Abstract
Flower color is one of the most important qualitative characteristics of ornamental horticultural plants, which mainly depends on anthocyanins and carotenoids. Modification of flower color in ornamental plants involves many aspects, including complicated structural gens, transcription factors and metabolic pathways. Anthocyanins are important secondary metabolites in the flavonoid biosynthesis pathway, which mainly derive from six anthocyanidins. Anthocyanin has antioxidant activity, protects plants and performs some other functions. Studying the genes and regulatory factors in the biosynthesis pathway will contribute to cultivating novel flower color plants and to improving plants’ resistance. To date, a series of structural and regulatory genes that are involved in anthocyanin synthesis and color formation have been reported, whose functions have also been revealed. Some color-related transgenic flowers have been successfully obtained. Carotenoids are the source of the yellow pigments in the petals of many flowers. The carotenoid biosynthesis pathway as well as its major enzymes and genes are conducive to regulate its biosynthesis via genetic engineering methods and thereby changing the color of flowers. In this paper, we review the plant flower color in terms of mechanisms of plant pigment formation, classification of flower color, biosynthesis pathway, and genes related to anthocyanin formation. Subsequently, we analyze the regulating mechanism of the expression of these genes, including transcription regulation and post-transcription regulation. The methods and achievements in flower color improvement in recent years are summarized. Finally, analyzing the molecular regulatory network in flower color formation provides a theoretical basis for the improvement and modification of flower color.
Introduction
Flower color is one of the most important characters of plants, with anthocyanin being an important secondary metabolite in plants that is formed by the catalysis of a series of enzymes [Citation1]. Anthocyanin has certain nutrient and pharmacological values and is mainly distributed in the vacuoles of flowers and fruits. Different anthocyanin contents produce different colors in plants, such as orange, red, purple and blue [Citation2]. The formation of anthocyanin is influenced by external environmental conditions and is mediated by the development of the plant [Citation3]. Additionally, anthocyanin can help plants resist low temperatures, drought and fungal infections; prevents ultraviolet damage and insect attacks, and induces insects to pollinate and spread seeds [Citation4, Citation5].
With social and economic development, the demand for flowers is increasing, as is the aesthetic level. Colorful flowers attract higher consumer purchases and account for increasing market shares. Therefore, culturing new plants with different flower colors is becoming increasingly important. In recent years, many researchers have been devoted to studying the flower colors of plants. Relatively comprehensive research on the formation and classification of flower colors and the regulatory mechanisms of the anthocyanin synthesis pathway have been achieved. On the basis of full understanding of the molecular biological basis and regulatory mechanisms of flower color production, it will be possible to modify the color of plants through genetic engineering techniques in the future.
Principle of flower color formation
When light radiates onto petals and penetrates through the pigmentary layer, some light is absorbed, while some is reflected by the spongy parenchyma and then moves through the pigmentary layer, thus producing visible colors. These colors are manifested as flower colors of ornamental plants [Citation6]. The flower color of ornamental plants is related to the categories and contents of the pigments (including relative contents of multiple pigments) in the petal cells, as well as to the physical behaviors and color formation caused by the internal or surface structure of the petals. Among these, anthocyanin plays a leading role in flower color. An increase or decrease in anthocyanin content in petals can change the flower color [Citation7].
The color formation is influenced by many factors, including the following: (1) Collaborative color formation: one pigment can cooperate with other pigments or compounds to develop colors together. Owing to the coexistence of these pigments, petals present various transition colors. For example, the yellow color of the tulip is the result of the cooperation of anthocyanin and carotenoids. Some anthocyanins can produce a phthalein reaction with organic acids (e.g., coumaric acid). (2) Chelation: there are many heavy metal ions in enchylema, including Al, Fe, Mg and Mo. Thus, the pigment is often integrated. Chelation of anthocyanin changes the flower color to purple to some extent. (3) pH value: pH changes in the cytochylema often induce changes in flower color. Anthocyanin is greatly influenced by pH and can produce red flowers under acidic conditions, light purple in a neutral state, and blue under alkaline conditions. (4) Internal and external factors: temperature, light, humidity and atmosphere determine the different categories and contents of anthocyanin [Citation8–10]. Several experiments have shown that ozone can change the pigment contents in plants by triggering the stress response, thus influencing flower color [Citation11]. The lack of phosphorus might increase the accumulation of anthocyanin [Citation12]. The red leaf lettuce often develops poor leaf colors under low illumination strength and has high expression of genes related to anthocyanin biosynthesis under high irradiance, which further proves the influences of light intensity on anthocyanin biosynthesis [Citation13].
Categories of flower color
The flower color of plants is developed by flavonoids, carotenoids, betalains and other pigments [Citation14]. Among them, carotenoids and flavonoids have extensive distribution in two pigment groups in plants [Citation1, Citation15]. Betalains only exist in a few genera of Caryophyllales. Some colorful plants contain more than one pigment. For instance, a recent study on the oriental lily and the Asian lily reported that the color of the oriental lily is mainly developed by anthocyanin and only a few varieties contain carotenoids. For the Asian lily, the pink petals are mainly developed by anthocyanin, the yellow and orange petals are caused by carotenoids, whereas the red petals contain both anthocyanin and carotenoids [Citation16]. Therefore, the flower color of many plants is a reflection of the combination of multiple pigments.
Flavonoids
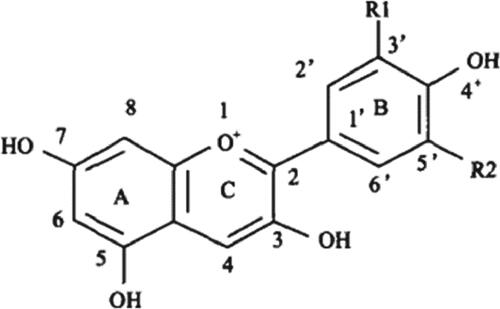
Flavonoids are an important secondary metabolite in plants. They can be divided into chalcone, aurone, flavone, flavonol, flavanone, dihydrochalcone, catechin, flavan-3-4-alcohol, double flavonoids, isoflavone, proanthocyanidin and anthocyanin [Citation17]. Anthocyanin is a type of soluble pigment that is extensively distributed in plants. The basic structure of anthocyanin is 3,5,7-trihydroxy benzene-2-phenyl benzofuran (). Anthocyanin is formed by the modification of anthocyanidin elements using glycosyl, acyl and methyl groups, which creates diversified anthocyanin structures, and thus changeable flower colors. Most anthocyanins are substituted with hydroxyls on the carbon loci 3, 5 and 7 of the flower color groups. Different locations of the substituent groups present different flower colors, such as orange-yellow, red, purple and blue [Citation2]. Anthocyanin often produces a purple color as hydroxylation progresses; however, it develops red as methylation and glycosylation continue and turns blue as a response to acylation.
Anthocyanidin in higher plants can be divided into six classes, including pelargonidin (Pg), cyanidin (Cy), delphinidin (Dp), peonidin (Pn), petunidin (Pt) and malvidin (Mv). The first three classes are common and the other three classes are formed by the methylation of B-ring groups. The blue color in flowers deepens with an increase in B-ring hydroxylation, whereas the blue color weakens and the red color deepens with the intensification of methylation of the B-ring hydroxyls. Pg, Cy and Dp present brick-red, red-pink and bluish violet colors, respectively. Pn, Mv and Pt create colors from amaranth to bluish violet [Citation18]. Previous studies have shown that spots on the petals and perigone in the red lily were caused by the accumulation of Cy. The flower color of the lily was found to be positively correlated with the content of anthocyanin, i.e., the higher the anthocyanin content, the deeper the color [Citation19].
There are three major biosynthesis mechanisms of anthocyanin, namely, the synthesis pathway of pelargonin (from orange red to erythrine), the synthesis pathway of cyanin (from pink to red), and the synthesis pathway of delphinin (bluish violet). Different synthesis pathways lead to different flower colors. The lily, tulip and carnation have no blue flowers due to the absence of the delphinin synthesis pathway. Without the synthesis pathway of pelargonin, Petunia and Iris tectorum Maxim. have no brick red flowers. There is no pink Gentiana because of the lack of the cyanin synthesis pathway [Citation2, Citation20, Citation21]. These all confirm that anthocyanin, an important secondary metabolite in metabolic pathways of flavonoids, is vital to the color development of plants.
