ABSTRACT
In this study, we present initial data for the potential anti-cancer effects of standardized Bulgarian Petasites hybridus root extract against breast cancer cells. The results showed that Butterbur extract (BE) caused a dose-dependent selective reduction in viability with concomitant increase of apoptosis in human breast cancer cells after 72 h treatment. We found the highest cytotoxicity of BE to MDA-MB-231 cells (IC50 – 520 μg/mL), followed by the MCF-7 cancer cell line (IC50 – 865 μg/mL). At the same time, the extract exhibited very low cytotoxicity to the non-tumorigenic L929 cell line (two-fold higher inhibitory concentration – 1252 μg/mL). As a result of our experiments we made the conclusion that Butterbur root extract shows selective high cytotoxicity and nucleus alterations in cancer cells comparing to non-cancerous cells.
Introduction
The utilization of medicinal plants for the production of natural compounds with important therapeutic properties has gained increasing attention over past decades due to growing tendency to replace synthetic drugs with natural ones. In this respect, there is an increase in the science-based evidence of the potential of secondary plant metabolites as safe and non-toxic anti-tumour agents in prolonged use.
The genus Petasites Mill (Asteraceae) is widespread in Europe, Northwest Asia and North America and has a long history of use in folk medicine. Among the numerous species in the genus, Petasites hybridus (L.) (P. Gaertn., B. Mey. & Scherb), Petasites formosanus (Kitamura) and Petasites japonicus (Sieb. еt Zucc.) Maxim are subject to particular attention by researchers due to their use in the ethnomedicine of different countries. Representatives of genus Petasites contain high levels of bioactive substances with promising therapeutic potential [Citation1]. Studies have shown that they exhibit anti-inflammatory, analgesic, antioxidant, diuretic, anti-spasmodic, anti-tumour and other activities [Citation1, Citation2]. Among many secondary metabolites of the species of this genus such as polyphenol acids, flavonoids, lignans, thanines and small concentrations of pyrrolizidine alkaloids, the pharmacological effects are mainly attributed to eremophilane type sesquiterpenes and sesquiterpene lactones [Citation3–6].
The Petasites extracts are successfully used for the prevention of gastric ulcer, urinary tract irritation and respiratory problems [Citation2]. They also find an increasingly widespread pharmacological application for the prophylaxis and treatment of migraine and tension headaches, bronchial asthma and allergic rhinitis – diseases affecting a large number of people [Citation7]. The active ingredients and action mechanism are the subject of intense research in clinical trials. Studies suggested that petasin, s-petasin and sesquiterpene lactones are able to block leukotrienes synthesis [Citation8] and histamine binding to H1-receptors in vitro [Citation9] and can block voltage gated calcium channels in vascular muscle cells causing vasorelaxation [Citation10].
Unlike these relatively well-studied effects associated with smooth muscle spasm, a survey of the literature data showed that very little is known with regard to the anticancer activities of Petasites extracts and the results are somewhat controversial depending on the plant species and tissue origin of cancer [Citation11]. Most of the investigations on the antitumor effects of Petasites species involved treatment with isolated and purified bioactive compounds. Petasin, s-petasin and iso-s-petasin have been shown to exert a strong antiproliferation effect on cancer cells with or without inducing apoptosis [Citation12–14]. Marked cytotoxic activity and potential anti-tumour properties were reported for bakkenolide-A, a sesquiterpenes lactone present in different Petasites species [Citation15]. A new benzofuran derivative isolated from P. hybridus roots showed moderate inhibitory activity on human breast cancer MCF-7 cells proliferation [Citation16, Citation17]. But, to this day there are no data whether petasines obtained from Butterbur root extract could work on breast cancer. The purpose of this study was to investigate the anticancer activity of standardized Butterbur extract from Bulgarian populations of Petasites hybridus species. We established its effect on the triple negative breast cancer cell line MDA-MB 231 and human breast adenocarcinoma cell line MCF-7 in terms of inhibition of cell proliferation and induction of apoptosis. Non-tumorigenic L929 cells were used as a control. The cytotoxicity of the extracts was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-dye reduction assay (MTT assay). The effect on the cell nuclei was investigated via DAPI staining.
