Abstract
Cell senescence is a continuous irreversible process, ending with cell death. Long-term in vitro cultivation leads to decreased proliferation ability, disruption in cellular morphology and function in somatic cells. The aim of the present study was to investigate the markers of cell cycle arrest and senescence after long-term cultivation of periodontal ligament stem cells (PDLSC). The cells were isolated from routinely extracted third molars via enzymatic digestion and cultured continuously up to passage 16. Cell count and population doubling were evaluated at each passage. The enzymatic activity of telomerase and β-galactosidase were assessed, as these are well-known markers for cell senescence and aging. The results demonstrated two peaks with significantly increased cellular proliferation rate at passage 6 and passage 12. Slight differences in the proliferative ability were identified between cells from 1st and 16th passages without any statistical significance. Significant decrease in telomerase activity was observed starting immediately after the first passaging. β-Galactosidase activity was found to be uninterrupted following long-term in vitro cultivation. Our study indicates that the PDL stem cells did not show a significant decrease in the proliferation ability up to the 16th passage.
Introduction
Cell senescence is a continuous irreversible process, ending with cell cycle arrest. It has been reported that cell cycle arrest of somatic cells occurs after approximately 40-60 population doublings [Citation1]. Cell senescence was first described by Hayflick [Citation2]. He stated that in long-term cell cultivation accumulation of debris, impaired cell activity and decreased proliferation rate are observed. Disruption in cell morphology and function are also identified.
Mesenchymal stem cells (MSC) are found in multiple tissues and organs and are considered a promising tool for tissue regeneration. However, the amount of the isolated stem cells is usually limited. Therefore, the application of stem cells in regenerative medicine for therapeutic purposes must be preceded by in vitro cultivation and multiplication. The primary cell culture usually needs to go through several passages (10 cycles in average) in order to obtain a sufficient number of cells for stem cell therapy. According to the available literature data, following a limited number of cell divisions, the MSC, like the somatic cells, enter a state of irreversible cell growth arrest [Citation3]. Cells at a growth arrest stage are not able to maintain tissue regeneration due to the impaired proliferation, migration and differentiation function. Stem cells were first described nearly 50 years ago and their properties are still debated especially the details regarding senescence; therefore the clinical application of these cells is not a routine practice yet.
Promising sources of stem cells are dental tissues. MSC of dental origin are investigated by a large number of scientific teams. The main aim is the introduction of the dental stem cells as a tool for tissue regeneration and the management of degenerative, neoplastic and inflammatory diseases. Teeth structures are considered an easily accessible source for isolation and further multiplication of MSC due to the numerous indications for routine removal of healthy teeth in the dental practice (i.e. supernumerary teeth, lack of space for orthodontic treatment, the natural exfoliation of deciduous teeth, etc.). Stem cells are isolated from several dental structures as follows: periodontal ligament (PDL), pulp of primary and deciduous teeth, apical papilla of teeth with non-fully-developed roots and dental follicle. The very basic properties of all MSC, including those of dental origin are currently well investigated. It is reported that these cells are non-differentiated and are capable of unlimited number of cell divisions, colony forming and multilineage differentiation [Citation4]. Periodontal ligament (PDL) is a structure consisting of ground connective tissue substance, collagen fibers and cells. It is situated between the root cementum and the alveolar socket wall providing the tooth stability and nutrition. PDL contains several cell types including fibroblasts, cementoblasts, osteoblasts, as well as non-differentiated stem cells capable of maintaining tissue regeneration [Citation5]. The PDL stem cells (PDLSC) properties make them ideal candidates for tissue regeneration. In order to be used in the clinical practice, these cells should be first isolated and multiplied in vitro, as sufficient quantities are needed. However, a question arises about the maintenance of stem cell properties following long term in vitro cultivation. Our aim is to investigate the markers of cell cycle arrest and senescence after long-term cultivation of PDL cells.