Anthocyanin in higher plants is the coordination effect product of multiple key enzymes in the biosynthesis pathway of flavonoids. The biosynthesis pathway of flavonoids can be divided into three stages. In the first stage, phenylalanine is deaminated by phenylalanine ammonia-lyase into trans-cinnamic acid. The trans-cinnamic acid further produces p-coumaric acid by hydroxylation based on cinnamic acid 4-hydroxylase. Later, p-coumaric acid produces p-coumaroyl coumaric acid (CoA) by catalysis of the 4-coumaric acid: CoA ligase. This stage can be observed in many secondary metabolic pathways. The second stage starts from CoA to the flavanonols. It is the key reaction in the metabolism of flavonoids. During this stage, substrates produced in the first stage produce naringenin by the catalysis of chalcone synthase (CHS) and chalcone isomerase (CHI). Naringenin further produces dihydrokaempferol (DHK), which is an essential precursor substance for various anthocyanins via the catalysis of flavanone 3-hydroxylase (F3H). The third stage is the synthesis and modification of different anthocyanins. Dihydroquercetin (DHQ) and dihydromyricetin (DHM) are formed upon hydroxylation in different loci of DHK by the flavonoid 3′-hydroxylase (F3′H) and flavanone 3′, 5′hydroxylase (F3′5′H). In addition, DHK, DHQ and DHM in the third stage are further reduced to various colorless anthocyanins by dihydroflavonol-4-reductase (DFR). The colorless anthocyanins are changed into colorful anthocyanins because of the catalysis by anthocyanidin synthase (ANS). Finally, more stable anthocyanins are formed through modifications, such as glycosylation of UDP-glucose: flavonoid-3-O-glucosyltransferase (UF3GT or 3GT), acylation of acyltransferase (AT) and methylation of methyltransferase (MT). These stable anthocyanins are transferred by glutathione S-transferase to the vacuole and concentrate in the vacuole [Citation22]. As a type of major flavonoid pigment, anthocyanins control the pink-red, red, violet and blue colors of flowers. The synthesis of anthocyanin is regulated by many key enzymes (), such as CHS, CHI, DFR, F3H and ANS. The genetic engineering of flower color modification is realized by encoding genes of these key enzymes.
Figure 2. General anthocyanin biosynthetic pathway in higher plants [Citation23–25].
![Figure 2. General anthocyanin biosynthetic pathway in higher plants [Citation23–25].](/cms/asset/76e13563-45c0-4f51-b616-752581bb4029/tbeq_a_1960605_f0002_b.jpg)
PAL: phenylalanine ammonia-lyase; C4H: cinnamic acid 4-hydroxylase; 4CL: 4-coumaric acid: CoA ligase; CHS: chalcone synthase; CHI: chalcone isomerase; F3H: flavanone-3-hydroxylase; F3′H: flavonoid 3′-hydroxylase; F3′5′H: flavanone 3′, 5′hydroxylase; DFR: dihydroflavonol-4-reductase; 3GT: flavonoid-3-O-glucosyltransferase; AT: acyltransferase; MT: transmethylase
Carotenoids
Carotenoids are an important component of plant photosynthesis that can change plant organs from yellow to red [Citation26]. They are the source of the yellow pigments in the petals of many flowers. The interpretation of the carotenoid biosynthesis pathway, as well as its major enzymes and genes, are conducive to regulate its biosynthesis via genetic engineering methods and thereby change the color of flowers.
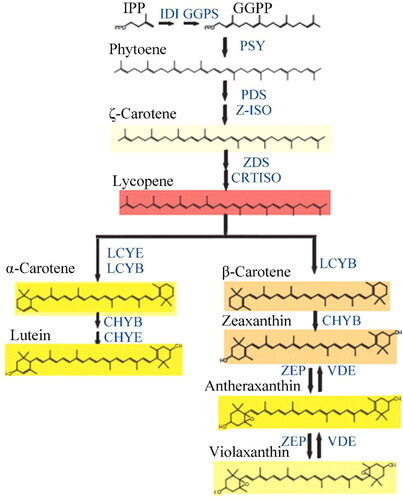
In the carotenoid biosynthesis pathway of plants [Citation1] (), the precursor of carotenoids is IPP (isopentenyl pyrophosphate). It is catalysized by GGPS (geranylgeranyl diphosphate synthase) and other enzymes to generate GGPP (geranylgeranyl pyrophosphate). Under the catalysis of various enzymes, phytoene, ζ-carotene, lycopene, α-carotene and β-carotene are generated through a series of chemical reactions by GGPP. Then, lutein was further synthesized from α-carotene, and zeaxanthin, antheraxanthin and violaxanthin were further synthesized from β-carotene. Enzymes involved in the transformation from GGPP to carotene include PDS (phytoene desaturase), ZDS (ζ-carotene desaturase), PSY (phytoene synthase), LCYE (lycopene ε-cyclase) and LCYB (lycopene β-cyclase).
Phytoene, a leuco-compound, is firstly synthesized in the pathway of carotenoid synthesis. The product from the first dehydrogenation is ζ-carotene, which is further stripped of four hydrogens under the catalysis of ZDS to form the pink lycopene. LCYE and LCYB can catalyze the conversion of lycopene into the α-carotene enzyme. Then, through a continuous hydroxylation reaction, lutein formed from α-carotene. Lycopene is catalyzed by LCYB to produce β-carotene. After a continuous hydroxylation reaction and cyclization reaction, zeaxanthin, antheraxanthin and violaxanthin were produced. The enzyme involved in both of these reactions is ZEP and this whole cycle is called the Lutein cycle [Citation27].
IPP: isopentenyl pyrophosphate; IDI: isopentenyl-diphosphate δ-isomerase; GGPP: geranylgeranyl pyrophosphate; GGPS: GGPP synthetase; PSY: phytoene synthase; PDS: phytoene desaturase; ζ-carotene ZDS: ζ-carotene desaturase; Z-ISO: ζ-isomerase; CRTISO: carotenoid isomerase; LCYE: lycopene ε-cyclase; LCYB: lycopene β-cyclase; CHYB: β-carotene desaturase; CHYE: ε-carotene desaturase; ZEP: zeaxanthine peroxidase; VDE violaxanthindeepoxidase
Carotenoids also exist in lily petals. Some yellow tepals include antheraxanthin, violaxanthin and lutein, whereas the orange and red lily petals contain capsorubin [Citation17]. A previous study showed that the carotenoid content differed significantly at different positions in lily flowers and the carotenoid content in the anther was higher than that in the other components [Citation28]. The capsorubin synthetase gene of the lily has stable overexpression in the callus of I. germanica. Xanthophylls, the precursor of capsorubin, turn the callus of transgenes from normal yellow to reddish-orange. A previous study increased our understanding of the molecular and genetic mechanisms of K-carotene synthesis in Lilium longiflorum, as well as the transgenetic modification of flower color and fruit color in several plants [Citation29].
Other water-soluble pigments related to alkaloids
Other water-soluble pigments related to alkaloids include betaine, berberine and papaverine. Betaine can be further divided into betacyanin that presents red and purple, and betaxanthin which presents yellow colors. Berberine develops the deep orange color in berberis and papaverine produces the yellow color of Meconopsis Vig.
The biosynthesis pathways of flavonoids and carotenoids are clearly understood. However, the biosynthesis of alkaloid pigments has rarely been discussed to date. In contrast, the anabolic pathway of the anthocyanin has been explored in depth via abundant biochemical experiments. The metabolic pathways that controls flower color are very complicated. Studies have focused on identifying the specific biochemical material that determines flower colors. Subsequently, genetic engineering of the metabolic pathway that forms this biochemical substance is performed. This includes analyzing enzymes that catalyze different reaction steps, the genes that encode these enzymes and the regulation of gene expression.
Genes related to the anthocyanin synthesis pathway
Flower color genes can be divided into seven classes, namely, structural genes, modification genes, regulatory genes, genes influencing pigment concentration, genes related to flower structure (producing pigments at specific positions of petals), genes or factors influencing flower color (cooperative coloring and metal ions), and genes controlling morphological features (e.g., shape and distribution of pigment cells) [Citation30].
Although the synthesis and accumulation of anthocyanin are closely related to multiple genes and other influencing factors during plant development, structural genes and regulatory genes are the major determinants of anthocyanin synthesis. Transcription factors encoded by regulatory genes can control the expression intensity of structural genes, which determine the synthesis pathways of anthocyanin [Citation31, Citation32]. Some structural genes and regulatory genes related to the metabolism of anthocyanin have been separated and cloned by protein purification, transposons tags, polymerase chain reaction, identification, and screening means. The direct change in flower color and improvement in the ornamental value and quality of the plants are realized by regulating the expression levels of transcription factors based on key gene-directed mutagenesis and transgene technology.
Structural genes
Structural genes encode anthocyanin biosynthesis enzymes directly. CHS, CHI, F3H, F3′H, DFR and ANS are structural genes in the anthocyanin synthesis pathway, which have been studied in many plants [Citation33, Citation34]. The regulation of these enzymes via genetic engineering can influence anthocyanin biosynthesis, thus changing flower colors or improving the variety of flowers. Abe et al. [Citation35] and Nakano et al. [Citation36] constructed genes related to anthocyanin and carotene synthesis in the petals of the Asian lily onto the same molecular linkage map, which provided a powerful tool for the screening and control of flower colors. Several genes related to anthocyanin synthesis in the lily have been isolated and identified [Citation37–40]. The flower color of the lily is mainly controlled by the flavonoid biosynthesis pathway and key enzymes related to isoflavones biosyntheses, such as CHS, CHI, F3H, FLS and DFR [Citation41]. Identification of CHS and DFR, which are key enzymes throughout the synthesis pathway of flower colors, laid the foundation for the molecular study of flower colors in the lily plant.
