?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Peracetic acid (PAA) and hydrogen peroxide (H2O2) were more potent at pH 8.2, while linear alkylbenzene sulfonate (LAS) showed higher potency at pH 5.0 against Pseudomonas fluorescens ATCC 13525. The aim was to understand the changes in the cellular redox status, ultrastructure and morphology underlying the synergistic bacterial control effects of selected pH values alone and treatments. The minimum inhibitory concentrations of PAA and H2O2 at pH 8.2, and MIC of LAS at pH 5.0 were tested during the stationary growth phase of planktonic cells. pH 8.2 alone mainly elevated the protein carbonyls level and decreased the levels of low molecular weight thiols (LMWT), which could potentiate the effect of H2O2 and PAA, while pH 5.0 alone largely decreased the total thiol level that could facilitate LAS action. Free radicals were only detected with LAS and PAA treatments. H2O2 and PAA increased the levels of protein carbonyls, while reduced LMWT levels. LAS increased the levels of protein carbonyls, while reduced the total thiol level. H2O2, PAA and LAS were also found to increase SOD and decrease catalase specific activities. Each treatment showed distinct alterations and disruption in cytoplasmic structures. We suggest that exposing bacteria to the test oxidants and LAS at the selected pH ranges resulted in high generation of reactive species which activated complex oxidative processes and antioxidant defense pathways causing an imbalance of the cellular redox homeostasis that led to deformity and collapse of ultrastructure at late stage of oxidative damage, and eventual control of bacterial growth.
Introduction
Controlling bacterial growth and resistance is essential in several areas (i.e. hospitals, industry, consumer homes, etc.). There are several cleaning disinfecting products containing bleaching agents and surfactants, e.g. hydrogen peroxide, peracetic acid and anionic surfactants, formulated at various pH ranges to clean and sanitise materials/surfaces through removal of bacterial colonies that inhabit different environments e.g. plants, soil, and wet surfaces. However, the biological synergy between the mechanism of actions of these disinfectants and direct effects of pH is still an emerging research question.
The pH is found to play a major role in determining the efficacy of hydrogen peroxide, peracetic acid, anionic surfactants by i) its direct effects on biomolecules, cellular redox homeostasis, metabolic processes, cell structure and morphology, hence cell viability, and/or ii) effects on potency of these disinfectants through influencing the concentration of unionized forms of disinfectants that cross the biological membrane and/or the degree of availability of the active species of these chemical agents in situ that exert the intrinsic bacterial control effects via targeting specific cellular redox biomolecules and ultrastructure.
One of the primary oxidants that ever existed on earth is the prevalence of O2 in its atmosphere, along with the highly abundant iron metal, which is readily reactive with oxygen giving rise to hydroxyl radicals and superoxide anions that inflict damage on biological molecules e.g. DNA, protein and lipids. In order for living organisms to shield themselves from such oxidative stress events, they are compelled to develop certain mechanisms like superoxide dismutase (SOD) and other enzymes, small protein molecules like glutaredoxins and thioredoxins, besides other molecules such as glutathione, in addition to the two major transcriptional regulators that govern bacterial genetic responses to oxidative stress (OxyR and SoxRS) [Citation1–4].
Escherichia coli, one of the leading examples of aerobic bacteria, utilizes oxygen for its nutrient’s oxidation as well as their various respiratory processes. Thus we can find that highly reactive hydroxyl radicals (•OH), hydrogen peroxide and superoxide anion radicals (O2•−) are continuously generated reactive oxygen species (ROS) in aerobic cells. Such by-products emerge from sequential and univalent reductions of molecular oxygen, which is mainly catalyzed by several membrane-attached respiratory chain enzymes [Citation5].
Intracellular ROS such as O2•− can cause oxidative stress when their concentration levels exceed the cellular capacity, however oxidative stress can also arise from exposure to environmental factors like ionizing radiation and ultraviolet light. As these ROS affect biomolecules, much of the imposed damage by H2O2 is generated from hydroxyl radicals coming from Fenton reaction, which requires divalent metal ions like iron or copper and a reducing equivalent (e.g. NADH) for metal regeneration.