Materials and methods
Cell isolation
PDL cells were isolated from intact routinely extracted impacted or partially impacted third molars of healthy donors (aged 18–40 years) after obtaining informed consent as previously described [Citation6]. Briefly, the extracted teeth were stored in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Eugene, OR, USA) supplemented with antibiotics and immediately transported to the laboratory for further processing. The teeth were rinsed three times with phosphate buffered saline (PBS) (Lonza, Verviers, Belgium) and the PDLs were scraped with sterile scalpel blades from the middle third of the roots, followed by enzymatic digestion with 3 mg/mL collagenase type I and 4 mg/mL dispase (Sigma-Aldrich) for 1 h at 37 °C in an incubator. The cell suspension was centrifuged and the supernatant was discarded. The cells were then seeded into cell culture dishes (2 cm) (Greiner Bio One, Frickenhausen, Germany) containing DMEM supplemented with 1% antibiotic-antimycotic (Sigma-Aldrich, St. Louis, MO, USA) and 20% heat inactivated fetal bovine serum (FBS) (Sigma Aldrich, St. Louis, USA). In some cases, after removing the crown and the pulp of the tooth, the roots were placed in collagenase/dispase for 1 h and then transferred in a cell culture dish to allow the cells to migrate. Three to four days after seeding the first fibroblast-like stem cells were found attached to the bottom of the petri dishes. The first cell colonies were seen 5–7 days after seeding. The cells were cultivated at standard cell culture conditions at 37 °C temperature in humidified atmosphere of 5% CO2 and 95% air and the cell culture media was replaced every 2nd or 3rd day. The cell growth and proliferation were daily monitored by phase contrast microscopy (Leica DMRE, Leica Microsystems GmbH, Germany).
Cell cultivation and multiplication
After reaching 80–90% confluence cells were detached from the cell culture dishes via tripsinization and then transferred to cell culture flasks for further multiplication and passaging. The cell layer was first washed with sterile PBS followed by incubation with 0.5–1 mL 0.025% trypsin/EDTA (Lonza) for 10–15 min at 37 °C. The cells were then thoroughly washed three times with PBS in order to collect all detached cells. The cell suspension was centrifuged for 4 min at 3000 rpm, resuspended with DMEM, supplemented with 10% FBS and seeded in cell culture flasks (Greiner Bio One, Frickenhausen, Germany) at a density from 5 × 103 to 1 × 104 cells/cm2. Cells from 1st to 16th passage were used in the current experiments. Each experiment was carried out at least three times.
Cells cryopreservation
PDLSC of each passage (from passage 1 to passage 16) were cryopreserved in order to obtain sufficient number of cells for the experiments in the present study. In each passaging the detached cells were counted and the cell proliferation rate was calculated. After reaching 80–90% confluence the cells were collected, centrifuged and the cell number was counted. A sufficient number or cells were seeded in a new cell culture flask for further cultivation, while the rest of the cells were cryopreserved. For this purpose the cells were resuspended in a solution consisting of 60% DMEM, 20% FBS and 20% DMSO (SERVA Electrophoresis GmbH, Heidelberg, Germany) with a final cell volume 1 × 106 cells per cryotube (TPP Techno Plastic Products AG, Trasadingen, Switzerland). The cryotubes were first stored at –80 °С for 24 h and were transferred in liquid nitrogen for long-term storage.
Cell proliferation
After reaching 80% confluence the cells were trypsinized and counted via Hemocytometer. The cells were counted under a phase contrast microscope. The cell population doubling was evaluated via the following formula: 3.32(lg (living cells count on detachment/seeded living cells number)).
Telomerase activity assay
Cryopreserved PDL stem cells from early and late passages were thawed and the cells were immediately lysed using RIPA buffer (Sigma) and protease inhibitor (Thermo Scientific). The total protein amount was identified in all samples using Nanodrop 1000 (Thermo Scientific). The telomerase enzymatic activity in early and late cell passages was evaluated using Human TERT/Telomerase Reverse Transcriptase ELISA Kit (ELISAGenie, Dublin, Ireland) following the manufacturer’s instructions. The analysis of the data was performed via microplate reader (Varioscan, Thermo Scientific) at 450 nm wavelength.
