Abstract
Melatonin has been approved as a sleep medication in Europe since 2007. The pharmacological dosages of this substance are much higher than the physiological ones, so it is no surprise that it has potential for many adverse effects. In children melatonin has been used for many years off-label, but in 2018 the Committee for Medicinal Products for Human Use (CHMP) granted paediatric-use marketing authorization for melatonin. We used the European Medicines Agency (EMA) database to compare the distribution of melatonin adverse drug reaction (ADRs) between the pediatric and the adult population. The results will serve as a basis for future research involving melatonin use in specific populations. All ADR reports received in EMA up to 23.03.2019 were analyzed for overall numbers, age and gender. The nature of the ADRs and the most frequently reported drug substances and drug event combinations were evaluated using MedDRA 21.0. As of 27th March 2019, EudraVigilance contained 1061 spontaneous reports. After excluding all the cases with other suspected or concomitant drugs, we are left with 177 cases. In children ADRs were generally associated with central nervous system (CNS) excitability, whereas in adults there was mainly inhibition. The present study focused on the ADR reports of melatonin in one of the largest databases. Future research of melatonin in the pediatric population is needed, because the current SmPCs lack such information, and in the cases of melatonin use in children are increasing in recent years.
Introduction
In today’s fast-paced society, in the big cities that never sleep, insomnia in children and adolescents is a serious emerging problem. Behavioural insomnia of childhood, including bedtime refusal and night waking, affects between 20% and 30% of toddlers and up to 5% of adolescents. Beyond these problems, insomnia ranges from 1% to 6% in the pediatric general population [Citation1].
For many, Мelatonin (MLT) may seem like the perfect solution for such problems, but it is still a hormone and this should be addressed when it is prescribed. It is also known as 5-methoxy-N-acetyltryptamine, it is a hormone with a vast amount of functions in all living creatures from algae to humans [Citation2]. In higher animals, melatonin is produced from pinealocytes in the epiphysis, as well as from the retina and gastrointestinal tract [Citation3, Citation4]. The production of melatonin is directly connected to the suprachiasmatic nucleus (SCN) of the hypothalamus, which receives information from the retina about the daily change of light and darkness. SCN contains the endogenous circadian (master) oscillator, the system that regulates the circadian rhythm, and it is what drives the daily cycle in most components of the paracrine and endocrine systems [Citation5]. Melatonin is a key component in this cycle, and it is even referred to as the chemical expression of darkness [Citation5, Citation6]. Melatonin is produced from the amino acid tryptophan (by serotonin synthesis) from the enzyme arylalkylamine N-acetyltransferase (AANAT) [Citation5].
The melatonin produced in the pineal gland acts as an endocrine hormone (secreted in the blood), while the melatonin produced by the retina and the gastrointestinal tract acts as a paracrine hormone [Citation3–5]. Many of the biological effects of melatonin are mediated by the activation of melatonin receptors, and others come from its role as a very efficient antioxidant, protecting the nuclear and mitochondrial DNA [Citation7]. Melatonin is also produced by some plants (e.g. rice) and can be ingested. Ingested melatonin can reach and connect to melatonin-binding sites in mammalian brains [Citation8].
Melatonin was discovered in 1958 in bovine samples. In 2007 the pharmaceutical product Circadin® containing this hormone obtained marketing authorization in several European countries indicated ‘as monotherapy for the short-term treatment of primary insomnia characterized by poor quality of sleep in patients who are aged 55 years or over’ [Citation9]. Today it is one of the most popular natural products—fourth most popular bought by US adults (after fish oil/omega 3-fatty acids, glucosamine/chondroitin, and probiotics) and second only to fish oil in children, with dramatic increase of market share from 0.1% in 2007 to 0.7% (419,000) in 2012. In the United States it is not FDA-approved for any use, but it is sold over-the-counter (OTC). In Europe, it is approved for treating insomnia in people over the age of 54 and in many European countries it has OTC status, while, in the United Kingdom, it is a prescription-only medication. Between 2016 and 2021 OTC melatonin use in adults in the United States is expected to grow more than twofold, with a market share of over 850 million US dollars in 2016 and expected 1500 million in 2021 [Citation10].