The investigated Butterbur root extract showed selective cytotoxic effect on cancerous cell lines in the same doses that inhibit only slightly the viability of the normal cells. The high cytotoxic effect of the BE on the cancerous cells was confirmed by the initiation of apoptotic alterations in the cells. Based on these findings, the investigated standardized Butterbur extract (15% petasines) from Bulgarian populations of Petasites hybridus is suggested as suitable for further study as anti-tumour therapy of breast cancer.
Materials and methods
Extracts
Butterbur powered extract, standardized to minimum 15% petasines, was provided by Rumex Ltd. (Bulgaria). The lipophilic extract was obtained from subterranean plant parts (‘radix petasitidis’) from Bulgarian populations of Petasites hybridus, using liquid-liquid extraction and after complete removal of the pyrrolizidine alkaloids.
Cell lines
Two breast cancer cell lines, MDA-MB-231 (epithelial, high invasive) and MCF-7 (epithelial, non-invasive), were purchased from American Type Culture Collection (ATCC, USA) and were cultivated in freshly prepared DMEM high glucose (HiMedia Laboratories Pvt Ltd, India) culture medium. The medium was supplemented with 10% Fetal Bovine Serum (FBS), Penicillin-Streptomycin-Amphotericin B and l-Glutamine (all from PAN Biotech., Germany). Especially, for the MCF-7 cell line the culture medium was added insulin, sodium pyruvate and 7.5% sodium bicarbonate solutions (all from Merck, Germany). Mouse fibroblast L929 cells (ATCC, USA) were also cultivated in DMEM medium supplemented with 10% FBS, Penicillin-Streptomycin-Amphotericin B, L-Glutamine and 7.5% sodium bicarbonate solutions. All cell lines were grown in an incubator at 5% CO2, 37 °C and optimum humidity.
Thin layer chromatography
The powered Butterbur extract (BE) was dissolved in ethanol (100% g), sonicated and heated at 50 °C for 30 min. For thin layer chromatography 6 µL of BE were applied on a silica gel 60 F254, HPLC plates (Merck, Germany) and the chromatogram was developed with toluene/ethyl acetate (93:7, v/v). Detection was performed with Vanillin/H2SO4 reagent and heating at 120 °C for 10 min. Тhe spots containing separated petasines were visualized in visible light and in UV light at 365 nm.
High performance liquid chromatography (HPLC) analysis
Apparatus
The analysis was performed on HPLC system Waters Alliance e2695 Separations Module with 4-channel degasser, Quaternary HPLC Pump, Autosampler with 100 μL loop, column thermostat and UV-VIS detector Waters 2998 PDA (Waters Corp., Milford, MA, USA).
Chromatographic conditions
The analysis was performed in a Venusil XBP C18 (L) column (250 mm × 4.6 mm × 5 µm) (Agela Tech., USA) with particle characteristics: 5 µm, 150 Å, 200 m2/g. The isocratic mode was used with methanol, acetonitrile and water (32:31:37) as described by Wildi et al. [Citation4] at column temperature of 30 °C . The eluent flow rate was 1.0 mL/min and the injection volume, 50 μL; i.e. 50 μL of sample solutions were injected into the HPLC systems for analysis. Data acquisition was up to 30 min. The contents of the six main sesquiterpene esters were quantified against an external petasin standard (Sigma Aldrich, USA), measuring the peak area of the sesquiterpene esters at 235 nm. The software for system control, data acquisition and data processing was Waters Empower 3 v.7.20.00.00 (Waters Corp., Milford, MA, USA).