β-Galactosidase activity assay
Detection of β-galactosidase activity of PDL cells from early and late passages was analyzed by ELISA Kit for Galactosidase β (GLb) (Cloud Clone Corp, Katy, TX, USA) following the manufacturer’s instructions. For this purpose cells were first lysed in RIPA buffer and protease inhibitors. The total protein amount in each sample was then determined using Nanodrop 1000. The analysis was performed on a microplate reader (Varioscan, Thermo Scientific) at 450 nm wavelength.
Statistical analysis
Each experiment was performed in triplicate and the obtained data was presented as the mean ± standard deviation. The statistical analysis was made via Students t-Test using Statistical Package for the Social Sciences (SPSS, IBM Inc., Amronk, USA). The difference between the experimental groups was considered statistically significant at the p < 0.05 level.
Results
Cell proliferation and apoptosis
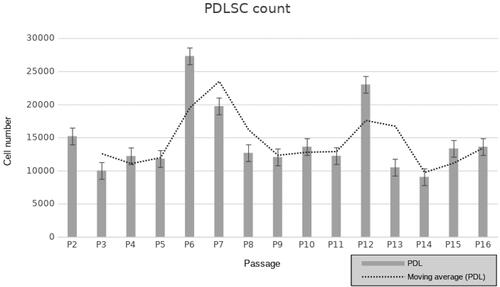
The isolated PDL stem cells were cultivated at standard cell culture conditions for a period of 7–8 months until reaching 16th passage. Cells were passaged when 80–90% confluence was reached. On each passage the population doubling time was evaluated. Fluctuations in cell proliferation rate were revealed in human PDL stem cells following long-term cultivation. The results demonstrate 2 peaks with significantly increased cellular proliferation rate at passage 6 and passage 12 (). Slight differences in the proliferative ability were identified between cells from 1st and 16th passages without any statistical significance. Limited number apoptotic cells were identified in early and late cell passages after Trypan blue staining, and less than 1% apoptotic cells were seen in each passage. No significant difference was found in cell apoptosis between early and late passages of human PDL stem cells.
Telomerase activity assessment
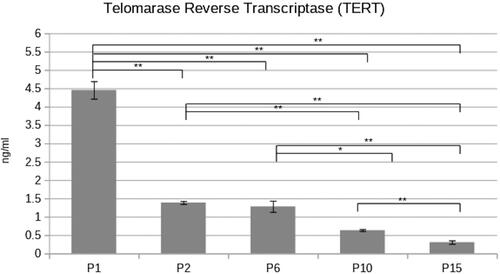
The telomerase activity in human PDL stem cells from early and late passages was investigated. Cells from passages 1, 2, 6, 10 and 15 were selected for the current experiment.
The results demonstrated a significant decrease in telomerase activity starting after the first passage. The most significant decrease in the enzymatic activity was observed between the 1st and the 2nd passage. In the following investigated passages, the telomerase activity differences are again statistically significant but at a lesser degree ().
β-Galactosidase activity assessment
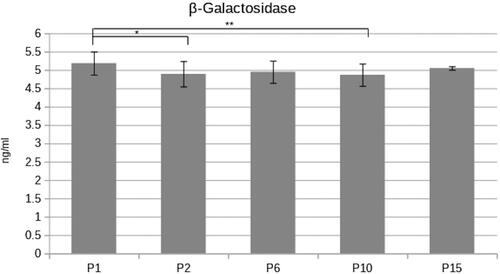
As shown in statistically significant differences were found only between the β-galactosidase enzymatic activity of PDL stem cell passages P1/P2 and P1/P10. Although statistically significant, the differences in the enzymatic activity were minor.
Discussion
A main issue in stem cell therapy is the limited number of primary cells isolated from the tissues. Long term in vitro cultivation and the subsequent preparation for local application for therapeutic purpose is a time-consuming and usually protracted procedure. Concern arises about the risk of irreversible cell properties impairment during the culturing due to the factors of the local environment or as a result of stem cell properties disruption following long-term in vitro cultivation. Stem cell senescence and aging are currently not well investigated. Madeira et al. [Citation7] reported that long term in vitro cultivation of bone marrow MSC induces morphological changes and proliferation ability loss after the 7th passage. The links between the donor age and the induction of cell senescence have also been investigated [Citation8,Citation9].