Since lipids are a primary target for ROS, free radicals eagerly attack polyunsaturated fatty acids in biological membranes, initiating lipid peroxidation, thus decreasing membrane fluidity, altering their properties and remarkably disrupting membrane bound protein. This cascade of reactions will eventually result in a higher production of free radicals which will boost oxidative stress, while giving various lipid degradation products, e.g. aldehyde, which also have damaging effects on biological molecules [Citation6]. Among the most studied aldehydes are malondialdehyde (MDA) and 4-hydroxyalkenals, especially 4-hydroxynonenal (HNE). These aldehyde molecules have a relatively longer life than free radicals, allowing them to diffuse away from their site of generation and possibly attack remote targets, therefore acting as ‘secondary toxic messengers’ [Citation7].
Several classes of protein damage have been also described, involving disulphide bonds reduction, metal catalyzed oxidation of amino acid residues close to their metal binding sites, prosthetic group and metal clusters alteration, and protein cross-linking and peptide fragmentation. All these modifications lead to protein loss of function, thus blocking replication and triggering mutations [Citation8, Citation9].
Specific cellular molecules are present to maintain redox hemostasis at pace through scavenging ROS. Some of these free-radical scavengers are non-enzymatic like α-tocopherol, ascorbic acid, β-carotene and glutathione (GSH). NADH and NADPH are not free-radical scavengers, although they can be attacked by free radicals. Glutathione is present in higher concentrations; it maintains the cellular redox environment in balance while kept in its reduced form by glutathione reductase using NADPH as a reducing equivalent. Moreover, certain enzymes reduce the steady-state levels of ROS like SODs and catalases, glutathione peroxidase, hydroperoxidase II, glucose-6-phosphate dehydrogenase and DT-diaphorase [Citation10–14].
Several primary structures of proteins in prokaryotic cells are covalently repaired by certain catalysts. Among these main modifications are the reduction of oxidized disulphide bonds: (i) electron transfer from NADPH to thioredoxin via a flavin carrier is conducted by thioredoxin reductase; (ii) glutaredoxin utilizes GSH as an electron donor for disulphide bond reduction, and (iii) protein disulphide isomerase catalyzes disulphide exchange reactions with inactive protein substrate. Oxidation of methionine residues at the doorway site to methionine sulfoxide occurs favourably, which suggests that methionine residues could act as a last line of defense against protein oxidation [Citation1,Citation15,Citation16].
There are a few studies on the effects of hydrogen peroxide, peracetic acid and anionic surfactants, especially surfactants on the bacterial cell redox status and ultrastructure. Therefore, in this paper, we attempted to unveil the potential synergistic bacterial control effects of selected pH ranges alone and these disinfectants at those pH values by dissecting and studying the effects on cell redox status, morphology and cytoplasmic ultrastructure, which could allow the development of better antibacterial products/combinations.
Materials and methods
Bacteria
Gram-negative aerobic, non-pathogenic, acidophilic and psychrophilic rod-shaped bacteria Pseudomonas fluorescens (P. fluorescens Migula ATCC ® 13525™) were tested. This bacterial strain was selected since it colonizes soil and water surfaces, and more resistant than gram-positive bacteria due to the outer membrane composed of lipopolysaccharides, proteins and phospholipids, which gives the advantage for further biological studies of bacterial control mechanisms of the selected chemicals applied onto surfaces. In 100 mL Erlenmeyer flasks, 0.4 mL bacteria (in glycerol at stationary phase) was inoculated into 49.6 mL TSB 100% (tryptic soy broth, Sigma-Aldrich) and incubated at 35.1 °C. After 16.5 h, bacteria were at early stationary growth phase and the net OD600 nm was adjusted to 1.0 (∼ 109 CFU/mL) as stock suspension [Citation17]. The tested planktonic cells of stationary growth phase are of high interest since they show resistance to disinfectants and are physiologically similar to biofilm cells.
