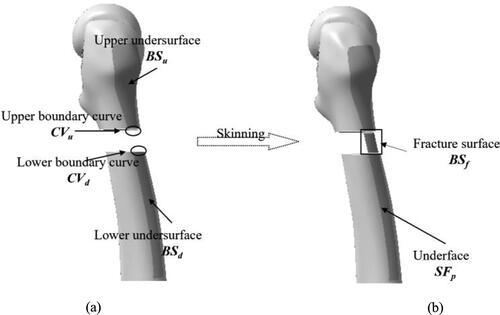
First, we project the sketch contour along the sagittal direction onto the lateral surfaces of restored fracture segments, and we cut surfaces with projection curves to obtain the upper undersurface BSu and lower undersurface BSd as shown in . Then, we create boundary curves CVu and CVd by cutting fracture boundaries of the upper and lower. Next, we create the fracture surface BSf with the skinning method based on the boundary curves CVu and CVd. Finally, we join the three surfaces CVu, BSf and CVd to obtain the undersurface of the plate SFp.
Creation of solid model of plate
The solid model is generated by stretching the undersurface outwards with certain distances. By using the instantiation of contour feature and thickness feature, the solid model of conventional plate can be quickly generated as shown in .
Results
To evaluate and verify the feasibility and effectiveness of the feature-based methods proposed in this work, the experiments including feature templates design, plate feature instantiation, plate shape modification and algorithm time consuming were carried out respectively.
Experiment environment
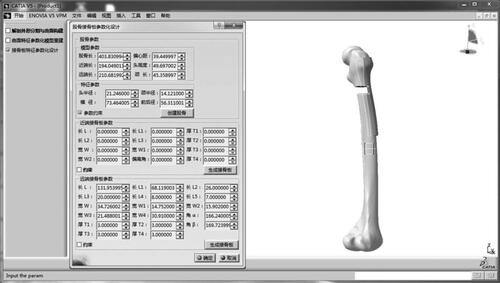
shows typical implementation interfaces of the developed prototype system platform. The resorted model of individual femur was constructed by inputting a few available anatomic feature parameters measured from a simulation fracture model based on the feature surface parameterization template, and then a personalized anatomic plate for proximal femoral fracture (AO/OTA 32C2 type) was automatically generated according to a selected feature template suitable for a specific type of fracture.
Generation of feature plate
The bone plate design for proximal intertrochanteric fractures was tested using a simulated fracture (AO/OTA-31A3 type) mesh model as input. Based the method suggested in our previous study [Citation3], several valid anatomic feature parameters of the fractured model were measured including femoral head radius (20.246 mm), distal length (207.882 mm), and anterior and posterior diameter of the condyle (55.911 mm). The conventional anatomic plate for proximal fracture was generated rapidly based on the feature instantiation of the predefined template. The thickness features were all set to the default value, namely 5 mm as shown in .
Shape modification of plate
In consideration of the need for local shape modification of the bone plate in the surgical fixation of individual femoral fractures, the semantic editing function of the feature shape of the bone plate created above was tested with experiments.
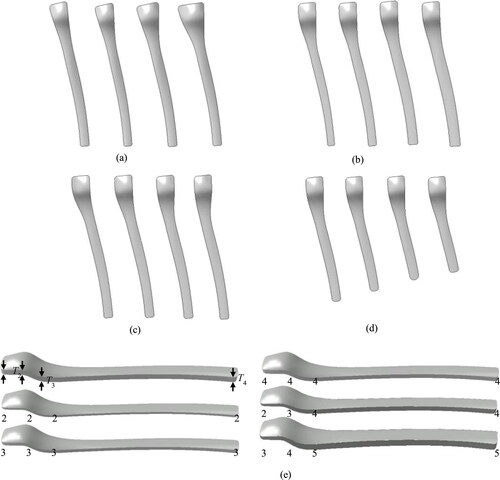
Through the modification of the features values of the outline sketch and solid that is width, angle, length and thickness in high-level, the detailed shapes of the undersurface feature and the thickness of the solid model were modified intuitively by a general operator, and the modified shapes are shown in .