Chalcone synthase
CHS is a rate-limiting enzyme of the isoflavones biosynthesis pathway that catalyzes the transformation from hydroxyl cinnamoyl-CoA to tetrahydroxy chalcone [Citation42]. In addition, it is the first key enzyme of isoflavones synthesis and has specific expression in flowers [Citation43]. Changes in CHS expression might induce alterations in flower colors [Citation44]. Sense and antisense plant expression vectors of CHS in the lily have been used to transform the tobacco leaf disk using the agrobacterium-mediated method. The petal color remained the same in sense genetically modified tobacco; however, the petal colors of some plants of antisense genetically modified tobacco became shaded [Citation45]. Thus, the genetic transformation of CHS is one of the effective regulatory means of flower color development. The CHS promoter deletant of lily was transferred into Arabidopsis to detect GUS (β-glucuronidase) activity, which demonstrated that the CHS promoter can drive the expression of an exogenous gene in transgenetic plants and presents evident specificity in tissues and organs. The TACPyAT framework of the CHS promotor plays a key role in the regulation of specific expression in organs [Citation46]. The transcriptional level of CHS was relatively low in the green pulp of grapes, which might be an important factor restricting the accumulation of anthocyanin in the grape pulp [Citation47].
Chalcone isomerase
CHI is the key enzyme during the early synthesis of isoflavones. It regulates the isoflavones metabolic pathway with CHS, and plays an important role in fruit development, pigment accumulation in flower organs, and resistance to UV damage in the leaves [Citation48]. Dou Xiaoying [Citation49] cloned CHI from the tepals of the oriental lily ‘Sorbonne’, which proved the time and spatial specificity of LhCHI expression. The expression level of CHI is bound to the flower color of the plants. The pollen of Petunia changes from a yellow to green color upon the downregulation of CHI-A expression [Citation50]. The petal colors and pollen of the tobacco plant fade owing to the inhibited expression of CHI [Citation51]. The inhibited expression of CHI in Dianthus caryophyllus and cyclamen using genetic engineering produce increased accumulation of chalcone in petals, thus developing yellow flowers [Citation52, Citation53]. The post-transcription silencing of the DvCHS2 gene in the white part of the two-color Dahlia pinnata plays a key role in color development [Citation54].
Flavanone 3-hydroxylase
F3H is the housekeeping enzyme that synthesizes Pg and the key enzyme during the early synthesis of anthocyanin. As an oxidative glutaric acid-dependent oxygenase, F3H requires Fe2+, oxygen and 2-oxoglutarate as the assisting factor. Based on its substrate specificity, F3H catalyzes hydroxylation at the C3 position of 4,5,7-trihydroxy flavanones, thus generating DHK. DHK is an essential precursor for the synthesis of anthocyanin. Hence, the F3H gene plays an important role in the color development of plants [Citation55]. The relevant gene of F3H was cloned from the snapdragon for the first time [Citation56]. Subsequently, studies on cloning and expression of F3H have been reported successively in many plants, such as the turnip, Fagopyrum tataricum and Malus spectabilis [Citation57–59]. In genetic engineering studies, changing the flower color using F3H has also shown great progress in some plants. For example, a white Torenia fournieri was achieved by processing the F3H of the bluish-violet flowers using RNA interference technology [Citation60]. The expression of endogenous F3H genes is inhibited by the introduction of the antisense F3H into the orange carnation, which contains pelargonin only, thus creating a cream or yellow carnation [Citation61]. AcF3H is expressed in all organs of the orchid. It has high expression during early flower development, but low expression during the partial or full opening of the flower [Citation62].
Flavonoid 3′-hydroxylase
F3′H is the key enzyme that synthesizes the red Cy in the anthocyanin synthesis pathway. As a monooxygenase of the cytochrome P450 family, it must use NADPH as the accessory factor. F3′H generally has expression in all tissues, reaching a peak during the budding period with anthocyanin accumulation. The expression of F3′H decreases as the flower becomes more mature. The F3′H transcription level influences anthocyanin synthesis and it is crucial to the development of the pink-red color in ornamental plants. F3′H is the most significant enzyme during the synthesis of Cy and can develop pink-red inflorescence [Citation63]. F3′H is mainly expressed in copies in the tulip [Citation64]. Two alleles have been discovered in Dahlia and Lilium [Citation37, Citation65]. LhF3′H not only represents high expression in organs with anthocyanin accumulation but expressions have been detected in the ovary and leaves. The abundance of F3′H is low during the early developmental stage of the perianth and continues to be transcribed during the post-flowering stage [Citation19].
Flavonoid 3′,5′-hydroxylase
F3′5′H is the key enzyme in the biosynthetic pathway of delphinidin. As a monooxygenase of the cytochrome P450 family, F3′5′H must use NADPH as the accessory factor. The absence of blue plants in nature is caused by the lack of F3′5′H [Citation20]. Isoflavones are one of the major anthocyanins in plants and also play an important role in multiple functions of plants, such as fungus infection prevention [Citation66] and plant fertility [Citation39]. Xu et al. [Citation67] cloned the flavonoid 3′,5′ hydroxylation enzyme genes nf1 and nf2 that control flower color from the purple petals of the Petunia using RT-PCR (reverse transcription-polymerase chain reaction). Meanwhile, the plants expression vector was constructed and the genetic transformation of Lilium longiflorum was undertaken using the agrobacterium-mediated method, thus obtaining transgenetic plants. The transgenetic plants were detected by Southern hybridization. Moreover, the transfer of F3′5′H, which is the key enzyme that controls the synthesis of blue anthocyanins, into L. longiflorum produced a positive PCR of 23/57 resistance seedlings and 16/23 seedlings passed the Southern hybrid identification. Qi Yinyan et al. [Citation68] placed F3′5′H genes of the butterfly orchid and DFR of the hyacinth into lilies for instantaneous expression. They observed purple cells in the epidermis cells of the lily petals, indicating that genetic engineering might produce blue flowers.
Dihydroflavonol 4-reductase
DFR is one of the key enzymes in the isoflavones synthesis pathway and is a member of the ADPH-dependent short-chain reductase family [Citation69] that maintains the conservative “VTGAAGFIGSWLIMRLLERGY” NADP structural domain in different species [Citation70]. The catalysis substrates, DHM, DHQ and DHK, produce colorless delphisine, colorless anthocyanin and colorless pelargonidin, respectively. Thus, different types of anthocyanosides are formed to develop different flower and fruit colors [Citation71, Citation72]. The anthocyanin derived from delphisine is the major type that controls blue flowers. The amino acid residues at position 134 in the binding region of the DFR substrate are mainly Asp and Asn, which determine the substrate specificity. Differences in the specificity and expression levels of these amino acid residues induce a diversity of colors. DFR in Petunia mainly catalyzes DHM to produce colorless delphisine and then catalyzes DHQ, but it will not influence DHK [Citation73]. The DFR gene is the key gene in the anthocyanin synthesis pathway. L. longiflorum with a yellow anther lacks anthocyanin in its petals and anther due to DFR mutation [Citation74]. Research on the two-color Chinese herbaceous peony has demonstrated that the significant differential expression of PsDFR might play an important role in the development of the two colors in this plant [Citation75].
Anthocyanidin synthase
ANS, also known as leucoanthocyanidin oxygenase, is a 2-ketoglutaric acid double oxygenase. As a key enzyme at the end of the anthocyanin synthesis pathway, ANS can catalyze the transformation of leucoanthocyanidin into colored anthocyanin [Citation76, Citation77]. ANS has a domain of Fe2+-dependent 2-ketoglutarate [2OG-Fe(II)] dioxygenase family genes. Rosati et al. [Citation78] cloned the ANS gene and promoter from Forsythia suspensa and concluded that the lack of ANS gene expression caused no accumulation of anthocyanin in the petals. A previous study on MaANS in Muscari botryoides discovered that its promoter contained many cis-acting elements (TATA-box and CAAT-box); anaerobic responsive element ARE and a GC motif, light, MeJA (methyl jasmonate) and salicylic acid response elements; and MYB binding elements. It also executes many functions [Citation79]. The ANS gene might help plants to respond to adverse stress and participate in metabolic pathways of phytohormones. ANS expression is regulated by MYB transcription factors. ANS is expressed in all tissues; however, the expression levels vary. ANS expression in deep or bright-colored tissues is high. ZjANS shows high expression in purple ear tip and stolon but presents low expressions in green tissues [Citation80]. Expressions of NtANS1 and NtANS2 in tobacco are upregulated during the flower maturity stage; however, their expression levels are downregulated slightly during the late stage of flower development [Citation81]. ANS mutation in raspberry causes the yellow raspberry [Citation82].
