Abstract
CO2 application is one of the important ways to promote crop growth and increase yield, especially for facility crop cultivation. Since carrot is a widespread vegetable, the area of off-season facility cultivation has been increasing in recent years. Jasmonic acid (JA) is a signal molecule that responds quickly to external stimulation in plants, so it is very important to study the JA metabolism under CO2 enrichment. In this study, the JA content and the biomass were measured during the growth and development of carrots under natural conditions and CO2 enrichment in greenhouse. The results showed that the JA content in carrot leaves under CO2 enrichment was higher than that of the control at all stages except harvest stage, but the leaf biomass and root yield were increased at all stages. RNA sequencing identified 482 differentially expressed genes (DEGs). By analyzing the JA synthesis pathway, five DEGs were obtained. In addition, GO annotation found 21 genes that respond to JA or are related to JA-mediated signal transduction. Among them, seven genes are directly related to growth and development, five genes are related to hormonal regulation, and six genes are related to stress. This study showed that JA was highly responsive to CO2 enrichment: JA not only regulated carrot growth, but also responded to abiotic stress. This research provides new insights into the process of plants adapting to high levels of CO2.
Keywords:
Introduction
Carrot (Daucus carota L. var. sativa D.C.) belongs to Umbelliferae. Carrot was originally cultivated in Afghanistan around the ninth century and is now widely cultivated all over the world. Many nutrients such as carotenoids and vitamins accumulate in the carrot fresh taproot [Citation1], hence, carrot is known as “little ginseng.”
CO2 is not only one of the main raw materials for photosynthesis, but also a regulator of stomatal response and the distribution of photosynthesis in plants. Facility cultivation is carried out in a semi-closed state, where the concentration of CO2 is often in a state of severe deficit, which has become one of the main limiting factors for yield improvement in facility crop cultivation [Citation2]. It has been demonstrated in many crops that CO2 enrichment promotes photosynthesis in C3 and C4 crops, especially the net photosynthetic rate (Pn), and increases the yield [Citation3,Citation4]. Elevated CO2 concentration significantly advanced the flowering and fruiting time, improved the fruit setting rate, increased the yield, increased the content of lycopene and enhanced the antioxidant capacity in tomato [Citation5].
A large number of studies have shown that jasmonates (JAs) have a broad spectrum of physiological effects, which can regulate the growth and development of plants, such as root growth, tuber formation, fruit maturation and senescence, etc. [Citation6]. Moreover, JAs are also an important regulator of environmental and biotic stress in plants, which can induce the expression of a series of plant defense genes, the synthesis of defense response chemicals, and regulate the immunity and stress response of plants [Citation7]. In recent years, JA as a new type of phytohormone of interest has received extensive attention from botanists. Related enzymes in its synthesis pathway have also been identified one after another, including lipoxygenase (LOX), allene oxide synthase (AOS) and β-oxidase and so on [Citation8].
Studies have shown that plant hormones can produce changes under elevated CO2 concentration. In Arabidopsis, the concentrations of all plant hormones increased except abscisic acid [Citation9]. Wang et al. [Citation10] found that jasmonic acid (JA) may regulate carrot growth in a stage-dependent and organ-specific manner. The JA content in leaves of rice seedlings was significantly increased, while that in roots was significantly decreased [Citation11]. Previous studies mostly focused on ABA, IAA and other plant hormones, while JA was relatively less studied. The accumulation and potential role of JA in the growth and development of carrots under CO2 enrichment remain unclear. The mechanism of how CO2 and JA integrate and regulate plant metabolic reactions is also still unclear.
In this experiment, the changes in JA content of carrot leaves at different developmental stages were measured under CO2 enrichment. JA metabolism pathway was analyzed, related genes were discovered. Our work will provide novel insights into JA accumulation and its potential role in the growth and development of carrots under CO2 enrichment.
Materials and methods
Plant material and experimental treatments
The experiment was conducted in a solar greenhouse at the Horticultural Station of Shanxi Agricultural University from September 2019 to January 2020. The carbon-enriched zone (the CO2 concentration was 800 ± 50 µmol·mol−1, referred to hereafter as “elevated CO2”) and control zone (natural environment, referred to as “ambient CO2”) in the solar greenhouse were separated by a plastic film. The equipment and gas source used in the CO2 automatic release system were the same as those of Song et al. [Citation12]. A CO2 automatic release system was installed in the carbon- enriched zone with liquid CO2 cylinder as the gas source. CO2 release control was monitored by a GMM220 sensor (VAISALA Company, Finland) and an automatic control system (Handan Jinanxinqu Shengyan Electronic Science and Technology Co., Ltd, china). CO2 was uniformly applied through pipelines and circulating fans.