MTT Assay
To observe the effect on cell viability of Butterbur root extract we used an MTT assay based on the conversion of tetrazolium salt from oxidoreductase enzymes to insoluble formazan (colour compound) and measurement of the absorption intensity of staining as described by Mosmann [Citation18]. MDA-MB-231, MCF-7 and L929 cells were treated with different concentrations of BE (2, 4, 8, 40, 200, 250, 500, 1000 and 5000 μg/mL) for 72 h. After the incubation time 20 μL of 5 mg/mL of MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Merck, Germany) reagent were added to each well in 96-well plates. The cells were incubated for 3 h at 37 °C. The formazan crystals were dissolved in a 5% solution of formic acid in isopropanol. The absorbance was measured at 570 nm wavelength using a microplate reader Tecan Infinite F200 PRO (Tecan, Australia). All data were normalized to control cells incubated with cell culture medium containing dimethyl sulfoxide (DMSO) in the same concentration as that contained in the sample with higher BE (5000 μg/mL in 1% DMSO). The half-maximal inhibitory concentration (IC50) values were calculated using nonlinear regression using GraphPad Prism 5 program. Two independent experiments with six repeats were performed.
Cell morphology analysis
The changes in cell morphology were observed under an inverted microscope (MEIJI, Japan) with objective 10× (Phase DM, MEIJI, Japan) and camera Optikam B1 Digital (Optica, Italy) after 72 h of treatment.
DAPI staining
To observe the apoptotic alterations in cell nuclei, after the cell treatment as described above, they were fixed in 3% paraformaldehyde for 20 min, rinsed with PBS and stained with DAPI (1 μg/mL final concentration, Merck, Germany). The apoptotic cells were identified as those with condensed and fragmented nuclei. Fluorescent nuclei were visualized using fluorescence microscope (Jenalumar, Carl Zeiss, Germany) with objective HI100×/1.30.
Data analysis
The results are expressed as the mean values with standard deviation (±SD) of the indicated numbers of determinations. The statistical significances of differences were determined by analysis of variance (ANOVA) with Turkey’s post-hoc test, and differences were considered statistically significant at p < 0.05 level. Analyses were performed using GraphPad Prism software version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Determination of the sesquiterpene type of Butterbur root extract
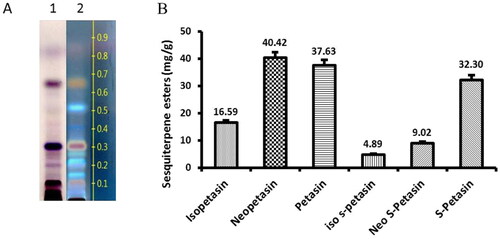
For the determination of the sesquiterpene type, the extract was characterized by thin layer chromatography and by high performance liquid chromatography (HPLC) analyses. The UV absorbing spots with blue and green-blue colour in the Rf range from 0.15 to 0.4 confirm the petasin chemotype of Butterbur extract (BE) (). shows the contents of the six main sesquiterpene esters found in the powder Butterbur root extract by HPLC analysis.
Figure 1. Sesquiterpene types in Butterbur extract. (A) Thin layer chromatogram of Butterbur extract: zones containing separated petasines as detected by viewing in daylight (1) or by UV light at 365 nm (2) as blue and green-blue fluorescent zones in the Rf range from 0.15 to 0.4; (B) Contents of the six main sesquiterpene esters (in mg/g) found in the powder Butterbur extract by HPLC analysis.
Cell viability and morphological examination of cells treated with Butterbur root extracts
MTT assay was performed to determine the effect of BE on the cell proliferation and to obtain IC50 for MDA-MB 231, MCF-7 and L929 cells. All cells were treated with BE in a concentration range from 2 μg/mL to 5000 μg/mL. Non-treated cells were used as a control.
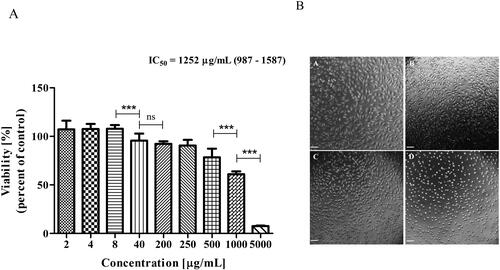
The obtained IC50 value of Butterbur root extract for L929 cells was 1252 μg/mL (95% Confidence Interval (CI) from 987 to 1587) (). At this concentration, morphological alterations, such as cell rounding and shrinkage (with apoptotic fragmentation), could be seen in the light microscopy images ().