Flavonoid 3-O-glucosyltransferase
Flavonoid 3-O-glucosyltransferase (3GT) is the enzyme that is encoded by the last structural gene in the anthocyanin synthesis pathway. It can catalyze unstable anthocyanins to form stable anthocyanin. Thus, 3GT transfers glucose on UDP-glucose onto the C3 hydroxy of anthocyanin molecules, which is beneficial for the stability of the anthocyanin mechanism [Citation83]. Despite increasing water solubility and stability of anthocyanin, 3GT is very important in changing flower color [Citation84]. Currently, 3GT genes in the anthocyanin metabolic process have been separated successfully from many plants, including Litchi chinesis Sonn [Citation85], and Freesia hybrida [Citation86]. Overexpression of Fh3GT1 intensifies the accumulation of anthocyanin and flavonol and adjusts the gene expression levels related to flavonol biosynthesis in transgenetic Petunia [Citation87].
In conclusion, structural genes adjust flower or fruit colors indirectly by facilitating or inhibiting CHS, CHI and ANS expression to increase or decrease anthocyanin content. Among the structural genes related to flower color, F3′H and F3′5′H determine the position and degree of hydroxylation of dihydroflavonol, so that the synthesized categories of anthocyanins are changed. Hence, flower colors are changed. Therefore, F3′5′H is called the “blue gene” and F3′H is called the “red gene.” Existing studies on the modification of blue flowers mainly focus on these two genes. CHS and DFR can influence the accumulation of anthocyanin indirectly because of regulation by the MYB transcription factor. Flower color modification based on DFR is another major research direction.
Regulatory genes
The regulatory gene is a gene that controls expression intensity and is a form of a structural gene. It is responsible for encoding regulatory factors and controlling the transcription and activity of multiple enzymes in biosynthesis pathways. MYB [Citation88–90], bHLH [Citation91] and WDR [Citation92] are several major transcription factors that adjust anthocyanin synthesis. MYB or MYB-bHLH-WDR complexes (MBW) are combined onto the promoter of structural genes to regulate downstream genes [Citation1]. The interaction of these three transcription factors is larger than the effect of a single factor. Transcription factors can activate or inhibit the transcription of genes independently or can combine with other proteins to regulate transcription.
MYB transcription factors
MYB, an important transcription factor in plants, is a type of DNA-binding protein and possesses a highly conservative MYB structural domain. Generally, each MYB structural domain covers three highly conservative tryptophan residues and maintains the “helix-loop-helix” structure of the MYB protein domain. MYB transcription factors can be divided into three types, including R3-MYB with one structural domain, R2R3-MYB with two structural domains, and R1R2R3-MYB with three structural domains. MYB transcription factors related to anthocyanin synthesis generally cover R2 and R3 sequences (R2R3-MYB) or contain R3 sequences (R3-MYB) [Citation93].
The synthesis of flavonoids in Arabidopsis thaliana is regulated by the MYB gene. The EBGs, early flavonoid synthesis gene, is mainly regulated by AtMYB11/PFG1, AtMYB12/PFG1 and AtMYB111/PFG3 [Citation94]. The late biosynthesis genes (LBGs) are mainly regulated by AtMYB75/PAP1, AtMYB90/PAP2 and AtMYB114 [Citation95]. The expression of AtMYB3, AtMYB4, AtMYB7 and AtMYB32 genes inhibited anthocyanin synthesis in A. thaliana [Citation96]. The expression of AtMYB123/TT2, AtMYB21 and AtMYB24 can promote anthocyanin accumulation in A. thaliana [Citation97]. In the plant, MYB transcription factors exert a positive regulating effect. In the mature developmental stage of the nectarine, abundant anthocyanins accumulate after ultraviolet irradiation, during which PpMYB10 plays an important role. PpMYB16 and PpMYB111 in Prunus persica are highly homologous with MdMYB17 and MdMYB111 in the apple. They are also phylogenetically clustered with the inhibitory regulators of A. thaliana (). However, the PpMYB16 and PpMYB111 expression levels decrease gradually with the continuous accumulation of anthocyanin, which can relieve the accumulation of anthocyanin [Citation98]. Therefore, there are few MYB transcription factors that have a negative regulatory effect on anthocyanin synthesis. In lily growth and development, anthocyanin accumulation and gene transcription in bud development is regulated by the LhMYB12 transcription factor [Citation99]. LhMYB12 can not only activate CHS and DFR promoters directly [Citation37] but also inhibits anthocyanin biosynthesis by obstructing its transcription under high temperatures [Citation100]. CHS, DFR and ANS expression in the colored parts of the lily are 7–30 times higher than those in the uncolored parts. Moreover, the expression level of LhMYB12 in the colored part is more than twice as high as that in the uncolored part [Citation101]. Based on the comparison between the green ovary and purple ovary of the hybrid Asian lily, LhMYB12-Lat is the key transcription factor that determines the anthocyanin distribution pattern in the ovary [Citation102]. The space-time transcription analysis of LhMYB6 and LhMYB2 in the Asian lily reveals that LhMYB6 and LhMYB2 facilitate anthocyanin biosynthesis and determine the specific accumulation of anthocyanin in tissues and organs [Citation103]. Under illumination conditions, LrMYB15 has expression in the outer petals, leaves and buds of the lily [Citation104], and LrMYB18 is significantly conducive to the formation of pigment spots in lily petals [Citation105]. MYB12 transcription factors in all lily species can be inherited stably during hybridization [Citation106].
Figure 4. Phylogenetic relationships between Arabidopsis thaliana MYB transcription factors and anthocyanin-related MYBs of other species (Modified from Ravaglia et al [Citation98]). Purple frame: MYB gene that regulates flavonoid synthesis pathway in early and late-stage; Green frame:MYB gene that inhibits anthocyanin synthesis; Red frame: MYB gene that promotes anthocyanin synthesis.
![Figure 4. Phylogenetic relationships between Arabidopsis thaliana MYB transcription factors and anthocyanin-related MYBs of other species (Modified from Ravaglia et al [Citation98]). Purple frame: MYB gene that regulates flavonoid synthesis pathway in early and late-stage; Green frame:MYB gene that inhibits anthocyanin synthesis; Red frame: MYB gene that promotes anthocyanin synthesis.](/cms/asset/65527f53-01f4-46a7-a953-cdcee347e34c/tbeq_a_1960605_f0004_c.jpg)
BHLH transcription factors
With their helix-loop-helix (HLH) structure, bHLH transcription factors can participate in the regulation of multiple physiological pathways, such as the development of flower organs and hormone response. The regulation effect of bHLH is very important for the regulation of anthocyanin synthesis pathways [Citation107]. Liu et al. [Citation108] discovered from anthocyanin synthesis regulation in Chinese bayberry fruits that MrbHLH1 can form a transcriptional complex with MrMYB1, which can activate certain structural genes. Consequently, anthocyanin synthesis is activated. However, highly homologous MrbHLH2 cannot activate anthocyanin biosynthesis. In lilies, the transcriptional regulation of LhbHLH is induced by illumination. Additionally, LhbHLH1 and LhbHLH2 both participate in anthocyanin biosynthesis, with the latter easily influenced by light [Citation109]. LhMYB6, LhMYB12 and LhbHLH2 cooperate to regulate the expressions of structural genes LhCHSa, LhDFR and LhCHSb in the tepals of lilies, which facilitate anthocyanin biosynthesis [Citation16]. In the strawberry, four bHLH transcriptional factors (FabHLH25, FabHLH29, FabHLH80 and FabHLH98) participate in anthocyanin biosynthesis and hormonal signal transduction in fruits [Citation110].
WD40 transcription factors
The WD40 repeat protein generally contains 4–16 WD elements tandem repeats, with each WD element having a conservative sequence composed of 40 amino acid residues. This sequence starts from the Gly-His at 11–24 residues at the N-end and ends with WD (Trp-Asp, WD) at the C-end, and can regulate anthocyanin biosynthesis on the transcription level [Citation111]. NtTTG2 protein, including a conserved WD40 domain, affects the formation of flower color in tobacco petals by regulating the expression of ANS. ANS expression in the NtTTG2 deletion mutant is inhibited, thus influencing the accumulation of anthocyanin [Citation112]. TTG1, a WD40 protein transcription factor in Arabidopsis, regulates the time-space expression of structural genes of anthocyanin via the cooperation of different bHLH transcription factors and MYB transcription factors [Citation113]. The overexpression of FtWD40 transcription factors of buckwheat in tobacco deepens the flower color. Its expression reaches the maximum in flowers and DFR and ANS expression in the petals are increased to some extent, indicating the transcriptional activity of FtWD40 [Citation114]. Mutation of one nucleotide in the violet mutant produces the loss of WD regulation functions and zero expression of DFR, which interrupts the anthocyanin biosynthesis pathways. Hence, violets produce white flowers [Citation115].