Until late 2018, there was no registered melatonin formulation intended for use in the children population. Circadin was used in France under Temporary Recommendation for Use (RTU) for children over 6 years of age, in disturbances of the sleep-wake cycle related to behavioural disorders (Rett, Smith-Magenis and Angelman syndromes, tuberous sclerosis complex and autism spectrum disorders) [Citation11]. In 2018 EMA issued a Pediatric Use Medical Authorization(PUMA) for melatonin containing drug (Slenyto) intended for children suffering from ASD with sleep disturbances [Citation12]. Until this day the usage of melatonin in healthy kids is done only off label. This leads to the question: is it safe to use melatonin so freely without strict regulations?
Little is known regarding the possible negative effects of melatonin among healthy children. In the 1960s it was demonstrated that micro-amounts of melatonin injected in adolescent and prepubertal rodents led to a highly significant decrease in ovarian weight and a delay in spontaneous vaginal opening and onset of estrus [Citation13]. In these early studies on melatonin and reproductive processes, it was often termed an ‘anti-gonadal hormone’ [Citation14]. In the 1991 review, Russell Reiter wrote, ‘In particular, melatonin as a mediator of photoperiodically induced changes in pubertal development and seasonal reproduction in nonhuman mammals has been repeatedly confirmed. Considering the pronounced effects of the pineal gland and melatonin on reproductive physiology in these nonhuman mammals, to assume they would not have some sexual effects in humans would almost seem naive. Whereas only 30 years ago the pineal was generally considered to be vestigial, it now appears it may be functionally involved with every organ system in the body’ [Citation15]. Regarding the comparison between the children and adult populations there are data about significant differences between the melatonin concentrations—The daytime melatonin levels are already declined to adult values within onset of puberty (27 ± 5.5 at Tanner II), whereas nighttime levels decrease during puberty. No sex difference in serum MLT is observed. The data are compatible with the concept of the inhibiting effect of MLT on sexual maturation [Citation16]. This direct connection of melatonin to the hypothalamic-pituitary-gonadal axis can explain the significant alterations in the reaction when comparing children and adults. A better understanding of risks specific to age and gender groups could allow more appropriate use of melatonin for treatment of insomnia in this population.
Therefore, the two main aims of this study were, first, to evaluate which neurological and psychiatric ADRs are more commonly reported for melatonin in children than adults, and second, to describe what the general problems in reporting the melatonin ADRs are.
In our study, we have taken the information about melatonin collected in the EudraVigilance pharmacovigilance database for analysis. EudraVigilance is a centralized European database for managing and analyzing information on suspected adverse reactions to medicines that have been authorized or being studied in clinical trials in the European Economic Area (EEA) [Citation17].
Materials and methods
Ethics statement
The study used data freely available data from EudraVigilance pharmacovigilance database. No personal information was disclosed.
Database
In our study, we did a retrospective analysis of the AE reports for ‘melatonin’ from the beginning of the report collection in 2006 until the 23rd of March 2019 which are publicly available in the EudraVigilance database at www.adrreports.eu portal [Citation17].
We selected only the AE reports where melatonin was the only suspected drug of interest. The reports with other suspected drugs and with concomitant medications were excluded from the analysis because in those cases it is impossible to determine the correlation and causation between the ADRs and the drugs involved. The extracted line listings and reports were then filtered according to keywords corresponding to neurological and psychiatric reactions, which were in turn selected from the MedDRA 22.0 [Citation18]. The ADR reports were assigned to ‘reaction groups’ used in the EV database (‘Nervous system disorders’ and ‘Psychiatric disorders’). Age group, patient sex, outcome and information about overdose were included in the analysis. EMA applies an algorithm that screens the data in EudraVigilance for duplicate reports, but not all will be captured. The results of this analysis, therefore, might contain a small amount (in the order of 10%) of duplicates [Citation19, Citation20].
Statistical analysis
Data were downloaded from the database and with the use of Microsoft Excel were filtered and sorted. The EV database contains categorical variables, namely: ‘Primary Source Qualification’ (non-/healthcare professional), ‘Patient Age Group’ (divided into eight categories: 0–1 month, 2 months–2 years, 3–11 years old, 12–17 years old, 18–64 years old, 65–84, more than 85 years old and unspecified), ‘Patient Sex’, ‘Reaction List’ (including outcome and seriousness criteria), ‘Suspect Drug List’, ‘Concomitant Drug List’. Data have been presented by means of descriptive statistics. The detailed statistical analysis was performed using GraphPad Prism 8 software. Differences in proportions between the groups were compared with the Fisher’s exact test, due to small sample sizes. Differences were considered statistically significant at the p < 0.05 level.