Figure 2. Effect of Butterbur extract on L929 cell line. (A) Cell viability after 72 h treatment of Butterbur extract on L929 cell line. The values represent the respective IC50 concentration with 95% confidence limits. The data were analyzed using GraphPad Prism 5.0. Statistical analysis was performed using Tukey’s Multiple Comparison Test. *** – p < 0.0001, (B) Light microscopy images of L929 cells – (a) control, (b) cells treated with 500 μg/mL BЕ, (c) cells treated with 1000 μg/mL BЕ and (d) cells treated with 5000 μg/mL BЕ for 72 h. Bar is 50 μm.
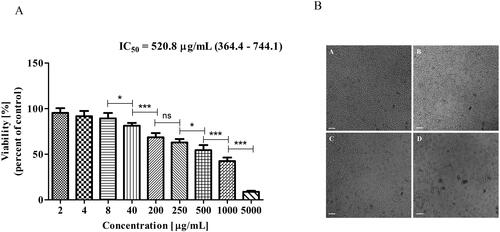
In contrast, when MDA-MB-231 cells were treated with extracts, the IC50 was approximately twice lower − 520 μg/mL (95% CI = 364–744) (). The light micrographs revealed morphological changes at 500 mg/mL, such as cell shrinkage, and at 1000 mg/mL already many fragmented (apoptotic) cells could be seen ().
Figure 3. Effect of Butterbur extract on MDA MB 231 cell line. (A) Cell viability after 72 h treatment of Butterbur extract on MDA MB 231 cell line. The values represent the respective IC50 concentration with 95% confidence limits. The data were analyzed using GraphPad Prism 5.0. Statistical analysis was performed using Tukey’s Multiple Comparison Test. * – p < 0.05, ** – p < 0.001, *** – p < 0.0001, (B) Light microscopy images of MDA-MB-231 cells – (a) control, (b) cells treated with 500 μg/mL BЕ, (c) cells treated with 1000 μg/mL BЕ and (d) cells treated with 5000 μg/mL BЕ for 72 h. Bar is 50 μm.
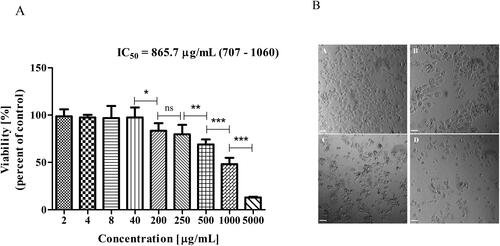
The other breast cancer cell line MCF-7, showed a higher IC50 value than MDA-MB-231– 865 μg/mL (95% CI = 707–1060) (), but still lower than the IC50 value obtained for the non-cancerous cell line L929 (). Morphological changes, such as cell shrinkage, were also observed at 1000 μg/mL ().
Figure 4. Effect of Butterbur extract on MCF-7 cell line. (A) Cell viability after 72 h treatment of Butterbur extract on MCF-7 cell line. The values represent the respective IC50 concentration with 95% confidence limits. The data were analyzed using GraphPad Prism 5.0. Statistical analysis was performed using Tukey’s Multiple Comparison Test. * – p < 0.05, ** – p < 0.001, *** – p < 0.0001, (B) Light microscopy images of MCF-7 cells – (a) control, (b) cells treated with 500 μg/mL BЕ, (c) cells treated with 1000 μg/mL BЕ and (d) cells treated with 5000 μg/mL BЕ for 72 h. Bar is 50 μm.