The carrot inbred line “Tianhong No. 1-1” was presented by Carrot Breeding Team of College of Horticulture, Shanxi Agricultural University (Shanxi, China). Seeds were sown on September 29th 2019. CO2 treatment began on October 31st 2019, from 9:00 to 11:00 a.m. (on sunny days); at this time the seedlings had four leaves. The treatment was paused on snowy days; there were 48 days for treatment in total. The plants were commonly cultivated.
Determination of biomass index
Taproot and shoot fresh weight, and the total biomass of the control and the treatment plants were measured 15, 31, 45, 61, and 70 days following the application of CO2. The experiment was set up in 3 biological replications per treatment and 15 plants were sampled for each biological replication.
Determination of JA content
Samples were taken at 5, 15, 31, 45, and 61 days after the CO2 application, respectively. Three independent replications were used for each treatment, and there were 15 plants for each replication, on each plant, the fourth leaf was picked respectively. Conventional enzyme-linked immunoassay [Citation13] was used for the determination.
Transcriptome analysis and DEGs identification
Samples were obtained at 10:00 a.m. on December 28th 2019 (a sunny day, 61 days after CO2 application). Three biological replicates were prepared. The specimen was immediately frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted from each sample using an RNeasy Plant Mini Kit (QIAGEN,74903) following the manufacturer’s instructions. cDNA library construction, sequencing, gene expression analysis and functional annotation was conducted by Biomarker Technologies Co. Ltd, Beijing, China. The detailed methods of these steps used were the same as those previously described by Sun et al. [Citation14].
Reverse transcription quantitative real-time polymerase chain reaction
To validate the RNA sequencing results, reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was conducted with an SYBR Premix ExTaq kit, and selected 6 genes (gene24757, gene946, gene2438 and gene13390 were involved in JA synthesis, gene14276 and gene15015 were randomly selected). Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) was used to design specific primers. The ACTIN gene was used as a reference gene. The sampling method and time were the same as those for the transcriptome. The methods of reverse transcription and RT-qPCR are the same as those used by Sun et al. [Citation14], and the relative gene expression was calculated using the 2−△△Ct method.
Statistical analysis
Values represent the mean values with standard deviation (± SD) of three replicates. One-way analysis of variance (ANOVA) was performed by the Statistical Analysis System (SAS, North Carolina, USA).
Data availability
The transcriptome sequencing data from this study have been deposited in the National Center for Biotechnology Information Sequence Read Archive database, and are accessible through accession number PRJNA705229 (https://www.ncbi.nlm.nih.gov/sra/PRJNA705229).
Results
Effect of CO2 enrichment on biomass yield
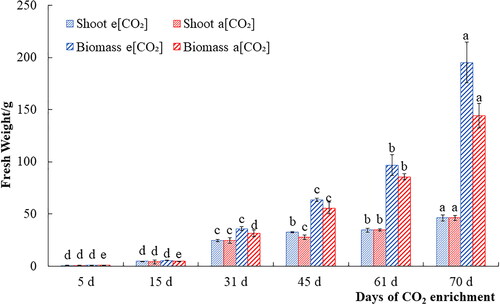
Elevated CO2 helps crops to absorb more nutrients and water from the soil, and speed up the turnover rate of roots, thus promoting the growth and development of roots [Citation15]. shows the comparison of the shoot fresh weights and total biomass between carbon-rich and control treatments in 6 development stages of carrots. As can be seen from the figure, comparing with the control, the production of elevated CO2 increased at each development stage. Within 31 days of CO2 enrichment, the growth rate of the aboveground parts was faster and the yield increased significantly. After 60 days of application, the roots grew rapidly. The roots were nearly mature at 70 days following treatment, and the roots of plants grown in carbon-rich conditions weighed 149 g on average, while those of the control plants were only 98 g, the difference was statistically significant (p < 0.05), but at this time, the growth of stems was relatively slow. It is consistent with the theory that the rapid growth of the shoots turns into roots after the period when the mass aboveground is equal to that belowground in carrots. These results indicated that CO2 enrichment promoted the morphology and yield improvement of carrot.