Others
As a type of specific transcription factor in plants, the SPL transcription factor has a highly conservative squamosa promoter-binding protein (SBP) structural domain, which is composed of approximately 80 amino acid residues and contains one new zinc finger and a nuclear localization signal [Citation116, Citation117]. The mechanism of action is interpreted by the regulation of gene expression through binding between the SPL transcription factor and the promoter region of the downstream target gene [Citation118, Citation119]. DFR genes are the binding loci of SPL, which show a negative correlation between them. Specifically, high expression of SPL inhibits DFR gene expression, thus reducing anthocyanin accumulation [Citation120].
MicroRNA (miRNA) is a type of non-coding single-chain RNA molecule that exists extensively in eucaryotes. It degrades or inhibits mRNA translation through a complementary pairing of target mRNAs, thus inhibiting transcriptional gene expression. However, previous studies on the color of plants have mainly focused on the physiological, biochemical and transcription levels, but only at the post-transcription level. Recently, research on miRNA in the regulation of plant color has achieved some progress and lays the foundation for further regulation of plant color at the post-transcription level. Researchers have discovered that the post-transcription miRNA level plays an irreplaceable role in the formation of plant color, especially in anthocyanin biosynthesis [Citation121]. miR858 in the tomato plant inhibits SIMYB7-like transcription. The endogenous miR585 is closed in the constructed transgenetic plants and the SIMYB7-like transcription is significantly increased, accompanied by upregulated ANS expression [Citation122]. It inhibits the expression of target genes by inhibiting the transcription or translation of transcription factors related to anthocyanin synthesis; therefore, the plant color is regulated [Citation123].
Previous studies have concluded that miR156 inhibits anthocyanin biosynthesis via the targeted SPL transcription factor [Citation124]. miR828 regulates PAP1/MYB75, PAP2/MYB90 and MYB113 expression under phosphorous deficient conditions by mediating the targeted gene TAS4, thus influencing the accumulation of anthocyanin [Citation31]. miR858 participates in the regulation of anthocyanin synthesis via the target MYB12 [Citation125]. miR165/166 can inhibit anthocyanin synthesis [Citation126]. Under phosphorus-deficient conditions, miR778 overexpression can facilitate anthocyanin accumulation [Citation127].
The SPL transcription factor is the target gene of miR156 [Citation128, Citation129]. A total of 17 SPL transcription factors have been detected in Arabidopsis, in which 11 are target genes of miR156 [Citation130, Citation131]. Gou et al. [Citation124] found that the target gene SPL9 of miR156 in Arabidopsis inhibited anthocyanin synthesis and influenced the transcription activity of the MYB-bHLH-WD40 complex via interaction with PAP1. Thus, the expression ANS and DFR, which are anthocyanin synthesis genes, is inhibited. Luo et al. [Citation132] showed that miR156 inhibited the expression of MYB genes via SPL9, which hindered anthocyanin accumulation. In addition, Franco-Zorrilla et al. [Citation133] and Gou et al. [Citation124] investigated the effects of miR156 on anthocyanin synthesis by transforming Arabidopsis plants using the constructed overexpression vector and silencing vector of miR156. The results showed that the anthocyanin content in overexpression plants was four times higher, while the anthocyanin content in silencing plants was only 1/10 that of the control group. This demonstrates that miR156 can influence plant color indirectly via the regulation of anthocyanin accumulation. Based on the analysis of the Arabidopsis mutant under adverse stress conditions, Cui et al. [Citation134] showed that the negative correlation network was composed of miR156, SPL and DFR. miR156 and DFR expression increased under adverse stress, whereas SPL expression decreased, resulting in anthocyanin accumulation. The formation of yellow petals of Chinese herbaceous peony might be caused by miR156 regulation over SPL1 [Citation135].
The silencing and expression analysis of two lncRNAs (LNC1 and LNC2) in Hippophae rhamnoides showed that both LNC1 and LNC2 could be used as endogenous targets to simulate the effects of miR156a and miR828a in reducing SPL9 expression and inducing MYB114 expression. Accordingly, anthocyanin content is increased or decreased [Citation136].
With the rapid development of modern genetic engineering technology, the regulation of plant color based on miRNA has attracted increasing attention [Citation137]. Some associated remarkable fruits have been achieved; however, they have mainly focused on model plants, such as Arabidopsis. Nevertheless, there have been few studies dedicated to ornamental plants. Therefore, studies on color regulation of ornamental plants based on miRNA must be undertaken in the future.
Problems and future prospects
China has implemented several studies on the genetic engineering of flower culture and has achieved some progress in this field. Yuan Lin et al. [Citation138] transferred the Roseal gene related to anthocyanin synthesis into the Sorbonne seedless scales of the oriental lily using the agrobacterium-mediated method, which was optimized with the genetic transformation system. This study preliminarily showed that the Roseal gene was integrated into the genomic DNA of the Sorbonne lily. This study aimed to optimize several important factors that influenced the Agrobacterium-mediated transformation in the Sorbonne lily and constructed a fast and effective gene transformation system. In addition, it further laid the foundation for analyzing molecular breeding and visual gene functions related to flower colors and provided a new way for improved flower breeding. In addition, abundant transgenetic flowers have been developed via genetic engineering. In recent years CRISPR technology has been widely used in various fields and has played an important role in changing plant color [Citation139]. The technique of CRISPR/Cas9 mediated gene knockout in tomato, make the color of transgenic tomato fruit change from wild red to yellow and orange [Citation140]. The CRISPR/Cas9 technology encodes the active site of the enzyme produced by the DFR-B gene, then the violet color of Japanese morning glory Ipomoea (Pharbitis) nil turns to white [Citation141]. Nevertheless, there are few associated studies and greater attention should be given to this area of research in the future.
Recently, anthocyanin metabolism and the molecular region have been explored via mutant and transgenic technologies. Structural genes and regulatory genes related to anthocyanin have been separated, identified and successively cloned. Although previous studies have discovered anthocyanin biosynthesis pathways, the synthesis and metabolism of anthocyanin are very complicated and the entire network system of anthocyanin synthesis has not been completely comprehended. Among the three types of pigments, the greatest attention has been given to flavonoids and carotenoids. However, the collaborative color developing mechanism of the three pigments and the influence of multiple regulatory factors on flower color remain unknown. Therefore, modern transgenetic technology, sequencing technology, RNA interference technology, bioinformatics analysis technology, genomics, transcriptomics and proteomics technologies should be applied in future studies. Anthocyanin synthesis and its regulation mechanism, phenotype, genetic pattern of flower color inheritance, especially the genetic mechanism of collaborative coloring, influences of environmental and chemical factors on anthocyanin formation and stability, genetic stability of transgenetic plants, and genetic pollution should be studied. These problems must be solved in the current genetic breeding of ornamental genes. Great effort should be made to solve the following problems: regulation mechanism and system in anthocyanin synthesis, relationship and influencing mechanism of anthocyanin metabolism and other metabolism behaviors, influences of biological environment and non-environmental environment on anthocyanin synthesis, and the modification and transfer of anthocyanin. Results from such studies would provide theoretical support for breeding improvement and development of anthocyanin plants.
Further studies on the regulation of plant color based on miRNA are required. New methods and techniques that have been proposed provide reliable technical support for further studies. Studying the regulation of the model plant color based on miRNA provides abundant references for future studies on ornamental plants. It is believed that targeted modification of plant color at the post-transcription level and the creation of rare species that do not exist in nature could be realized by miRNA technology in the not-so-distant future.
Conclusions
This review described the genes and metabolites related to anthocyanin biosynthesis, and analyzed the molecular regulatory network in flower color formation, including the regulation and influence of anthocyanin biosynthesis pathways. In addition, the methods and achievements in flower color improvement in recent years were summarized, which provided a theoretical basis for the improvement and modification of flower color.
The developmental opportunities brought by genetic engineering should be seized and full use should be taken of the advantages in the rich germplasm resources of plants and fundamental studies. Considering the flower breeding status, key attention should be paid to fundamental studies in a small range, such as constructing the transformation receptor system, exploring the transformation method, studying the character control by genes, and identification of key genes. Genetic engineering of flowers gives promising developmental prospects for the improvement of flower colors. New flower colors could be obtained by targeted modification and the innovation of ornamental plant resources creates an advanced means for breaking the inter-species hybridization barriers. Subsequently, gene transformation and genetic engineering in flowers and other characters of ornamental plants should be undertaken, which are conducive to development and vitalization of the floriculture industry.
Authors’ contributions
XJY, TTW and MZH carried out reference collecting and literature analysis and writing the first draft of the manuscript; YBZH helped to review and editing the manuscript; MI has been involved in revising it critically for important intellectual content; LJCH and LZH had overall responsibility for this project, including project ideas, guidance on experimental design, data analysis, paper writing and revision. CH took part in the project administration and funding acquisition. All authors revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
All data generated during the study are interpreted in the manuscript.