The aim of the present study was to reveal the effect of long term in vitro cultivation on PDLSC. Two major issues regarding the regenerative potential of periodontal MSC have been discussed in the literature. These are the presumption of stem cell properties alterations in elderly patients [Citation10], as well as disruption of the stem cells properties following long-term cultivation in order to achieve cell multiplication [Citation9]. Most probably the high number of elderly patients diagnosed with moderate or severe periodontitis is strongly related to age-associated reduced number of periodontal MSC. Therefore, the regeneration ability of the periodontal structures is impaired and the tissue destruction cannot be properly managed [Citation10]. In elderly patients diagnosed with severe periodontitis, scaling and root planning, in combination with periodontal regenerative therapy (application of growth factors, bone grafts, platelet concentrates, etc.) often do not show significantly improved clinical outcomes. According to Oh et al. [Citation11], stem cell senescence in such cases would compromise the stem cells isolation, multiplication and preparation for clinical application.
Transplantation of long-term in vitro cultivated stem cells may cause adverse tissue reactions to the recipient due to significant impairment of the cell properties and disruption of their phenotypic characteristics [Citation3]. Currently the potential risks and complications have not been clearly defined, as the known data remain insufficient.
Proliferation is the cell function known to be most commonly affected by the aging in a negative manner [Citation12], as cell proliferation rate is expected to be significantly reduced after a certain number of population doublings. The local environment highly affects the cell functions, as well as their proliferation ability. In the present research we observed two peaks in cell proliferation rate of the MSC isolated from PDL. The first peak was seen at passage 6 and the second one, at passage 12. At the last investigated passage 16, the proliferation rate was decreased when compared to the early passages. The fluctuations in the cell count observed in our study and the cell proliferation rate respectively, could be explained by the cell cycle phases. Probably cells from the early passages are predominantly at G0/G1 phases, whereas cells in the passages with higher proliferation rate are expected to be in S and G2/M cell cycle phases. Further on, the proliferation rate is observed to be slightly decreased in the cells at the later passages. Similar findings have been described by Danisovic et al. [Citation13] who investigated the aging and cell cycle phases in cells derived from adipose tissue. Variations in cell proliferation rate among different cell passages were also described in their study.
According to the current data, some well-known markers for cell senescence monitoring have been described. Telomerase and β-galactosidase are main markers associated with cell aging and senescence, therefore the activity of these two proteins has been broadly investigated following long-term cultivation. Telomeres are short nucleotide sequences of linear DNA situated at the terminal ends of the chromosomes protecting them from harmful events, destruction and fusion between neighboring chromosomes. After each cell division, the chromosome end is shortened, as the telomeres become shorter, and could be restored only by the enzyme telomerase. We revealed that the telomerase activity is significantly decreased following long-term cultivation of PDLSC. The cells from the first passage demonstrated high enzymatic activity rate, whereas in the further passages the activity markedly decreased.
β-Galactosidase activity has also been investigated and has been found to increase with cellular propagation in different cultures, i.e. nerve cells [Citation14], fibroblasts [Citation15], etc. According to some reports, β-galactosidase is not considered the most accurate marker for cell aging and it is not required for senescence [Citation16]. Various environmental factors may significantly influence the activity of the enzyme rather than the cell aging [Citation17]. However, together with other experimental methods, β-galactosidase is still one of the most commonly investigated markers for cell aging. Both telomerase and β-galactosidase are broadly investigated in multiple studies that aim to reveal the effects of long-term cultivation on cell properties. Thus, these two enzymes were chosen for the experimental design in the present study. Although we discovered minor statistically significant decreases of β-galactosidase activity in P2 and P10 (), which can further be linked with an increase in cell proliferation rate in P6 and P12 (), no statistically significant increases were found throughout all PDL cell propagation.