Effect of CO2 enrichment on JA content
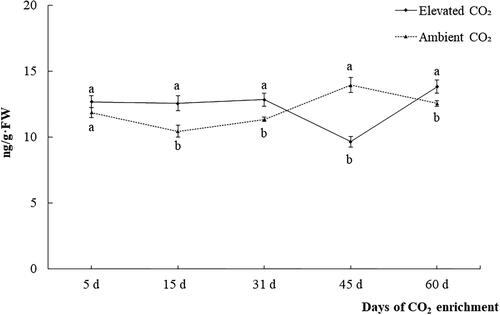
Under CO2 enrichment, The JA content in the leaves of carrots was higher than that of the control plants at each stage except for the harvest period (). The JA content generally showed a decreasing trend first, then increasing and then decreasing. In the process of development, the content of JA was the lowest at 15 days after CO2 enrichment, then gradually increased. The peak was reached at 45 days, it decreased again at 60 days, but was higher than that at 15 days.
Sequencing quality assessment
RNA sequencing (RNA-Seq) was used to compare the gene expression profiles of carrot leaves grown in CO2-enriched and control zones (). High-quality reads of the six samples were aligned to the carrot genome sequence. A total of 89.40%, 89.16%, 89.69%, 90.02%, 89.80% and 89.57% of the clean reads from the control 1, control 2, control 3, elevated CO2-1, elevated CO2-2 and elevated CO2-3 libraries, respectively, were mapped to the reference genome. The Reads Map to “+” accounted for 44.03%, 43.76%, 44.10%, 44.10%, 44.00% and 42.76% of the total mapped reads from the control sample and elevated CO2 sample libraries, respectively. The proportion of mapped genes was high, indicating that the sequencing libraries and reference genome were suitable for further analysis.
Table 1. Sequencing statistics and comparison of each library.
A false discovery rate (FDR) value ≤ 0.01 and a fold change (FC) value ≥ 2 were used as thresholds to identify significant differentially expressed genes (DEGs). The number of DEGs between the control sample and the enriched CO2 sample was 482. DEGs in which the FC were between 2 and 5 comprised the majority (291) and included 157 genes that were upregulated and 134 genes that were downregulated under CO2 enrichment. There were 107 genes with 10- to 50-fold expression changes. Only 30 genes exhibited a FC in expression that was greater than 50, of which 14 were upregulated and 16 were downregulated ().
Table 2. Statistical analysis of DEGs under CO2 enrichment in carrot.
KEGG significance analysis
To identify the biological pathways involved in CO2 enrichment, 482 DEGs were mapped to the referenced and canonical pathway in KEGG. A total of 59 KEGG pathways were assigned, of which 6 pathways showed significant differences between the control and elevated CO2 groups (p < 0.05) ().
Table 3. KEGG significant enrichment of DEGs.
Analysis of JA synthesis pathway
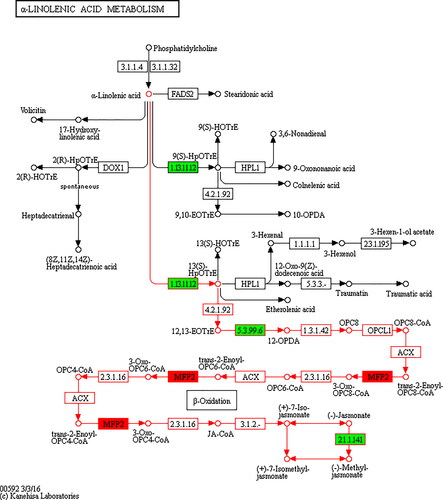
JA synthesis is essentially a series of enzymatic reactions starting with linolenic acid and linoleic acid released from the cell membrane as substrates. shows the KEGG pathway map of α-linolenic acid metabolism (KO00592), which includes the process of JA synthesis, marked in red. Genes encoding 11 key enzymes in JA synthesis have been isolated and identified by previous researchers [Citation16].