Induction of apoptotic changes in cells treated with Butterbur extract
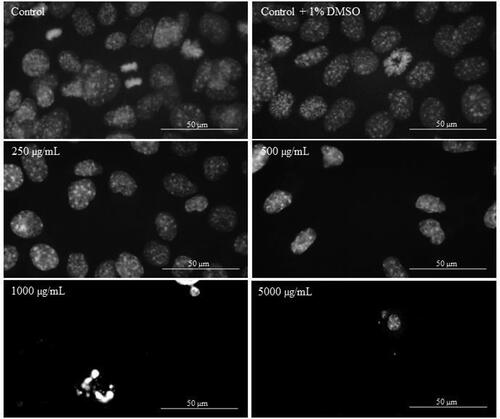
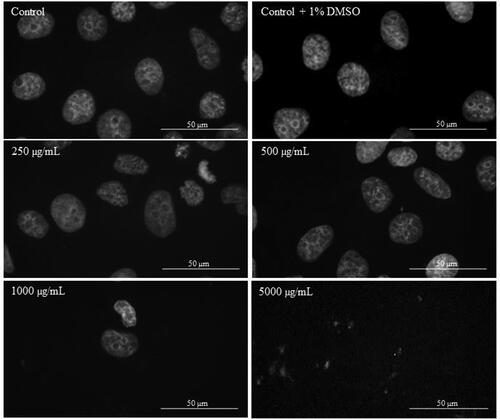
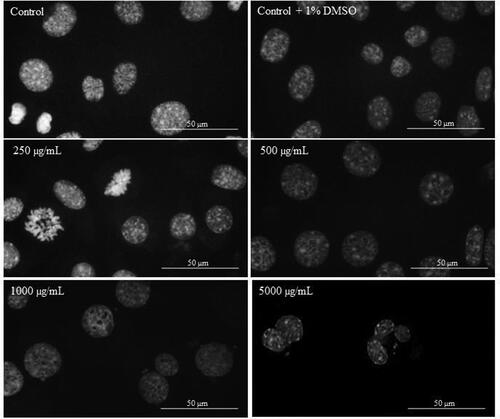
In order to investigate the apoptotic effect of Butterbur root extract on the nuclei of L929, MDA-MB-231 and MCF-7 cells, DAPI staining was performed. After the treatment with different concentrations of BE (from 2 μg/mL to 5000 μg/mL) for 72 h, cells were stained with DAPI and their nuclei morphology was examined by fluorescent microscopy analysis. The microscopic assessment demonstrated certain apoptotic features in cellular morphology. As can be seen from , no apoptotic changes were observed in the population of L929 cells treated for 72 h with different BE concentrations. Even in control (non-treated) cells and cells treated with 250 μg/mL of Butterbur root extracts (), mitosis in different stages was observed. In contrast, after treatment of MDA-MB-231 () and MCF-7 () cells with the extracts, a condensation of the chromatin in cell nuclei was observed at 1000 μg/mL. The treatment with the highest concentration of BE (5000 μg/mL) caused destruction of the cell nuclei in both cell lines ( and ).
Figure 5. DAPI staining of L929 cells treated with different concentrations of Butterbur extract for 72 h. Bar is 50 µm.
Discussion
The main active substances of P. hybridus (common butterbur) include petasin and its isomers (i.e. isopetasin and neopetasin) and s-petasin (methylthio derivative of petasin) and its isomers, iso-s-petasin and neo-s-petasin [Citation3]. Differences in sesquiterpene profiles in different populations of the species and the existence of hemovarieties with furanoeremofilanes (furanopetasin hemotype) have been established [Citation19–21]. It should be borne in mind that only the petasin chemotype is considered suitable for pharmaceutical purposes [Citation22]. In the studied Bulgarian extract, the petasin and its isomers (isopetasin and neopetasin) are in the higher content. On this basis, the present results indicate a selective cytotoxicity of Butterbur root extract to breast cancer cell lines, as the higher cytotoxicity was obtained for the more invasive cell line MDA-MB-231, compared with the less invasive MCF-7 cell line. However, for the non-cancerous cell line, the extract remained very weakly cytotoxic. Our findings are in accordance with other investigations, which show selective sensitivity of stomach, colon and uterine cancer cells to Petasites japonicus methanol extracts comparing to the normal liver epithelial cells [Citation11]. Previous studies reported that petasin, s-petasin and iso-s-petasin possess anti-proliferative activity on different cancer cells and some mechanisms of their effects were proposed [Citation12, Citation13] Cheng et al. [Citation12] showed that petasin is a potential antitumor agent against human neuroblastoma cell SK-N-SH Sk-N-SH by inhibiting the ERK1/2 phosphorylation. But the cytotoxic effect of s-petasin and iso-s-petasin on the proliferation of human prostate cancer cells is suggested to be related to caspase activation, BAX translocation and cytochrome c release [Citation14]. On the other hand, petasin was shown to inhibit the proliferation of colon cancer SW-620 cells via inactivating the Akt/mTOR pathway [Citation23]. The mechanism was suggested to be related to the activation of capsase-3, 8 and 9 proteins and the changes in phosphorylation of p38 MAPK, ERK1/2 and MEK. Based on the above results, a series of petasin derivatives were designed and synthesized [Citation24] for investigating the structure-activity relationship and discovering new antiproliferative agents.