Additional information
Funding
References
- Tanaka Y, Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol. 2008;19(2):190–197.
- Tanaka Y, Brugliera F . Flower colour and cytochromes P450. Philos Trans R Soc Lond B Biol Sci. 2013;368(1612):20120432.
- An JP, Liu YJ, Zhang XW, et al. Dynamic regulation of different light intensity-modulated anthocyanin biosynthesis by BT2-TCP46-MYB1 in apple. J Exp Bot. 2020;71(10):3094–3109.
- Fang H, Dong Y, Yue X, et al . The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019;42(7):2090–2104.
- Liu X, Zhang Q, Yang G, et al. Pivotal roles of tomato photoreceptor SlUVR8 in seedling development and UV-B stress tolerance. Biochem Biophys Res Commun. 2020;522(1):177–183.
- Xu JZ, Wang LH. Advances in the transformation of flower color gene in ornamental plant. Rev China Agricul Sci Technol. 2006;8(5):56–60.
- Li H, Tian J, Yao Y, et al. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol Biochem. 2019;139:141–151.
- Zhu YC, Zhang B, Allan AC, et al. DNA demethylation is involved in the regulation of temperature-dependent anthocyanin accumulation in peach. Plant J. 2020;102(5):965–976.
- Zheng T, Li Y, Lei W, et al. SUMO E3 Ligase SIZ1 stabilizes MYB75 to regulate anthocyanin accumulation under high light conditions in Arabidopsis. Plant Sci. 2020;292:110355.
- Stavenga DG, Leertouwer HL, Dudek B, et al. Coloration of flowers by flavonoids and consequences of pH dependent absorption. Front Plant Sci. 2020;11:600124.
- Wang H, Yang Y, Li M, et al. Effects of atmospheric ozone on anthocyanin and carotenoid of plants. Plant Physiol J. 2017a;53(10):1824–1832.
- Wang ZQ, Zhou X, Dong L, et al. iTRAQ-based analysis of the Arabidopsis proteome reveals insights into the potential mechanisms of anthocyanin accumulation regulation in response to phosphate deficiency. J Proteomics. 2018;184:39–53.
- Zhang Y, Xu S, Cheng Y, et al. Transcriptome profiling of anthocyanin-related genes reveals effects of light intensity on anthocyanin biosynthesis in red leaf lettuce. PeerJ. 2018d;6:e4607.
- Tanaka Y, Brugliera F. Flower color. In: Ains-worth C, ed. Flowering and its manipulation. London, UK: Black-Well; 2006:201–239.
- Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780.
- Yamagishi M, Shimoyamada Y, Nakatsuka T, et al. Two R2R3-MYB genes, homologs of Petunia AN2, regulate anthocyanin biosyntheses in flower Tepals, tepal spots and leaves of asiatic hybrid lily. Plant Cell Physiol. 2010;51(3):463–474.
- Madhuri G, Reddy AR. Plant biotechnology of flavonoids. Plant Biotechnol. 1999;16(3):179–199.
- Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008;54(4):733–749.
- Yamagishi M, Yoshida Y, Nakayama M. The transcription factor LhMYB12 determines anthocyanin pigmentation in the tepals of Asiatic hybrid lilies (Lilium spp.) and regulates pigment quantity. Mol Breeding. 2012;30(2):913–925.
- Liang CY, Rengasamy KP, Huang LM, et al. Assessment of violet-blue color formation in Phalaenopsis orchids. BMC Plant Biol. 2020;20(1):212.
- Diretto G, Jin X, Capell T, et al. Differential accumulation of pelargonidin glycosides in petals at three different developmental stages of the orange-flowered gentian (Gentiana lutea L. var. aurantiaca). PLoS One. 2019;14(2):e212062.
- Zhao Y, Dong W, Zhu Y, et al . PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach. Plant Biotechnol J. 2020;18(5):1284–1295.
- Butelli E, Titta L, Giorgio M, et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol. 2008;26(11):1301–1308.
- Nishihara M, Nakatsuka T. Genetic engineering of novel flower colors in floricultural plants: recent advances via transgenic approaches. Methods Mol Biol. 2010;589:325–347.
- Nishihara M, Nakatsuka T. Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol Lett. 2011;33(3):433–441.
- Niyogi KK. Safety valves of photosynthesis. Curr Opin Plant Biol. 2000;3(6):455–460.
- Goss R, Latowski D. Lipid dependence of xanthophyll cycling in higher plants and algae. Front Plant Sci. 2020;11:455.
- Liang J, Wang L, Ding R, et al. Analysis of key enzyme genes in carotenoid metabolism pathway of Lilium and cloning of LoLcyB gene. Mol Plant Breed. 2019;10(4):4520–4529.
- Jeknic Z, Morre JT, Jeknic S, et al. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium Thunb. Splendens’). Plant & Cell Physiol. 2012;53:1899–1912.
- Forkmann G. Flavonoids as flower Pigment the information of nature spectrum and its extension by genetic engineering. Plant Breed. 1991;106(1):1–96.
- Hsieh LC, Lin SI, Shih AC, et al. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009;151(4):2120–2132.
- Stommel JR, Lightbourn GJ, Winkel BS, et al. Transcription factor families regulate the anthocyanin biosynthetic pathway in Capsicum annuum. J Amer Soc Hort Sci. 2009;134(2):244–251.
- Zheng J, Wu H, Zhu H, et al. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019;223(2):705–721.
- Liu H, Su B, Zhang H, et al. Identification and functional analysis of a flavonol synthase gene from grape hyacinth. Molecules (Basel, Switzerland). 2019;24(8):1579.
- Abe H, Nakano M, Nakatsuka A, et al. Genetic analysis of floral anthocyanin pigmentation traits in Asiatic hybrid lily using molecular linkage maps. Theor Appl Genet. 2002;105(8):1175–1182.
- Nakano M, Nakatsuka A, Nakayama M, et al. Mapping of quantitative trait loci for carotenoid pigmentation in flower tepals of Asiatic hybrid lily. Scie Horticul. 2005;104(1):57–64.
- Lai YS, Shimoyamada Y, Nakayama M, et al. Pigment accumulation and transcription of LhMYB12 and anthocyanin biosynthesis genes during flower development in the asiatic hybrid lily (Lilium spp.). Plant Sci. 2012;193-194:136–147.
- Du F, Fan J, Wang T, et al. Identification of differentially expressed genes in flower, leaf and bulb scale of lilium Oriental hybrid ‘Sorbonne’ and putative control network for scent genes. BMC Genomics. 2017;18(1):899.
- Nakatsuka A, Izumi Y, Yamagishi M. Spatial and temporal expression of chalcone synthase and dihydroflavonol 4-reductase genes in the asiatic hybrid lily. Plant Sci. 2003;165(4):759–767.
- Wang Y, Cui J, Zhang K, et al. Molecular cloning and expression analysis of anthocyanidin synthase gene fragment in lilium. Chinese Agricul Sci Bull. 2013;29:162–166.
- Zhang M, Jiang L, Zhang D, et al. De novo transcriptome characterization of lilium ‘sorbonne’ and key enzymes related to the flavonoid biosynthesis. Mol Genet Genomics. 2015;290(1):399–412.
- Stich K, Eidenberger T, Wurst F, et al. Enzymatic conversion of dihydroflavonols to flavan-3,4-diols using flower extracts of dianthus caryophyllus L. (carnation). Planta. 1992;187(1):103–108.
- Johzuka-Hisatomi Y, Hoshino A, Mori T, et al. Characterization of the chalcone synthase genes expressed in flowers of the common and japanese morning glories. Genes Genet Syst. 1999;74(4):141–147.
- Li Y, Cui W, Qi X, et al. Chalcone Synthase-Encoding AeCHS is involved in normal petal coloration in actinidia eriantha. Genes-Basel. 2019;10(12):949.
- Chen J, An L, Wang T, et al. Cloning of chalcone synthase gene in lilium and expression analysis of flower colour changes in transgenic tobacco. Acta Bot Boreal Occident Sin. 2012;32(8):1511–1517.
- Liu Y, Lou Q, Xu W, et al. Characterization of a chalcone synthase (CHS) flower-specific promoter from lilium orential ‘Sorbonne’. Plant Cell Rep. 2011;30(12):2187–2194.
- Zhang K, Liu Z, Guan L, et al. Changes of anthocyanin component biosynthesis in ‘summer black’ grape berries after the red flesh mutation occurred. J Agric Food Chem. 2018b;66(35):9209–9218.
- Chao N, Wang R, Hou C, et al. Functional characterization of two chalcone isomerase (CHI) revealing their responsibility for anthocyanins accumulation in mulberry. Plant Physiol Biochem. 2021;161:65–73.
- Dou X, Lang L, Bao F, et al. Cloning and expression analysis of chalcone isomerase gene LhCHI in Oriental hybrid lily (Lilium spp.). J Northeast Forestry Univer. 2015;43(9):6–17.