A total of 4 enzymes are significantly enriched in the JA synthesis pathway () by using FC > 2 at FDR value ≤0.01 as the selection criteria. LOX (1.13.11.12) is located in the chloroplast and is the first key enzyme in the JA synthesis pathway. LOX catalyzes the transformation of α-linolenic acid into 3-HPOT (13S – hydroperoxyl - (9Z,11E,15Z) -octadecatrienoic acid), which is a necessary step in the JA synthesis [Citation17]. LOX enzymes are divided into two types according to the different catalytic sites: one is 13-LOX leading to JA synthesis pathway, and the other is 9-LOX, which catalyzes the synthesis of 9-HPOT. Under CO2 enrichment, gene 24757, which encodes 9-LOX, and gene 946, which encodes 13-LOX, and their expressions are in a down-regulated pattern. There are 18 CmLOX genes in the melon genome which are expressed in different ways during melon development and maturation [Citation18].
Table 4. Enzymes and genes that are significantly differentially expressed in the jasmonic acid synthesis pathway.
The catalysis of allene oxide cyclase (5.3.99.6, AOC) is essential for the metabolite to lead to the final JA product [Citation19], which can cyclize the unstable compounds generated in the AOS catalyzed reaction to generate the final precursor of JA product − 12-oxy-phytodienoic acid. In addition, AOC has more stringent catalytic properties than LOX, identifying only 12,13 (S) -Epoxy-9 (Z),11,15(Z) -Octadecatrienoic acid as substrates. In Arabidopsis, four genes encoded AOC enzymes, and their expression patterns were organ-specific [Citation20]. Two homologous genes were identified in cotton, which may play a role in cotton fiber development, especially in secondary cell wall thickening [Citation21].
Enyl CoA Hydrase (MFP2) is the key enzyme in three β-oxidation processes in the JA synthesis pathway, it catalyzes the second step of β-oxidation of fatty acids to generate acetyl CoA and energy [Citation16]. Gene2438 showed an up-regulated expression pattern, suggesting that it promoted the formation of JA.
Jasmonate O-methyltransferase (2.1.141) is the key enzyme that catalyzes the synthesis of methyl jasmonate from jasmonate into the methyl jasmonate synthesis pathway. It controls the transformation of JAs in plants, and then affects the resistance of plants to biotic and abiotic stresses [Citation22]. Overexpression of JA methyltransferase caused significant changes in the morphology of transgenic soybean leaves and roots. Most of the transgenic soybean leaves became slender, and the growth of primary roots was inhibited while the growth of lateral roots was promoted [Citation23]. In this study, the down-regulated expression of the gene encoding the enzyme indicated that jasmonate methyltransferase inhibited the formation of methyl jasmonate and affected the morphogenesis of carrots.
Screening of differentially expressed genes under CO2 enrichment
In addition to the 5 genes that are significantly enriched in the metabolic pathway of JA, based on the Biological Process of GO annotation (JA biosynthetic process (GO:0009695), JA metabolic process (GO:0009694), response to JA (GO:0009753), JA mediated signaling pathway (GO:0009867), 21 DEGs related to JA were identified, and their role in JA biosynthetic process in carrot was predicted by comparison with the homologous genes in Arabidopsis thaliana ().
Table 5. DEGs associated with JA in carrot.
The A. thaliana homolog of gene11023 is ABCG, and 16 ABCG homologous genes have been identified in cucumber recently. Among them, CsABCG36 (CsPDR8) and CsABCG40 (CsPDR12) were most abundant in roots, and were significantly affected by plant hormones and herbicides. ABCG proteins have diversified functions or different regulatory mechanisms [Citation24]. The Arabidopsis gene homologous to gene29846 is UGT85A2, which belongs to the plant glycosyltransferase family and has a wide variety of species with complex biological functions. Current studies have shown that glycosylation of small molecules in plants plays a very important role in maintaining cell balance, regulating plant growth and development, and improving its resistance to stress environment, which is induced by hormones [Citation25]. The Arabidopsis homolog of gene7457 is JAZ1, and jasmonate treatment can induce its proteasome degradation [Citation26]. After the Arabidopsis gene homologous to gene8628 is knocked out, the related functions of auxin are impaired [Citation27]. Gene2452, homologous to the Arabidopsis gene ERS1, belongs to one of the five gene families that control the perception of ethylene, and is the most conserved gene of ETR1 [Citation28]. Gene28379 is a protein phosphatase. In Arabidopsis, at least seven of the nine 2 C type proteins act as negative regulators of the ABA pathway [Citation29]. The Arabidopsis gene homologous to gene32272 is ATABCG40, which is an ABC transporter and acts as an ABA promoter in plant leaf guard cells, it is also expressed in other organs, but its function is still unclear [Citation30]. These 6 genes are all related to hormones. Gene7457 and gene28379 are down-regulated under CO2 enrichment, which is consistent with the results of plant hormone signal transduction (ko04075), and the other genes are up-regulated, which promoted the JA synthesis.