Results
Population characteristics
In the studied period, the total number of ADR reports for melatonin was 1,061. Overall, 631(59.5%) of the subjects were female, 406 (38.3%) of them were men and 24 (2.3%) reports did not specify the sex of those taking part. There were 631 reports related to neuro-psychiatric reactions. After excluding all the cases with other suspected or concomitant drugs we are left with 177 cases. Eighty-four of the cases were reported by a health care provider (HCP) and ninety-three were reported by non-HCP. Those reports contained a total of 597 individual AEs; including 311 AEs related to neuro-psychiatric reactions. demonstrates a detailed flowchart of the data selection used in this study.
Figure 1. Flowchart of inclusion and exclusion of adverse drug reaction reports included in the study.
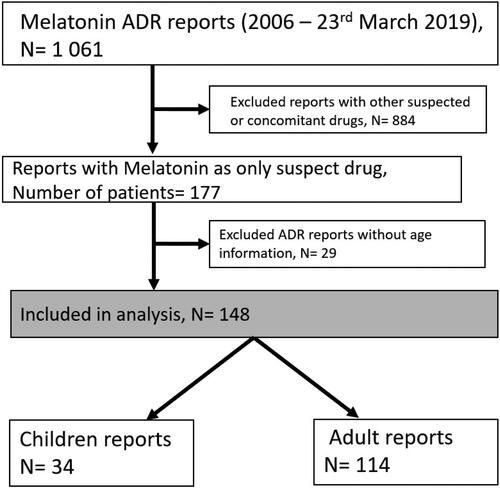
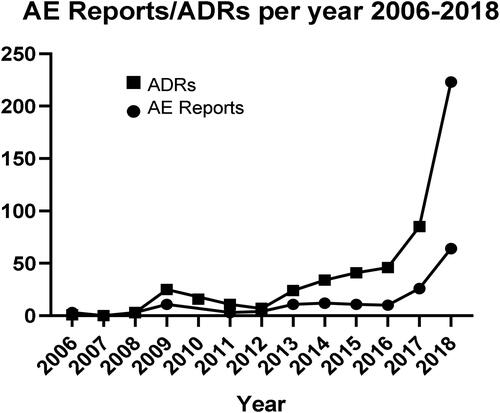
The mean number of AEs per report was 3.7 (range 1–13). As shown in , the number of reports collected in the EV database increased over time. Thirty-four (19.2%) cases concerned children, including 18 cases of children between 3 and 12 years of age and 16 cases of adolescents from 12 to 17 years old. The total number of AEs was 63. One hundred and fourteen (64.4%) cases concerned adults above 18 years old, with 188 individual AEs. This accounts for 83.6% of all the AE reports. In the other 29 (16.4%) cases there was no data regarding the age, with a total number of 60 AEs.
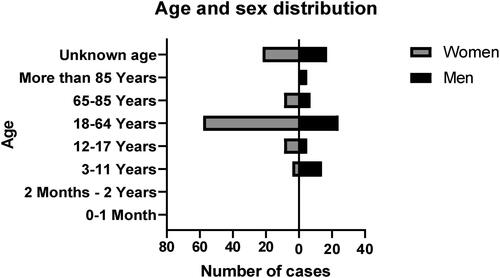
The gender was very well reported, with only 3 (1.7%) of cases missing information about it. There is a slight predominance of the females with a total of 102 (57.6%) cases against 72 (40.7%) male reports. Only the age groups of 3 to 11 years and over 85 years had a significant (77.8% and 100%, respectively) predominance of male reports ().
Adverse drug reaction frequencies
The relative proportions of pediatric and adult reports for each MedDRA preferred terms (PT) were compared. Generally, the most frequent symptoms reported in the analyzed AEs were ‘Dizziness’, ‘Headache’, ‘Somnolence’, ‘Insomnia’, ‘Nightmare’, ‘Depression’, ‘Agitation’, ‘Anxiety’, ‘Suicidal ideation’ and ‘Abnormal dreams’ (). There is no significant difference in the occurrence of those ADRs between child and adult populations.
Table 1. Top 10 most common reports of melatonin in a Preferred term (PT), with comparison between children and adult reports, with reported p values.
The 10 most common ADRs in children are shown in . There is statistical significance in the frequencies of PTs Aggression (p < 0.001), Abnormal behaviour (p = 0.038), Psychomotor hyperactivity (p = 0.011) in favor of the child population when compared to adult patients.