Figure 10. Shapes of the proximal bone plate after parameterized modification. Instances with four values of WP1 (16.0, 27.0, 29.0 and 32.5 mm) (a). Instances with four values of WP3 (7.0, 8.5, 10.0 and 12.0 mm) (b). Instances with four values of α1 (172.0, 170.0, 168.0 and 166.5°) (c). Instances with four values of LP (150.0, 145.0, 140.0 and 135.0 mm) (d). Instances with various values of T (e).
Computational consumption
The methodology and algorithms mentioned above were implemented in the software environment including Microsoft Visual Studio C++ 2008 and CATIA V5 R21 integrated with CAA and RADE toolkits by using a 3.0 GHz i7-9700 CPU with 16 GB RAM. Average values of time consumption in each stage were recorded and listed in Table 1, all steps were completed within one hour.
Method comparison
Compared with certain existing approaches, our method has a number of advantages as listed in .
Table 2. Comparison of existing methods of plate design.
Discussion
Individual differences in bone quality, fracture type and the degree of trauma should be fully taken into consideration during the design stage of personalized bone plate products. Under the premise of effective cooperation between doctors and designers, the efficient method reported in a previous study [Citation9] requires two or more days to finish the development of personalized anatomic bone plate. In addition, it is widely recognized that osteoporosis caused by stress concentration of the bone plate after implantation is the most significant cause of fracture treatment failure.
Because of the lack of information about the original normal anatomic structure of a specific femur in the fractured model, it is hard to achieve the automatic reduction of fracture segments directly. In view of the semantic advantage of feature technology in the shape representation of complex model [Citation17], feature parameterization was incorporated into the restoration of fractured model in this study. With a small amount of valid anatomic information extracted from a specific fractured femur, the individual reference model was constructed based on the constrained deformation of femur surface feature parameterized template, and used to guide the main fracture segment to automatically transform to the original anatomic position by adopting rigid registration algorithm based on feature points.
Due to the complex appearance of anatomic bone plate, the reusability of existing plate models is not effective as well, and the models can hardly be used in the plate development of the same type of fracture for a different patient directly. As a result, the conventional developments of new bone plate models need to start from scratch [Citation18]. Based on feature parameterization, design users can quickly realize the plate creation and the modification of the undersurface and solid features from a predefined template by using semantic feature parameters in high level to meet specific surgical requirements of individual patients.
According to the patient’s fracture model, the personalized anatomic plate can be quickly created. shows that the total design processes including parameter measurement and shape adjustment of plate can be implemented within one hour. The shortening of the design time of individual anatomic bone plate provides a favorable opportunity for the surgical treatment of femoral fracture as soon as possible.
On the other hand, our study has several limitations. Firstly, although the features of femur plates were represent in four levels, more features could be designed into the library to describe the detailed shape and structure to describe anatomic plates, especially for different types of plate templates. Secondly, simulated fracture models were used to perform the experiments and evaluate the feasibility and effectiveness, and actual models of patient’s fractured femur were not used in this study. Nevertheless, our study proposes a novel feature-based method for the convenient creation and modification of anatomic plates for individual femoral fracture treatment. Feature parameterization technology was incorporated into the design of the anatomic plate of femur. Combined with valid anatomic information of each specific patient and the requirements of surgical fracture fixation, the three-dimensional model of anatomic plate suitable for the bone and fracture features of patients can be rapidly generated and modified.