There are 3 tomato genes homologous to gene11578, which show a high degree of inducibility in many organs at various stages. ACO1 and ACO3 transcripts accumulate during the senescence of leaves, fruits and flowers. In addition, it seems that ACO1 is wound-induced in leaves [Citation31]. The Arabidopsis homolog of gene14303 is TCP20, which belongs to an ancient plant-specific gene family, and can regulate the development of buds, flowers and embryos [Citation32]. The Arabidopsis homologous gene of gene2347 belongs to the GATL family, which consists of 10 genes. AtGATL4 expression appears to be limited to grain pollen. Most AtGATL genes are strongly expressed in the vascular bundles of stems and hypocotyls [Citation33]. The Arabidopsis homolog of gene30019 is ATBS1 INTERACTING FACTOR 4 (AIF4). All AIFs (there are four genes in Arabidopsis) overexpressing plants showed intense dwarfism, negatively regulating cell elongation [Citation34]. Gene23004 encodes NAC protein and is a plant specific transcription factor, which is involved in leaf senescence, flower formation, seed development, root development and hormone signal transduction in plants [Citation35]. More and more members of the NAC family have been identified, such as 117 genes in the Arabidopsis family [Citation36]. In this study, the expression of NAC014 was up-regulated, and the specific function remains to be further studied. These 5 genes directly regulate the development of plants. In this study, the carrot samples were collected in the harvest period, which supported the reliability of the results.
The Arabidopsis homolog to gene16970 is CPK20, which is overexpressed in Arabidopsis and rice and may enhance disease resistance by activating a series of defense-related genes [Citation37]. The Arabidopsis homologous genes of gene22686 and gene29219 are PUB. The Arabidopsis genome contains at least 64 PUBs, most of them respond to biotic or abiotic stress, and only a limited number of biological functions are known [Citation38]. Gene7323 encodes proline oxidase, which is related to stress. This gene was identified in chickpeas and regulated pod dehiscence [Citation39]. The Arabidopsis homologous gene in gene26436 encodes methyltransferase (ATNAMT1), which catalyzes the carboxymethyl methylation of NA to produce MENA. NaMT1 fine-tuned the long-distance transportation of MeNA and was finally used in NAD production. The abiotic stress treatments (salt, abscisic acid and mannitol) that increase the degradation of NAD can induce the expression of NAMT1 [Citation40]. Gene2572, the Arabidopsis thaliana homolog is KCS1, encodes the ketoacyl-CoA synthase of 3-ketoacyl-CoA, is involved in the synthesis of long-chain fatty acids in vegetative growth tissues and plays a role in wax biosynthesis [Citation41]. These genes are all related to resistance, which is consistent with the fact that JA not only regulates plant development, but also has biological functions in response to stress.
The Arabidopsis homolog of gene23438 is AtWRKY50, and it may be involved in the production of secondary metabolites, especially hydroxylcinnamates, such as sinapinic acid and 1-O-myrosyl-β-D-glucose [Citation42]. Gene27896 encodes psaA protein. PsaA protein and psaB protein constitute the photosystem I reaction center. Gene15832, a new GH3-related gene (called DFL2), is highly expressed under continuous white, blue and far-red light, but its expression level is very low in red light and dark [Citation43]. In this study, the expression of gene23438 was up-regulated, and the expression of gene27896 and gene15832 were down-regulated, which induced the synthesis of secondary metabolites and inhibiting photosynthesis, these functions are the specific manifestations of the physiological effects of JA [Citation17].
RT-qPCR verification
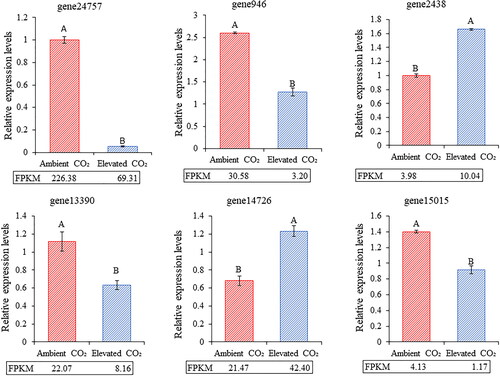
In the pathway of JA synthesis from α-linolenic acid, the expression level of some key enzyme-encoding genes directly affects the content of JA. As can be seen from , gene24757, gene946 and gene13390 were significantly down-regulated under the condition of CO2 enrichment, which resulted in the decreased activities of 9-LOX, 13-LOX and AOC, thus reducing the content of JA in leaves. Gene2438 was significantly up-regulated, and the activity of MFP2 increased, which promoted the content of JA in leaves. In addition, two genes were randomly selected to verify the reliability of the sequencing results. As a result, the fluorescence quantification results of the six genes were consistent with the sequencing results.