Table 2. Top 10 of the most common ADRs in children.
In adults, the most common ADRs in the analyzed data were Dizziness, Headache, Insomnia, Somnolence, Agitation and Abnormal dreams, but none of them were significantly more frequent compared to the pediatric population. shows the ten most commonly reported ADRs in adults.
Table 3. Top 10 of the most common ADRs in adults.
While there is significant overlap between the reports, some of the ADRs are reported only in children population, e.g. ‘Aggression’ and ‘Psychomotor hyperactivity’; and others, only in adults: ‘Abnormal dreams’ and ‘Restlessness’. When analyzing the ADR reports about epileptiformic events (PTs ‘Epilepsy’, ‘Seizure’, ‘Generalized tonic-clonic seizure’), there is significant difference (p < .05) between children (6.35%) and the adult (0.88%) population ().
Table 4. Reported epileptoformic events.
We recorded 2 AE reports with fatal outcomes (2 females, one child (12–17 years old) and one adult (18–64 years old)), another 23 subjects did not recover, 5 recovered with sequelae and 77 recovered completely or were reported as recovering. The further history of 70 subjects from the reports was unknown. The mean percentage of unknown outcomes in the period from 2006 to 2012 is 9.5%, and after 2012 it rose to 13.2%. The unknown outcomes were more frequently recorded in non-healthcare professional reports than in the reports from healthcare professionals: 72% versus 51%, respectively.
Discussion
Our study is, to the best of our knowledge, the first to compare the reported melatonin ADRs between age groups. There is plenty of information regarding the usage and safety of melatonin in children with neuropsychiatric pathology, but that is not the case with healthy children [Citation21–24]. Especially when it comes to safety, the data are insufficient.
Aggressive behaviour
We did not find significant differences between male and female patients, but we found some significant differences in the reported reactions between age populations. The most apparent one was for the PT ‘Aggression’. In our study we found data about increased risk of developing aggressive behaviour in children. Our analysis also showed increased reporting of ‘Abnormal behaviour’ in children, which according to MedDRA 22.0, is again part of Standardized MedDRA Queries family of Hostility/aggression.
In 2017, a study by Liu et al. [Citation25] was conducted that showed a link between melatonin and reactive aggression in humans. It was a double-blind, randomized, placebo-controlled human study including 64 young (mean age 21) healthy participants divided in 2 groups: one receiving melatonin and the other one, placebo. They were subjected to Taylor Aggression Paradigm (TAP), and given the opportunity to administer high or low punishments to an opponent; the participants who ingested melatonin selected the high punishment more often than those who ingested placebo [Citation25]. There have been a few more articles concerning rodent studies citing increased aggression in the animals receiving melatonin [Citation26, Citation27]. In 2020, Munley et al. [Citation28], showed that melatonin mediates seasonal transitions in aggressive behaviour and circulating androgen profiles in seasonal animals. When the non-aggressive long-day animals got injected in, they developed aggressive behaviour similar to that of the short-day animals. As the authors write, these findings suggest that melatonin regulates seasonal changes in peripheral steroidogenesis and aggressive behaviour in the test animals, although the exact mechanism is still object of research [Citation28]. Still there are no extensive studies on the effects of melatonin on healthy children in view of aggressive behaviour.
Epilepsy
We found a small number of cases reporting epileptiformic events, with significant predominance of child reports. There are conflicting data about the role of melatonin in epileptogenesis.
Available data show that melatonin concentrations in patients with difficult-to-treat epilepsy are significantly lower than those in controls. In the postictal period, however, they increase up to three times [Citation29]. A few studies indicate worsening of triggering of epilepsy in children and young adults, especially in high doses (5 mg daily) [Citation30, Citation31]. One of these studies was even terminated, with a significant decrease in the frequency and severity of seizures after discontinuation of melatonin usage [Citation31].
In contrast to these findings, some studies have shown the protective effects of melatonin against epileptogenesis, due to speculated increase in brain GABA concentrations [Citation32]. In 2013, Jain and Besag [Citation33] reviewed the literature on existing data on the role of exogenous melatonin in epilepsy. Twenty-six articles (from January 1990 to May 2012) reported a link between melatonin and epilepsy, with seven not providing relevant information. The studies either show a decrease in seizure frequency or no effect at all, but the authors conclude that the data are not enough to draw a conclusion [Citation33].