The blockage of the apoptosis-inducing pathway is one of the key features of cancerous cells and could be the important mechanism for resistance to chemotherapy [Citation25]. That is why the activation of apoptosis pathway is understood as a key mechanism by which cytotoxic compounds kill tumour cells. In this regard, the DAPI staining confirmed our findings for the selective sensitivity of Butterbur root extracts to cancer and non-cancerous cell lines. The relationship between decreased cell viability and induced apoptosis was confirmed by Kim et al. [Citation26] for Hep3B cells. The authors showed that apoptosis was induced by inhibition the Akt/mTOR and Wnt signalling pathways. Hwang et al. [Citation27] reported the induction of apoptosis by P. japonicus ethanol extract in cervical carcinoma HeLa cells. However, another study confirmed that the treatment with petasin can cause a strong anti-proliferative effect by provoking the G0/G1 cell cycle arrest and inhibition of ERK1/2 phosphorylation on SK-N-SH neuroblastoma cell line without causing apoptosis [Citation12]. Exploring the effect of s-petasin and iso-s-petasin as potential anticancer agents, Wang et al. [Citation14] summarized that these active substances may influence several cell processes, such a cell proliferation and induction of apoptosis, and ultimately induction of morphological changes. The results of our study are in full agreement with this consideration. Therefore, we can speculate that the observed anti-carcinogenic effect on breast cancer lines may be related to petasines, as the main active components () in the standardized Butterbur root extract obtained from Bulgarian populations of Petasites hybridus.
In the context of the increase in oncological diseases, especially breast cancer, as a worldwide trend, the conventional drugs used for their treatment are either not effective enough or are often accompanied by significant side effects and high resistance in prolonged use. Phytotherapy refers to the traditional herbal medicine for the treatment of diseases, which is currently considered a promising alternative to chemotherapy. In recent years, knowledge about the potential of secondary plant metabolites as safe and non-toxic antitumor agents has been accumulated. In vivo studies on the anti-cancer potential of Petasites extracts are necessary for health promotion and prevention of diseases [Citation28].
Conclusions
This study investigated the cytotoxic activity of standardized root extract from Bulgarian populations of Petasites hybridus species. Our results confirmed that the extract exhibited selective dose-dependent cytotoxicity on two breast cancer cell lines, MDA-MB-231 and MCF-7. The cytotoxicity was connected with triggering apoptosis. In conclusion, the present data proved that the Butterbur root extract of Bulgarian origin exhibits highly specific action to breast tumour cells and has a low effect on non-cancerous cells.
Disclosure statement
The authors report no conflict of interest
Data availability statement
All data that support the findings from this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Aydın A, Zerbes V, Parlar H, et al. The medical plant butterbur (Petasites): analytical and physiological (re)view. J Pharm Biomed Anal. 2013;75:220–229.
- Tys J, Szopa A, Lalak J, et al. A botanical and pharmacological description of Petasites species. Curr Issues Pharm Med. 2015;28(3):151–154.
- Debrunner B, Neuenschwander M. Isolierung, HPLC-trennung und quan-tifizierung der sesquiterpenfraktion von Petasites hybridus (L.) G. M. et SCH. Chimia. 1994;48564–48569.
- Wildi E, Langer T, Schaffner W, et al. Quantitative analysis of petasin and pyrrolizidine alkaloids in leaves and rhizomes of in situ grown Petasites hybridus plants. Planta Med. 1998;64(03):264–267.
- Wu TS, Kao MS, Wu PL, et al. The bakkenolides from the root of Petasites formosanus and their cytotoxicity. Chem Pharm Bull. 1999;47(3):375–382.