Bleaching agents
Hydrogen peroxide (30% w/w, Sigma-Aldrich) and peracetic acid (38–40% w/w, Merck Millipore) were investigated against P. fluorescens.
Surfactant
The anionic surfactant linear alkylbenzene sulphonate (LAS) (Calsoft F-90, powder, Pilot chemical company, USA) with CMC 1.9 µmol/L was used. The concentration of the surfactant was higher than the critical micellar concentration (CMC).
Determinaton of minimum inhibitory concentrations of tested disinfectants at selected pH values
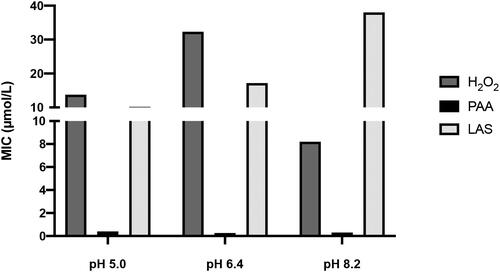
We determined the concentrations of H2O2, peracetic acid and linear alkylbenzene sulphonate (LAS) which inhibit growth and lead to no growth of P. fluorescens planktonic cells at early stationary growth phase in 30 min at pH values 6.4 (primary control), 5.0 and 8.2. These tested chemicals were preliminarily found to be more potent in inhibiting the growth of the selected bacterial strain at mild alkaline conditions (pH 8.2), except for LAS, which was more potent at pH 5.0.
The minimum inhibitory concentration (MIC) of each disinfectant was selected to test the cell oxidative/anti-oxidative status at the pH value giving the highest potency to the disinfectant. MIC is not lethal to cells but causes damaging effects that give a real picture of the cell redox status preceding cellular death.
Using serial dilution method, hydrogen peroxide, peracetic acid and LAS MIC values were tested in triplicates on a final bacterial concentration in each well corresponding to ∼108 CFU/mL.
Procedures: 10 µL of bacteria from the stock suspension (∼109 CFU/mL) were added in each well of the multiwell plates prefilled with 100 µL of each chemical with the proper concentration in 0.1 mol/L bicarbonate solution pH 8.2 (first three plates set), pH 5.0 (second three plates set) and pH 6.4 (third three plates set). The mixtures were incubated at room temperature for 30 min. After the treatment, 10 µL from the mixture of each well from each plate were transferred correspondingly in each well of different nine plates prefilled with 90 µL of the broth medium or neutralizer (Letheen broth, modified, hardy Diagnostic), and incubated at room temperature for 5 min to stop the reaction (cell oxidation). Then, 3 µL from the last mixture of each well were plated on agar dishes. After 18–24 h of incubation at 35.1 °C, the plates were checked to identify the minimum concentrations which inhibit visible bacterial growth [Citation18,Citation19].
Analysis of the cell redox status and ultrastructure
The effects of selected pH values alone and of MICs at the favourable pH were assayed [Citation20]:
Sterile TSB (100%) liquid medium (357 mL) was inoculated with 3 mL bacterial cells (stored in glycerol at stationary phase) in 500 mL Erlenmeyer flask, then mixed well and incubated at 35.1 °C for 16.5 h till reaching early stationary growth phase (net OD600 was adjusted to 1.0), then divided equally into 9 different 500-mL glass bottles — the bottles were prefilled in triplicates with 400 mL of 0.1 mol/L bicarbonate solution pH 8.2 as a control for experiments of H2O2 and peracetic acid, which were found to be more effective at alkaline conditions, pH 5.0 (control for experiments of LAS surfactant, which showed higher potency at acidic conditions) and pH 6.4 (0.1 mol/L bicarbonate solution with TSB medium 100% with bacteria at early stationary phase as the primary control).