We suggest that the observed variance in the reports about ADRs associated with CNS excitability could be due to differences in CNS maturation, regulation and the hormonal imbalance associated with growth and puberty, especially concerning the direct links between melatonin and the hypothalamus-pituitary axis [Citation13, Citation14, Citation34]. This axis not only regulates the gonads, but the entire primal functions of the organism, including the stress system. Stress is among the most frequently self-reported precipitants of seizures in patients with epilepsy [Citation35]. There are data that corticosterone imposes a risk for neuronal injury. Under conditions of repetitive stress, either external or hormonal dis-balances, the steroid levels accelerate epileptogenesis and lower the seizure threshold in various animal models of epilepsy [Citation36]. Although melatonin is a natural anti-stress hormone [Citation37], there is a possibility that the supplementation can cause a decrease in endogenous production, therefore suppressing the body’s response to different stressors, which, especially in puberty, are a very wide group of factors. Unfortunately, for now, there is not enough data to fully understand the role of melatonin in the regulation of the stress system and the discreet mechanisms behind its ubiquitous actions.
Conclusions
The present study focused on the ADR reports of melatonin in one of the largest databases, the EMA EudraVigilance. It confirmed that reports of neuropsychiatric reactions in children show some significant differences compared to those in adults. There is a definite predominance of excitatory CNS events in patients under 18 years old in contrast to more CNS inhibitory reactions in adults. Despite the common off-label usage of melatonin in children, the information and the number of reports is very low, and also very unclear. There were a lot of ill written reports, and the number of those that clearly implicate the role of melatonin in the adverse reactions is slim. We showed that future research of melatonin in the pediatric population is needed, because the current SmPCs lack such information, and in the last few years the cases of melatonin use in children are increasing.
| Abbreviations | ||
| AANAT | = | Arylalkylamine N-acetyltransferase |
| ADR | = | Adverse Drug Reaction |
| AE | = | Adverse Events |
| ASD | = | Autism spectrum disorder |
| CNS | = | Central nervous system |
| EEA | = | European Economic Area |
| EMA | = | European Medicines Agency |
| EV | = | EudraVigilance |
| GABA | = | Gamma Aminobutyric acid |
| HCP | = | health care provider |
| MedDRA | = | Medical Dictionary for Regulatory Activities |
| MLT | = | Melatonin |
| OTC | = | Over-the-counter |
| PT | = | MedDRA Preferred term |
| PUMA | = | Pediatric Use Medical Authorization |
| RTU | = | Temporary recommendation for usage |
| SCN | = | Superchiasmatic nucleus |
| SmPC | = | Summary of product characteristics |
| TAP | = | Taylor Aggression Paradigm |
Disclosure statement
The authors declare that they have no conflicts of interest.
Funding
National Science Fund of Bulgaria, Grant 13/16 21.12.2017.
Data availability
The data that support the findings reported in this study are available at European database of suspected adverse drug reaction reports—adrreports.eu.
References
- Owens J. Classification and epidemiology of childhood sleep disorders. Sleep Med Clin. 2007;2(3):353–361.
- Choi D. Potency of melatonin in living beings. Dev. Reprod. 2013;17(3):149–177.
- Chen CQ, Fichna J, Bashashati M, et al. Distribution, function and physiological role of melatonin in the lower gut. W J Gastro. 2011;17(34):3888–3898.
- Tosini G, Baba K, Hwang CK, et al. Melatonin: an underappreciated player in retinal physiology and pathophysiology. J Exp Eye Res. 2012;103:82–89.
- Maronde E, Pfeffer M, Olcese J, et al. Transcription factors in neuroendocrine regulation: rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J Neurosci. 1999;19(9):3326–3336.
- Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79(1–3):53–158.
- Fulia F, Gitto E, Cuzzocrea S. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J Pineal Res. 2001;31:343–349.
- Meng X, Li Y, Li S, et al. Dietary sources and bioactivities of melatonin. Nutrients. 2017;9(4):367.
- EMA.europe.eu. Еuropean medicines agency. Summary of product characteristics Cyrcadin [Internet], 2007 [cited 30 May 2019]. Available from: https://www.ema.europa.eu/en/documents/product-information/circadin-epar-product-information_en.pdf.
- BussinesWire.com. Businesswire melatonin supplements market - drivers and forecasts by Technavio [Internet], 2017 [cited 30 May 2019]. Available from: https://www.businesswire.com/news/home/20170825005517/en/Melatonin-Supplements-Market-Drivers-Forecasts-Technavio.