The differentiation properties of the stem cells following long-term cultivation are currently debated. It remains unclear if the cells are capable of retaining their multilineage differentiation properties after several weeks in vitro. According to studies with MSC, their ability to differentiate into osteoblast-like cells is maintained even after long-term cultivation [Citation18]. Other studies reported that stem cells gradually lose the ability to differentiate into various cell types following multiple passaging [Citation19]. According to many scientists, osteo-, adipo- and chondrogenic differentiation are reduced to varying degrees in late passage cells. Young et al. [Citation20] revealed that human PDL cell cultures retain their properties following eight passages. Straka et al. [Citation21] showed that long-term cultivation of PDLSC affects the morphology, as well as the proliferation and does not disturb cell cycle, apoptosis regulators and telomerase activity. However, further investigations are needed prior to their clinical application. Our previous research [Citation22] also demonstrated no significant signs of aging and senescence in PDL cells, as cells from early and late passages showed similar ability of markers expression and differentiation towards osteoblasts, chondrocytes and adipocytes.
Conclusions
Our study indicates that PDLSC do not enter cell proliferation arrest phase after long-term in vitro cultivation, as the cells did not show a significant decrease in the proliferation ability. The telomerase activity is known to be quite typical for neoplastic cells. Therefore, our data suggest lack of tumorigenic potential in human PDL cell cultures, as we revealed suppression of telomerase activity following continuous cultivation. However, the mechanisms of cellular senescence in human PDL cells remain elusive and further research is needed to clarify the biosafety of these cells prior to their application in regenerative medicine.
Disclosure statement
All authors declare no conflict of interest.
Data availability statement
Raw data were generated at the Medical University of Sofia. Derived data supporting the findings of this study are available from the corresponding author ZM upon reasonable request.
Additional information
Funding
References
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76.
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636.
- Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. IJMS. 2016;17(7):1164.
- Sharpe PT. Dental mesenchymal stem cells. Development 2016;143(13):2273–2280.
- Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant 2011;20(2):271–285.
- Mihaylova Z, Tsikandelova R, Sanimirov P, et al. Role of PDGF-BB in proliferation, differentiation and maintaining stem cell properties of PDL cells in vitro. Arch Oral Biol. 2018;85:1–9.
- Madeira A, da Silva CL, dos Santos F, et al. Human mesenchymal stem cell expression program upon extended ex-vivo cultivation, as revealed by 2-DE-based quantitative proteomics. PLoS One 2012;7(8):e43523.
- Feng XM, Xing J, Feng GJ, et al. p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev. 2014;141-142:46–55.
- Horibe H, Murakami M, Iohara K, et al. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS One 2014;9(5):e98553.
- Zhang J, An Y, Gao LN, et al. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012;33(29):6974–6986.
- Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20(8):870–880.
- Rinaldi S, Maioli M, Santaniello S, et al. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging 2012;7:191.
- Danisovic L, Oravcova L, Krajciova L, et al. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem cells. J Physiol Pharmacol. 2017;68(1):149–158.
- Geng YQ, Guan JT, Xu XH, et al. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem Biophys Res Commun. 2010;396(4):866–869.
- Yang NC, Hu ML . The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol. 2005;40(10):813–819.
- Lee BY, Han JA, Im JS, et al . Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006;5(2):187–195.
- Severino J, Allen RG, Balin S, et al. Is beta-galactosidase staining a marker of senescence in vitro and in vivo?Exp Cell Res. 2000;257(1):162–171.
- Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 2008;3(5):e2213.
- Suchánek J, Visek B, Soukup T, et al. Stem cells from human exfoliated deciduous teeth-isolation, long-term cultivation and phenotypical analysis. Acta Med (Hradec Kralove) 2010;53(2):93–99.
- Yang H, Gao LN, An Y, et al. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013;34(29):7033–7047.
- Straka M, Kupcová I, Varga I, et al. Effect of long-term cultivation on morphological and biological characteristics of human periodontal ligament stem cells. Neuroendocrinol Lett. 2016;37(5):361–367.
- Mihaylova Z, Stanimirov P, Miteva M, et al. Periodontal ligament stem cell properties after long-term in vitro cultivation. Problems Dent Med. 2019;1(45):44–50.