- van Tunen AJ, Mur LA, Recourt K, et al. Regulation and manipulation of flavonoid gene expression in anthers of petunia: the molecular basis of the point mutation. Plant Cell. 1991;3:39–48.
- Nishihara M, Nakatsuka T, Yamamura S. Flavonoid components and flower color change in transgenic tobacco plants by suppression of chalcone isomerase gene. FEBS Lett. 2005;579(27):6074–6078.
- Forkmann G, Dangelmayr B. Genetic control of chalcone isomerase activity in flowers of dianthus caryophyllus. Biochem Genet. 1980;18(5-6):519–527.
- Miyajima I, Maehara T, Kage T, et al. Identification of the main agent causing yellow color of Yellow-Flowered cyclamen mutant. Engei Gakkai Zasshi. 1991;60(2):409–414.
- Ohno S, Hori W, Hosokawa M, et al . Post-transcriptional silencing of chalcone synthase is involved in phenotypic lability in petals and leaves of bicolor dahlia (Dahlia variabilis) ‘Yuino’. Planta. 2018;247(2):413–428.
- Li W, Ning G, Zuo C, et al. MYB_SH[AL]QKY[RF] transcription factors MdLUX and MdPCL-like promote anthocyanin accumulation through DNA hypomethylation andMdF3H activation in apple. Tree Physiol. 2021;41(5):836–848.
- Martin C, Prescott A, Mackay S, et al. Control of anthocyanin biosynthesis in flowers of antirrhinum majus. Plant J. 1991;1(1):37–49.
- Shen H, Zhang J, Yao Y, et al. Isolation and expression of McF3H gene in the leaves of crabapple. Acta Physiol Plant. 2012;34(4):1353–1361.
- Zhang H, Huang Y, Yang C, et al. Molecular cloning and sequences analysis of flavanone 3-hydroxylase gene from fagopy rumtataricum. Acta Bot Boreal Occident Sin. 2010;30:447–452.
- Zhiru X, Guoxin C, Chunlei L, et al. Cloning, sequence analysis and expression of flavanone 3-hydroxylase gene in turnip. Mol Plant Breed. 2008;6:787–792.
- Ono E, Fukuchi-Mizutani M, Nakamura N, et al. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proc Natl Acad Sci U S A. 2006;103(29):11075–11080.
- Zuker A, Tzfira T, Ben-Meir H, et al. Modification of flower color and fragrance by antisense suppression of the flavanone 3-hydroxylase gene. Mol Breed. 2002;9(1):33–41.
- Khumkarjorn N, Thanonkeo S, Yamada M, et al. Cloning and expression analysis of a flavanone 3-hydroxylase gene in Ascocenda orchid. J Plant Biochem Biotechnol. 2017;26(2):179–190.
- He H, Ke H, Keting H, et al . Flower colour modification of chrysanthemum by suppression of F3’H and overexpression of the exogenous Senecio cruentus F3’5’H gene. PloS One. 2013;8(11):e74395.
- Yuan Y, Ma X, Tang D, et al. Comparison of anthocyanin components, expression of anthocyanin biosynthetic structural genes, and TfF3′H1 sequences between Tulipa fosteriana ‘albert heijn’ and its reddish sport. Scientia Horticul. 2014;175:16–26.
- Schlangen K, Miosic S, Halbwirth H. Allelic variants from Dahlia variabilis encode flavonoid 3’-hydroxylases with functional differences in chalcone 3-hydroxylase activity. Arch Biochem Biophys. 2010;494(1):40–45.
- Kemp M, Burden R. Phytoalexins and stress metabolites in the sapwood of trees. Phytochemistry. 1986;25(6):1261–1269.
- Xu B, JL, Jin Z. Cloning of flavonoid-3’,5’-hydroxylase gene and its transformation into lily (Lilium longiforum). Acta Horticul Sinice. 2005;32:1051–1055.
- Qi Y, Lou Q, Quan Y, et al. Flower-specific expression of the phalaenopsis flavonoid 3′, 5′-hydroxylase modifies flower color pigmentation in petunia and lilium. Plant cell. Plant Cell Tiss Organ Cult. 2013b;115(2):263–273.
- Martens S, Teeri T, Forkmann G. Heterologous expression of dihydroflavonol 4-reductases from various plants. FEBS Lett. 2002;531(3):453–458.
- Johnson ET, Yi H, Shin B, et al . Cymbidium hybrida dihydroflavonol 4-reductase does not efficiently reduce dihydrokaempferol to produce orange pelargonidin-type anthocyanins. Plant J. 1999;19(1):81–85.
- Shimada N, Sasaki R, Sato S, et al. A comprehensive analysis of six dihydroflavonol 4-reductases encoded by a gene cluster of the Lotus japonicus genome. J Exp Bot. 2005;56(419):2573–2585.
- Trabelsi N, D’Estaintot B, Sigaud G, et al. Kinetic and binding equilibrium studies of dihydroflavonol 4-reductase from Vitis vinifera and its unusually strong substrate inhibition. JBPC. 2011;2(3):332–344.
- Forkmann G, Ruhnau B. Distinct substrate specificity of dihydroflavonol-4-reductase from flowers of Petunia hybrida. Zeitschrift Für Naturforschung C. 1987;42(9-10):1146–1148.
- Suzuki K, Tasaki K, Yamagishi M. Two distinct spontaneous mutations involved in white flower development in Lilium speciosum. Mol Breed. 2015;35:193
- Zhang X, Zhao M, Guo J. Anatomical and biochemical analyses reveal the mechanism of double-color formation in Paeonia suffruticosa ‘Shima Nishiki’. 3 Biotech. 2018;8:1–9.
- Li H, Liu J, Pei T, et al. Overexpression of SmANS enhances anthocyanin accumulation and alters phenolic acids content in salvia miltiorrhiza and salvia miltiorrhiza bge f. alba plantlets. Int J Mol Sci. 2019;20(9):2225.
- Qi X, Shuai Q, Fan L, et al. Molecular cloning and expressional pattern of anthocyanidin synthase gene in two mulberry species with different fruit colors. Sci Sericul. 2013;39(1):5–13.
- Rosati C, Cadic A, Duron M, et al. Molecular characterization of the anthocyanidin synthase gene in forsythia × intermedia reveals organ-specific expression during flower development. Plant Sci. 1999;149(1):73–79.
- An W, Liu Y, Liu H, et al. Isolation and analysis of muscari armeniacum MaANS gene and its promoter. Acta Bot Boreal Occident Sin. 2015;35(9):1728–1734.
- Jh A, Js K, SK, et al. De novo transcriptome analysis to identify anthocyanin biosynthesis genes responsible for tissue-specific pigmentation in zoysiagrass (Zoysia japonica Steud.). PLoS One. 2015;10(4):e0124497.
- Lim S-H, Kim JK, Lee J-Y, et al. Petal-specific activity of the promoter of an anthocyanidin synthase gene of tobacco (Nicotiana tabacum L.). plant cell. Plant Cell Tiss Organ Cult. 2013;114(3):373–383.
- Rafique MZ, Carvalho E, Stracke R, et al. Nonsense mutation inside anthocyanidin synthase gene controls pigmentation in yellow raspberry (Rubus idaeus L.). Front Plant Sci. 2016;7:1892.
- Vogt T, Taylor LP. Flavonol 3-O-glycosyltransferases associated with petunia pollen produce gametophyte-specific flavonol diglycosides. Plant Physiol. 1995;108(3):903–911.
- Meng X, Li Y, Zhou T, et al. Functional differentiation of duplicated flavonoid 3-O-Glycosyltransferases in the flavonol and anthocyanin biosynthesis of freesia hybrida. Front Plant Sci. 2019;10:1330.
- Zhao ZC, Hu GB, Hu FC, et al. The UDP glucose: flavonoid-3-O-glucosyltransferase (UFGT) gene regulates anthocyanin biosynthesis in litchi (litchi chinesis sonn.) during fruit coloration. Mol Biol Rep. 2012;39(6):6409–6415.
- Sui X, Gao X, Wang Q, et al. cDNA cloning and characterization of UDP-glucose: anthocyanidin 3-O-glucosyltransferase in freesia hybrida. Plant Cell Rep. 2011;30(7):1209–1218.
- Sun W, Meng X, Liang L, et al. Overexpression of a freesia hybrida flavonoid 3-O-glycosyltransferase gene, Fh3GT1, enhances transcription of key anthocyanin genes and accumulation of anthocyanin and flavonol in transgenic petunia (Petunia hybrida). In Vitro Cell Dev Biol Plant. 2017;53:478–488.
- Jiu S, Guan L, Leng X, et al. The role of VvMYBA2r and VvMYBA2w alleles of the MYBA2 locus in the regulation of anthocyanin biosynthesis for molecular breeding of grape (vitis spp.) skin coloration. Plant Biotechnol J. 2021;19(6):1216–1239.