- Wang Y-L, Li RP, Guo ML, et al. Bakkenolides from Petasites tricholobs and their neuroprotective effects related to antioxidant activities. Planta Med. 2009;75(03):230–235.
- Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004; 63(12):2240–2244.
- Thomet OAR, Simon H-U. Petasins in the treatment of allergic diseases: results of preclinical and clinical studies. Int Arch Allergy Immunol. 2002;129(2):108–112.
- Berger D, Burkard W, Schaffner W. Influence of Petasites hybridus on dopamine-D2 and histamine-H1 receptors. Pharm Acta Helv. 1998; 72:373–375.
- Wang GJ, Lin YL, Chen CH, et al. Cellular calcium regulatory machinery of vasorelaxation elicited by petasin. Clin Exp Pharmacol Physiol. 2010;37(3):309–315.
- Kang H-G, Jeong S-H, Cho J-H. Antimutagenic and anticarcinogenic effect of methanol extracts of Petasites japonicus Maxim leaves. J Vet Sci. 2010;11(1):51–58.
- Cheng YF, Wang CF, Li WJ, et al. Antiproliferation effects of petasin on human neuroblastoma SK-N-SH cells. Acta Neuropharmacol. 2012;1:1–6.
- Yue H, Ren D, Wang D, et al. Petasin-induced apoptosis of myeloma RPMI 8226 cells and the mechanisms. J Xian Jiaotong Univ Med Sci. 2015; 36:395–399.
- Wang ZH, Hsu HW, Hou JC, et al. Cytotoxic effect of s-petasin and iso-s-petasin on the proliferation of human prostate cancer cells. Anticancer Res. 2015; 35:191–200.
- Jamieson GR, Reid EH, Turner BP, et al. Bakkenolide-A. Its distribution in Petasites species and cytotoxic properties. Phytochemistry. 1976;15(11):1713–1715.
- Khaleghi F, Faramar LBD, Charati R, et al. A new bioactive compound from the roots of Petasites hybridus. Phytochem Lett. 2011; 4(3):254–258.
- Soleimani A, Asadi J, Rostami-Charati F, et al. High cytotoxicity and apoptotic effects of natural bioactive benzofuran derivative on the MCF-7 breast cancer cell line. CCHTS. 2015;18(5):505–513.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Debrunner B, Neuenschwander M, Brenneisen R. Sesquiterpenes of Petasites hybridus (L.) G.M. et Sch.: distribution of sesquiterpenes over plant organs. J Pharm Biomed Anal. 1995;70(2):167–173.
- Novotný L, Toman J, Starý F, et al. Contribution to the chemotaxonomy of some European Petasites species. Phytochem Lett. 1966;5(6):1281–1287.
- Mihajilov-Krstev T, Jovanović B, Zlatković B, et al. Phytochemistry, toxicology and therapeutic value of Petasites hybridus subsp. ochroleucus (common Butterbur) from the Balkans. Plants. 2020;9(6):700–715.
- Chizzola R, Langer T, Franz C. An Approach to the inheritance of the sesquiterpene chemotypes within Petasites hybridus. Planta Med. 2006;72(13):1254–1256.
- Lyu X, Song A-L, Bai Y-L, et al. Inhibitory effects of petasin on human colon carcinoma cells mediated by inactivation of Akt/mTOR pathway. Chin Med J. 2019;132(9):1071–1078.
- Shi X, Zhang S, Wang J, et al. Synthesis and bioactivity of natural occurring petasin-like derivatives as antitumor agents. OJMC. 2015;05(02):23–31.
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485–495.
- Kim HJ, Park SY, Lee HM, et al. Antiproliferative effect of the methanol extracts from the roots of Petasites japonicus on Hep3B hepatocellular carcinoma cells in vitro and in vivo. Exp Ther Med. 2015;9(5):1791–1796.
- Hwang YJ, Wi HR, Kim HR, et al. Induction of apoptosis in cervical carcinoma HeLa cells by Petasites japonicus ethanol extracts. Food Sci Biotechnol. 2015;24(2):665–672.
- Kondo MH. Antioxidant compounds of Petasites japonicus and their preventive effects in chronic diseases: a review. Clin Biochem Nutr. 2020;67(1):10–18.