For experiments where the effect of minimum inhibitory concentrations at favourable pH was evaluated, 357 mL of TSB 100% liquid medium were inoculated with 3 mL bacterial cells then mixed well and incubated till early stationary growth phase, then divided equally into 9 different 500-mL glass bottles — 6 bottles were prefilled with 400 mL of 0.1 mol/L bicarbonate solution pH 8.2 containing 8.2 µmol/L hydrogen peroxide and 0.3 µmol/L peracetic acid separately in triplicates, while three bottles were prefilled separately with 400 mL of 0.1 mol/L bicarbonate solution pH 5.0 containing 10.2 µmol/L LAS (final concentrations in 440 mL total volume). The bottles were incubated at room temperature for 30 min. The bacterial cells from each bottle were harvested separately by centrifugation at 4,000 g at 4 °C for 10 min. After that, the harvested cells from each bottle were washed three times with 2 mL of 50 mmol/L phosphate buffer (pH 7.4), then small aliquots of pellets were re-suspended in 1 mL of 50 mmol/L phosphate buffer (pH 7.4) to be examined by transmission electron microscope and the rest of each pellet was re-suspended in 1 mL 50 mmol/L phosphate buffer (pH 7.4) in triplicates for homogenisation (cell disruption) through ultrasonication in ice bath using BANDELIN SONOPULS HD 2070 and HD 2200 (ultrasonic power 21 W, ultrasonic time 3 min, continuous mode) and then centrifuged at 12,000 g at 4 °C for 10 min. The homogenates supernatants were used for measuring protein carbonyls, LMWT, total thiols, catalase and SOD specific activities. Free radicals have been detected by electron paramagnetic resonance (EPR) in 20 μL samples supernatants containing equal concentrations of soluble protein (10 μg/mL). Total soluble protein concentrations in supernatants were measured in 30 μL samples by Bradford assay [Citation21] at 595 nm according to the manufacturer’s protocol (Sigma-Aldrich) using bovine serum albumin (BSA) standard curve equation.
Protein carbonyl assay (PCO)
2,4-dinitrophenylhydrazine (DNPH) solution (20 μL of 10 mmol/L prepared in 2 mol/L HCl) was added to 100 μL protein samples. The blank for each sample was prepared by adding 20 μL of 2 mol/L HCl (without DNPH) to 100 μL of protein samples. Samples were vortex-mixed and left in the dark at room temperature for 60 min, with regular vortex-mixing every 10–15 min intervals; 120 μL of 10% w/v TCA solution were added to protein samples (samples and blanks) and incubated on ice for 15 min. Samples and blanks were centifuged at 1,0000 g for 5 min at 4 °C. Supernatants were discarded, then protein pellets of samples and blanks were washed twice with 100 μL of 10% w/v TCA and vortex-mixed. Pellets were collected by centrifuging at 10,000 g for 5 min, at 4 °C. Supernatants were discarded, and protein pellets of samples and blanks were washed twice with 100 μL of 1:1 (v/v) ethanol:ethyl acetate and mixed by vortexing in order to remove any excess of free DNPH. Pellets of samples and blanks were collected by centrifuging at 10,000 g for 5 min at 4 °C and supernatants were discarded. Pellets of samples and blanks were left to vacuum dry for about 5 min to allow complete solvent evaporation. Protein pellets of samples and blanks were resuspended in 100 μL of 6 mol/L guanidine hydrochloride (dissolved in 50 mmol/L phosphate buffer, pH 2.3) and 700 μL of 50 mmol/L phosphate buffer (pH 2.3), and incubated at 37 °C for 15–30 min with vortex mixing. Once protein pellets were completely dissolved, carbonyl contents (expressed as nmol PCO/mg protein), were determined from the peak absorbance at 370/430 nm by using a molar absorption coefficient of 22,000 L/(mol cm) [Citation22–Citation24].
Thiols assay
Measuring protein thiols and low molecular weight thiols by spectrophotometer
In a polystyrene cuvette, 100 μL of resultant supernatant were added to 700 μL of 0.2 mol/L PB (pH 7.4), mixed with stirrer rod, then autozero and start, then 5 μL of 10 mmol/L 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB) were quickly added, mixed with rod, and the absorbance was recorded at 412 nm for 600 s (until plateau), then again 5 μL DTNB (10 mmol/L) were added after thiols consumption to be subtracted from sample absorbance as blank [Citation25].