- Song L, Wang X, Han W, et al. PbMYB120 negatively regulates anthocyanin accumulation in pear. Int J Mol Sci. 2020;21(4):1528.
- Qi Y, Gu C, Wang X, et al. Identification of the eutrema salsugineum EsMYB90 gene important for anthocyanin biosynthesis. BMC Plant Biol. 2020;20(1):186–186.
- Qi Y, Zhou L, Han L, et al. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol Biochem. 2020;154:396–408.
- Su W, Tao R, Liu W, et al . Characterization of four polymorphic genes controlling red leaf colour in lettuce that have undergone disruptive selection since domestication. Plant Biotechnol J. 2020;18(2):479–490.
- Ma D, Constabel CP. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019;24(3):275–289.
- Stracke R, Ishihara H, Huep G, et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50(4):660–677.
- Gonzalez A, Zhao M, Leavitt JM, et al. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53(5):814–827.
- Lepiniec L, Debeaujon I, Routaboul JM, et al. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol. 2006;57:405–430.
- Song S, Qi T, Huang H, et al. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in arabidopsis. Plant Cell. 2011;23(3):1000–1013.
- Ravaglia D, Espley RV, Henry-Kirk RA, et al. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013;13:68.
- Lai Y, Li H, Yamagishi M. A review of target gene specificity of flavonoid R2R3-MYB transcription factors and a discussion of factors contributing to the target gene selectivity. Front Biol. 2013;8(6):577–598.
- Lai Y, Yamagishi M, Suzuki T. Elevated temperature inhibits anthocyanin biosynthesis in the tepals of an Oriental hybrid lily via the suppression of LhMYB12 transcription. Scientia Horticul. 2011;132:59–65.
- Suzuki K, Suzuki T, Nakatsuka T, et al. RNA-seq-based evaluation of bicolor tepal pigmentation in Asiatic hybrid lilies (Lilium spp). BMC Genomics. 2016;17(1):611.
- Xu L, Yang P, Yuan S, et al. Transcriptome analysis identifies key candidate genes mediating purple ovary coloration in asiatic hybrid lilies. Int J Mol Sci. 2016;17:1881.
- Yamagishi M. Oriental hybrid lily sorbonne homolog of LhMYB12 regulates anthocyanin biosyntheses in flower tepals and tepal spots. Mol Breeding. 2011;28(3):381–389.
- Yamagishi M. A novel R2R3-MYB transcription factor regulates light-mediated floral and vegetative anthocyanin pigmentation patterns in Lilium regale. Mol Breed. 2016;36:1–14.
- Yamagishi M. Involvement of a LhMYB18 transcription factor in large anthocyanin spot formation on the flower tepals of the Asiatic hybrid lily (Lilium spp.) cultivar “grand cru”. Mol Breed. 2018;38:60.
- Yamagishi M, Nakatsuka T. LhMYB12, regulating tepal anthocyanin pigmentation in asiatic hybrid lilies, is derived from Lilium dauricum and L. bulbiferum. The Hortic J. 2017;86(4):528–533.
- Deng J, Li J, Su M, et al. A bHLH gene NnTT8 of nelumbo nucifera regulates anthocyanin biosynthesis. Plant Physiol Biochem. 2021;158:518–523.
- Liu X, Feng C, Zhang M, et al. The MrWD40-1 gene of chinese bayberry (myrica rubra) interacts with MYB and bHLH to enhance anthocyanin accumulation. Plant Mol Biol Rep. 2013;31(6):1474–1484.
- Nakatsuka A, Yamagishi M, Nakano M, et al. Light-induced expression of basic helix-loop-helix genes involved in anthocyanin biosynthesis in flowers and leaves of asiatic hybrid lily. Scientia Horticul. 2009;21:84–91.
- Zhao F, Li G, Hu P, et al. Identification of basic/helix-loop-helix transcription factors reveals candidate genes involved in anthocyanin biosynthesis from the strawberry white-flesh mutant. Sci Rep. 2018;8(1):2721.
- Wang G, Zhang ZR, Wang YF, et al. [Bioinformatics analysis of safflower WD40 transcription factor family genes]. Zhongguo Zhong Yao Za Zhi. 2020;45(14):3432–3440.
- Zhu Q, Li B, Mu S, et al . TTG2-regulated development is related to expression of putative AUXIN RESPONSE FACTOR genes in tobacco. BMC Genomics. 2013;14:806.
- Nesi N, Jond C, Debeaujon I, et al. The arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13(9):2099–2114.
- Yao P, Zhao H, Luo X, et al. Fagopyrum tataricum FtWD40 functions as a positive regulator of anthocyanin biosynthesis in transgenic tobacco. J Plant Growth Regul. 2017;36(3):755–765.
- Dressel A, Hemleben V. Transparent testa glabra 1 (TTG1) and TTG1-like genes in Matthiola incana R. Br. and related brassicaceae and mutation in the WD-40 motif. Plant Biol (Stuttg). 2009;11(2):204–212.
- Zhang D, Han Z, Li J, et al. Genome-wide analysis of the SBP-box gene family transcription factors and their responses to abiotic stresses in tea (Camellia sinensis). Genomics. 2019;112(3):2194–2202.
- Lei K, Ren J, Zhu Y, et al. SPL1 is involved in the regulation of rhizosphere acidification reaction under low phosphate condition in arabidopsis. Chinese Bull Bot. 2016;51(2):184–193.
- Dai F, Hu Z, Chen G, et al. Progress in the plant specific SBP-box gene family. Chinese Bull Life Sci. 2010;22(2):155–160.
- Lei K, Liu H. Research advances in plant regulatory hub miR156 and targeted SPL family. Chem Life. 2016;36(1):13–20.
- Feyissa BA, Arshad M, Gruber MY, et al. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019;19(1):434.
- Li Y, Cui W, Qi X, et al. MicroRNA858 negatively regulates anthocyanin biosynthesis by repressing AaMYBC1 expression in kiwifruit (actinidia arguta). Plant Sci. 2020;296:110476.
- Jia X, Shen J, Liu H, et al. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta. 2015;242(1):283–293.
- Tirumalai V, Swetha C, Nair A, et al. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J Exp Bot. 2019;70(18):4775–4792.
- Gou JY, Felippes FF, Liu CJ, et al. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell. 2011;23(4):1512–1522.
- Jaillon O, Aury JM, Noel B, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449(7161):463–467.
- Yan J, Gu Y, Jia X, et al. Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell. 2012;24(2):415–427.
- Wang L, ZengJ HQ, Song J, et al. miRNA778 and SUVH6 are involved in phosphate homeostasis in Arabidopsis. Plant Sci. 2015b;238:273–285.
- Li X, Hou Y, Xie X, et al . A blueberry MIR156a-SPL12 module coordinates the accumulation of chlorophylls and anthocyanins during fruit ripening. J Exp Bot. 2020;71(19):5976–5989.
- Wang Y, Liu W, Wang X, et al. MiR156 regulates anthocyanin biosynthesis through SPL targets and other microRNAs in poplar. Hortic Res. 2020;7(1):118.
- Gandikota M, Birkenbihl RP, Hohmann S, et al. The miRNA156/157 recognition element in the 3’ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007;49(4):683–693.
- Klein J, Saedler H, Huijser P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol Gen Genet. 1996;250(1):7–16.
- Luo Y, Guo Z, Li L. Evolutionary conservation of microRNA regulatory programs in plant flower development. Dev Biol. 2013;380(2):133–144.
- Franco-Zorrilla JM, Valli A, Todesco M, et al . Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39(8):1033–1037.
- Cui LG, Shan JX, Shi M, et al. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014;80(6):1108–1117.
- Zhao D, Wei M, Shi M, et al. Identification and comparative profiling of miRNAs in herbaceous peony (Paeonia lactiflora Pall.) with red/yellow bicoloured flowers. Sci Rep. 2017;7:44926.
- Zhang G, Chen D, Zhang T, et al. Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Res. 2018a;25(5):465–476.
- Cui J, Gao Z, Li B, et al. Identification of anthocyanin biosynthesis related microRNAs and total microRNAs in Lonicera edulis by high-throughput sequencing. J Genet. 2020;99:31.
- Yuan L, Wei C, Jia G. Study on transformation of Lilium orential Sorbonne with an anthocyanin regulatory gene Rosea1. Guangdong Agricul Sci. 2012;39(10):10–12.
- Zhang D, Li Z, Li J-F. Targeted gene manipulation in plants using the CRISPR/Cas technology. J Genet Genomics. 2016;43(5):251–262.
- Cheng Y, Jiao Y, Qiao N, et al. Targeting modification PSY1 gene in tomato using CRISPR/Cas9 system. China Vegetables. 2018;11:32–38.
- Watanabe K, Kobayashi A, Endo M, et al. CRISPR/Cas9-mediated mutagenesis of the dihydroflavonol-4-reductase-B (DFR-B) locus in the japanese morning glory ipomoea (pharbitis) nil. Sci Rep. 2017;7(1):10028.