Discussion
Elevated CO2 concentration has a great influence on photosynthesis, yield, transpiration and water use efficiency of plants, and these growth and development processes of plants are regulated sequentially or interlinked by several endogenous hormones [Citation11]. In this study, genes that respond to JA synthesis or signal transduction were discovered under CO2 enrichment. They are not only related to JA, but may also be related to other hormones (ethylene, auxin, abscisic acid or salicylic acid) (), the results were consistent with those of Wang [Citation11] on the changes in several hormone contents in rice in response to CO2 enrichment.
Some genes responded to stress as well as JA, CO2 is itself an environmental stimulus for plants, stress occurs when the concentration is inappropriate, which will cause plants to respond physiologically to adapt to the elevated CO2 concentration. JA is the signal molecule that provides plants the fastest response to external stimuli [Citation7], and will respond to environmental changes as soon as possible. CO2 enrichment can promote the function of JA in kidney beans and induce the production of volatiles [Citation44]. Studies on transcriptional and metabolic responses to physiological differences between optimal and superoptimal atmospheric CO2 concentrations in Arabidopsis have found that some genes that respond to high concentrations are regulated by stress, and both high concentrations of CO2 and abiotic stress are regulated by JA [Citation45]. The experimental results are consistent with it.
Under CO2 enrichment, the yield of stem, leaf and root was increased compared with that of the control during the growth and development process. Except for the harvest stage, the content of JA was higher than that of the control at each stage, which indicated that the content of JA was directly related to the development of carrot. The accelerated growth and development of A. thaliana in higher concentrations of CO2 may be partly attributable to the increased foliar concentration of plant hormones [Citation9]. Teng et al. [Citation9] verified the reliability of the experimental results. The decrease in JA content at the harvest stage may be due to the decrease in light energy interception capacity of plant stems and leaves. Most studies have shown that the initial promotion effect of long-term high-concentration CO2 treatment on plants will gradually disappear over time [Citation46]. The phenomenon of declining photosynthetic capacity of plants due to long-term living under high concentrations of CO2 is called photoresponse [Citation47]. It may also be the adaptation of carrots to the environment during the long-term application of elevated CO2, or the instinctive changes in the physiology of the plants that are about to be harvested. In addition, the JA content increased in a higher CO2 environment [Citation11]. A study on the effects of elevated CO2 on the susceptibility of tomato plants to Tomato yellow leaf curl virus (TYLCV) in two successive years in open top chambers in the field, showed that elevated CO2 reduced JA in uninfected plants while it increased JA and abscisic acid in virus-infected plants [Citation48]. Findings showed that elevated CO2 down-regulated the expression of JA dependent defense genes in wild-type Arabidopsis thaliana plants infested by aphids [Citation49]. In the study on the gene expression profile involved in JA biosynthesis and signal transduction during the growth and development of carrots, it was found that the JA content showed a trend of decreasing firstly and then increasing, and then decreasing and increasing again, as carrots are stage-dependent on JA [Citation10]. Therefore, it remains to be further studied whether carrot plants will be dependent on JA in different stages under CO2 enrichment, or whether there is a possibility of weakened JA response to environmental adaptation after long-term CO2 application, and whether changes in JA content are coordinated by biotic or abiotic stresses.
Conclusions
This study analyzed key enzymes of JA metabolism and expression of coding genes in carrot under CO2 enrichment by RNA-Seq, we found that expression of 5 genes is consistent with JA content. By exploring JA metabolism-related genes through GO annotation and also screening of 21 DEGs, in total, 26 genes were found related to JA metabolism or signal transduction. These results suggested that these genes are directly related to JA biosynthesis under CO2 enrichment, therefore JA not only regulates the growth and development of carrot, but also actively responds to changes in CO2 concentration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ma J, Li J, Xu Z, et al. Transcriptome profiling of genes involving in carotenoid biosynthesis and accumulation between leaf and root of carrot (Daucus carota L.). Acta Biochim Biophys Sin (Shanghai). 2018;50(5):481–490.