- ANSM.sante.fr [Internet] Agence nationale de securite du medicament et des produits de sante RTU- Protocole Circadin juillet 15 [Internet]. ANSM. 2015. [cited 30 May 2019]. Available from: https://ansm.sante.fr/var/ansm_site/storage/original/application/58c9957ff3346fd8dc4072da68e8a54f.pdf.
- EMA.europe.eu. European Medicines Agency. Assessment report Slenyto[Internet]. 2018. Publication number EMA/556280/2018 [cited 30 May 2019]. Available from: https://www.ema.europa.eu/documents/assessment-report/slenyto-epar-public-assessment-report_en.pdf.
- Wurtman RJ, Axelrod J, Chu EW. Melatonin, a pineal substance: effect on the rat ovary. Science. 1963;141:277–278.
- Kennaway DJ. Melatonin use in paediatrics. J Paediatr Child Health. 2015;51:584–589..
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180.
- Waldhauser F, Frisch H, Weissenbacher G, et al. Day- and nighttime serum melatonin in children and adults. Pediatr Res. 1981;15(12):1566–1566..
- EMA.europe.eu. European Medicines Agency. EudraVigilance [Internet]. 2017 [cited 30 May 2019]. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance.
- MedDRA.org. Medical dictionary for regulatory activities MedDRA term selection: points to consider. Based on MedDRA Version 22.0 [Internet]. Release 4.17. 2019 [cited 30 May 2019]. Available from: https://www.meddra.org/how-to-use/support-documentation.
- EMA.europe.eu. European Medicines Agency. Process description for managing duplicates in the context of the Medical literature monitoring (MLM) service [Internet]. 2015. [cited 30 May 2019] Publication number EMA/262834/2015. Available from: https://www.ema.europa.eu/documents/other/process-description-managing-duplicates-context-medical-literature-monitoring-mlm-service_en.pdf.
- Pinheiro LC, Candore G, Zaccaria C, et al. An algorithm to detect unexpected increases in frequency of reports of adverse events in EudraVigilance. Pharmacoepidemiol Drug Saf. 2018;27(1):38–45.
- Hoebert M, van der Heijden KB, van Geijlswijk IM, et al. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res. 2009;47(1):1–7.
- Mindell JA, Emslie G, Blumer J, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117(6):e1223–1232.
- Cummings C. Melatonin for the management of sleep disorders in children and adolescents. Paediatr Child Health. 2012;17(6):331–333.
- Barrett JR1, Tracy DK, Giaroli G. To sleep or not to sleep: a systematic review of the literature of pharmacological treatments of insomnia in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(10):640–647.
- Liu J, Zhong R, Xiong W, et al. Melatonin increases reactive aggression in humans. J Psychopharmacol. 2017;234(19):2971–2978.
- Jasnow AM, Huhman KL, Bartness TJ, et al. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male siberian hamsters (phodopus sungorus). Horm. Behav. 2000;38(2):102–110.
- Jasnow AM, Huhman KL, Bartness TJ, et al. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus). Horm. Behav. 2002;42(1):13–20.
- Munley K, Deyoe J, Ren C, et al. Melatonin mediates seasonal transitions in aggressive behavior and circulating androgen profiles in male Siberian hamsters. Hormones and Behavior. 2020;117:104608.
- Bazil CW, Short D, Crispin D, et al. Patients with intractable epilepsy have low melatonin, which increases following seizures. Neurology. 2000;55(11):1746–1748.
- Sandyk R, Tsagas N, Anninos PA. Melatonin as a proconvulsive hormone in humans. Int J Neurosci. 1992;63(1–2):125–135.
- Sheldon SH. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet. 1998;351(9111):251254.
- Niles LP, Pickering DS, Arciszewski MA. Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. J Neural Transm. 1987;70(1–2):117–124.
- Jain S, Besag FM. Does melatonin affect epileptic seizures?Drug Saf. 2013;36(4):207–215.
- Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461.
- Privitera M, Walters M, Lee I, et al. Characteristics of people with self-reported stress-precipitated seizures. Epilepsy Behav. 2014;41:74–77.
- Joëls M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009; 50:586–597..
- Pierpaoli W, Maestroni GJ. Melatonin: a principal neuroimmunoregulatory and anti-stress hormone: its anti-aging effects. Immunol Lett. 1987;16(3–4):355–361.