Conclusions
To improve the efficiency and quality of plate design, this study proposes an integrated computer-aided approach for parametric investigation of anatomic plate design. Based on the constraint deformation of femur template, a reference model was constructed, and major fracture segments were quickly restored to each original anatomic region by rigid registration to the reference model. The experimental results indicate that personalized anatomic plates can be constructed and edited quickly and intuitively with semantic parameters in high level, and contribute to the improvement of design efficiency and quality of customized plates for individuals. To improve the mechanical performance of the personalized plate for the treatment of distal femoral fractures, related issues, such as the dynamical influence of plate parameters on stress distribution and average stress in the plate and material parameterization should be researched in the future.
Data availability
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Changzhou science and technology support plan project (Grant CE20205006), Research Fund Project of Changzhou Vocational Institute of Engineering (Grant 11130300119006) and the Qing Lan Project of Jiangsu Province (Grant 2019-2022).
References
- Hierholzer C, von Rüden C, Pötzel T, et al. Outcome analysis of retrograde nailing and less invasive stabilization system in distal femoral fractures: a retrospective analysis. Indian J Orthop. 2011;45(3):243–250.
- Chen X. Parametric design of patient-specific fixation plates for distal femur fractures. J Eng Med. 2018; 232(9):901–911.
- Stoffel K, Dieter U, Stachowiak G, et al. Biomechanical testing of the LCP–how can stability in locked internal fixators be controlled?. Injury. 2003;34(supp-S2):11–19.
- Anitha D, Das D, Sun K, et al. Improving stability of locking compression plates through a design modification: a computational investigation. Comput Methods Biomech Biomed Eng. 2015;18(2):153–161.
- Partenheimer A, Goslin T, Muller M, et al. Management of bicondylar fractures of the tibial plateau with unilateral fixed-angle plate fixation. Der Unfallchirurg. 2007; 110(8):675–683.
- Arnone JC, El-Gizawy AS, Crist BD, et al. An integrated Computer-Aided engineering approach for parametric investigation of locked plating systems design. J Med Dev. 2013;7(2):1–8.
- Ren L, Zhang Y, Guo Z, et al. Computer-aided personalized anatomic plate of the distal femur. J Clin Rehabil Tissue Eng Res. 2011;15(13):2309–2312.
- Shen X, Zhang J, Du P. Personalized design for dissection steel plate based on CT picture and reverse engineering. Mach Des Manuf. 2011;7:161–163.
- Cronskar M, Rannar L, Backstrom M. Implementation of digital design and solid free-form fabrication for customization of Implants in trauma orthopaedics. J Med Biol Eng. 2011;32(2):399–401.
- Jun Y, Choi K. Design of patient-specific hip implants based on the 3D geometry of the human femur. Adv Eng Softw. 2010;41(4):537–547.
- Szymon R, Marc L. Efficient variants of the ICP algorithm. Third International Conference on 3-D Digital Imaging and Modeling, IEEE; 2001. p. 145–152.
- Chen X. Reconstruction individual three-dimensional model of fractured long bone based on feature points. Comp Appl Math. 2020;39(2):131–147.
- Chen X, He K, Chen Z, et al. Quick construction of femoral model using surface feature parameterization. Mol Cell Biomech. 2015;12(2):123–146.
- Park B, Bae J, Koo B, et al. Function-based morphing methodology for parameterizing patient-specific models of human proximal femurs. Comput-Aided Des. 2014;51:31–38.
- Chen X. Hierarchical rigid registration of femur surface model based on anatomical features. Mol Cell Biomech. 2020;17(3):139–153.
- Nobari S, Katoozian H, Zomorodimoghadam S. Three-dimensional design optimisation of patient-specific femoral plates as a means of bone remodelling reduction. Comput Methods Biomech Biomed Eng. 2010;13(6):819–827.
- Pernot J, Guillet S, Leon J. A shape deformation tool to model character lines in the early design phases. Shape Modeling International, IEEE; 2020. p. 165–172.
- Harith HH, Schmutz B, Schuetz M, et al. Quantitative fit assessment of a precontoured fracture fixation plate: its automation and an investigation on the borderline cases. AMR. 2011;339(1):685–689.