- Zhang ZH, Sun S, Liu Y, et al. Effects of CO2 enrichment on photosynthetic characteristics of greenhouse tomato during fruiting stage. Chin J Ecol. 2018;37(5):1398–1402.
- Kimball BA, Kobayashi K, Bindi M. Response of agricultural crops to free air CO2 enhancement. Adv Agron. 2002;77:293–368.
- Reddy AR, Rasineni GK, Raghavendra AS. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr Sci. 2010;99(1):46–57.
- Mamatha H, Rao NK, Laxman RH, et al. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (Lycopersicon esculentum Mill) cv. Arka Ashish. Photosynt. 2014;52(4):519–528.
- Yang DG, Zhang XD. Progress of jasmonates and its signal transduction pathway. Biotechnol Bull. 2009;(2):43–49.
- Wu JS, Chong K. The molecular biology research on the action of jasmonates. Chin Bull Botany. 2002;19(2):164–170.
- Jiang KJ, Pi Y, Hou R, et al. Jasmonate biosynthetic pathway: its physiological role and potential application in plant secondary metabolic engineering. Chin Bull Botany. 2010;45(2):137–148.
- Teng N, Wang J, Chen T, et al. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006;172(1):92–103.
- Wang G, Huang W, Li M, et al. Expression profiles of genes involved in jasmonic acid biosynthesis and signaling during growth and development of carrot. Acta Biochim Biophys Sin (Shanghai). 2016;48(9):795–803.
- Wang Q. Study on the effects of high-concentration carbon dioxide on the endogenous hormones and organic acid content of rice seedlings [dissertation]. Shenyang (China): Shenyang Normal University; 2019.
- Song H, Li Y, Xu X, et al. Analysis of genes related to chlorophyll metabolism under elevated CO2 in cucumber (Cucumis sativus L.). Sci Hortic. 2020;261:108988.
- Deng A, Tan W, He S, et al. Monoclonal antibody-based enzyme linked immunosorbent assay for the analysis of jasmonates in plants. J Integr Plant Biol. 2008;50(8):1046–1052.
- Sun M, Qi X, Hou L, et al. Gene expression analysis of Pak Choi in response to vernalization. Plos One. 2015;10(10):e0141446.
- Wang WM, Wang C, Li C. Effects of elevated atmospheric CO2 concentrations on growth of plants. Acta Bot Bor Occid Sin. 2000;20(4):676–683.
- Chen H, Tan XF. Identification of linolenic acid metabolism pathway based on transcriptome data of Vernicia fordii kernels during tung oil synthesis stage. Sci Sil Sin. 2015;51(3):41–48.
- Gong CR, Li YM, Yang LJ. Relationship between lox activity and SA and JA accumulations in tobacco leave under water stress. Sci Agri Sin. 2003;36(003):269–272.
- Zhang C, Jin Y, Liu J, et al. The phylogeny and expression profiles of the lipoxygenase (LOX) family genes in the melon (Cucumis melo L.) genome. Sci Hortic. 2014;170:94–102.
- Lian QL, Li XX, Zhong XH. Cloning and expression analysis of allene oxide cyclase gene GhAOC from Gladiolus hybridus. J China Agri Univ. 2012;17(5):46–53.
- Stenzel I, Otto M, Delker C, et al. ALLENE OXIDE CYCLASE (AOC) gene family members of Arabidopsis thaliana: tissue- and organ-specific promoter activities and in vivo heteromerization. J Exp Bot. 2012;63(17):6125–6138.
- Wang LM, Zhu YM, Tong XC, et al. Molecular cloning and characterization of an Allene Oxide Cyclase gene associated with fiber strength in cotton. J Integ Agri. 2014;13(10):2113–2121.
- Chen LL, Wang YR, Guo YF, et al. Cloning and expression analysis of jasmonic acid carboxyl methyltransferase gene (JMT) in sugarcane (Saccharum spp. hybrids). J Agri Biotechnol. 2020;28(11):1936–1946.
- Xue RH, Zhang B. Increased endogenous methyl jasmonate altered leaf and root development in transgenic soybean plants. J Genet Genomics. 2007;34(4):339–346.
- Adam R, Magdalena AW. M. Genes encoding cucumber full-size ABCG proteins show different responses to plant growth regulators and Sclareolide. Plant Mol Biol Report. 2016;34:720–736.
- Sun Y. Study on stress tolerance of Arabidopsis glycosyltransferase gene [dissertation]. Jinan (CHN): Shandong University; 2013.
- Chini A, Fonseca S, Fernández G, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448(7154):666–671.
- SchererGuenther FE. Patatin like phospholipase A genes from Arabidopsis are involved in auxin functions. Catedra Nova. 2003;2013:123–128.
- Hall AE, Findell JL, Schaller GE, et al. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 2000;123(4):1449–1458.
- Rodrigues A, Adamo M, Crozet P, et al. ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein Kinase1 Signaling in Arabidopsis. Plant Cell. 2013;25(10):3871–3884.
- Kuromori T, Miyaji T, Yabuuchi H, et al. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci U S A. 2010;107(5):2361–2366.
- Barry SC, Cooper DNW, Hamilton TA, et al. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9(4):525–535.
- Guan P, Wang R, Nacry P, et al. Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc Natl Acad Sci U S A. 2014;111(42):15267–15272.
- Kong YZ, Zhou GK, Yin YB, et al. Molecular analysis of a family of Arabidopsis genes related to galacturonosyltransferases. Plant Physiol. 2011;155(4):1791–1805.
- Ikeda M, Mitsuda N, Ohme-Takagi M. ATBS1 INTERACTING FACTORs negatively regulate Arabidopsis cell elongation in the triantagonistic bHLH system. Plant Signal Behav. 2013;8(3):e23448.
- Chen XL, Wang AX, Zhang ZZ, et al. Genome-wide identification and bioinformatics analysis of NAC gene family in tomato. Plant Physiol Commun. 2014;50(4):461–470.
- Fang ZH, Liu JN, Zhang Y, et al. Bioinformatics analysis of NAC gene family in bothriochloa ischaemum. Acta Agres Sin. 2020;28(05):98–106.
- Fu L, Yu X, An C. OsCPK20 positively regulates Arabidopsis resistance against Pseudomonas syringae pv. tomato and rice resistance against Magnaporthe grisea. Acta Physiol Plant. 2014;36(2):273–282.
- Jung C, Zhao P, Seo JS, et al. PLANT U-BOX PROTEIN10 regulates MYC2 Stability in Arabidopsis. Plant Cell. 2015;27(7):2016–2031.
- Aguilar-Benitez D, Rubio J, Millán T, et al. Genetic analysis reveals PDH1 as a candidate gene for control of pod dehiscence in chickpea. Mol Breeding. 2020;40(4):40.
- Wu R, Zhang F, Liu L, et al. MeNA, controlled by reversible methylation of nicotinate, is a NAD precursor that undergoes long-distance transport in Arabidopsis. Mol Plant. 2018;11(10):1264–1277.
- Todd J, Post-Beittenmiller D, Jaworski JG. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J. 1999;17(2):119–130.
- Hussain RMF, Kim HK, Khurshid M, et al. Overexpression of AtWRKY50 is correlated with enhanced production of sinapic derivatives in Arabidopsis. Metabolomics. 2018;14(3):25.
- Takase T, Nakazawa M, Ishikawa A, et al. DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol. 2003;44(10):1071–1080.
- Ballhorn DJ, Reisdorff C, Pfanz H. Quantitative effects of enhanced CO2 on jasmonic acid induced plant volatiles of lima bean (Phaseolus lunatus L.). J Appl Botany Food Qual. 2012;84(1):65–71.
- Fatma K, Zhao W, Richards JT, et al. Transcriptional and metabolic insights into the differential physiological responses of Arabidopsis to optimal and supraoptimal atmospheric CO2. Plos One. 2012;7(8):e43583.
- Zhang FF, Wang YL, Huang ZZ, et al. Effects of CO2 enrichment on growth and development of impatiens hawkeri. SciWorldJ. 2012;2012:601263.
- Zhao TH, Wang MY, Zhang WW, et al. Effects of elevated atmospheric CO2 concentration on plant photosynthesis. Ecol Environ. 2006;15(5):1096–1100.
- Huang L, Ren Q, Sun Y, et al. Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol (Stuttg). 2012;14(6):905–913.
- Sun Y, Guo H, Zhu-Salzman K, et al. Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 2013;210:128–140.