For the experiments where both the hidden and the exposed protein thiols were measured, 100 μL of supernatant were mixed with 50 μL of sodium dodecyl sulphate (SDS) (10% w/v) for 2 min before analysis.
Measuring low molecular weight thiols by spectrophotometer
The supernatant (70 μL) was mixed with 70 μL of 10% w/v TCA to deproteinize the sample, then the mixture was centrifuged for 2 min at 14,500 g. In a polystyrene cuvette, 100 μL of the deproteinized supernatant were added to 700 μL of 0.2 mol/L Na2HPO4, mixed with rod, then autozero and start, then 2.5 μL DTNB (10 mmol/L) were quickly added and mixed with rod, and the absorbance (412 nm) was recorded until a plateau was reached, then 2.5 μL DTNB (10 mmol/L) were added again to be subtracted from sample reading as blank [Citation25]
Catalase specific activity
In a quartz cuvette, 333 μL of H2O2 solution (50 mmol/L) were mixed with 567 μL of 0.2 mol/L phosphate buffer (pH 7.0), then 100 μL of cells homogenate supernatant were mixed quickly with the reaction mixture and the rate of decrease of absorbance of hydrogen peroxide at 240 nm (ε = 0.0436 L/(mol.cm)) was measured for 1 min by Jasco V-530 UV/VIS spectrophotometer [Citation26–Citation28]. One unit of catalase activity is defined as the amount of enzyme required to decompose 1.0 µmole of H2O2 per minute at pH 7.0 at 25 °C.
SOD specific activity
SOD activity was measured by the xanthine + xanthine oxidase (XOD) + nitroblue tetrazolium (NBT) method.
For the reaction, 900 μL of 50 mmol/L sodium carbonate/bicarbonate buffer (pH 10.2) containing 200 μmol/L xanthine, 50 μmol/L NBT and 200 μmol/L tripotsassium ethylenediaminetetraacetate (K3EDTA) were mixed with 100 μL XOD working suspension. After 2 min, 100 μL enzyme sample supernatant were quickly added and mixed, and the decrease in absorbance was recorded for 3 min at 560 nm [Citation29].
The SOD activity was expressed as U/mg soluble protein (one unit is the amount of SOD that inhibits the rate of formazan dye formation by 50% per minute).
Free radicals detection
Continuous wave (CW) X-band (9 GHz) EPR measurements were carried out on a Bruker E580 ELEXSYS Series using the Bruker ER4122SHQE cavity filling in a 1 mm ID quartz capillary tube and then it was placed inside standard suprasil electron paramagnetic resonance (EPR) tubes with an ID = 4 mm. Low temperature was controlled with a Bruker ER 4111VT variable temperature unit. The samples (10 µg/mL soluble proteins) were frozen inside liquid nitrogen before recording the EPR spectra.
All measurements were performed at 150 K and the spectra were acquired with 3516 G central field and 1000 G scan width, with 3440 G central field and 200 G scan width. The experimental conditions in all cases were: ν = 9.67 GHz microwave frequency, 2 G modulation amplitude and 5 mW microwave power. All spectra were baseline corrected.
Transmission electron microscopy
The pooled bacterial pellets from 1 ml 50 mmol/L phosphate buffer pH 7.4 were fixed with 2.5% (v/v) glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.2) for 2 h at 4 °C, post-fixed with buffered 1% (w/v) osmium tetroxide (OsO4) for 1 h and processed by standard dehydration by a graded series of ethanol (50–100 °C). Specimens were embedded in pure Epon resin through a graded series of ethanol:resin mixture. Polymerization was done in an oven at 60 °C for 48 h.
Thick sections (60–70 nm) were cut with an Ultracut E (Reichert-Jung, Wien AT) ultramicrotome, stained with uranyl acetate and lead citrate and examined by a Tecnai G2 Spirit (FEI, Eindhoven NL) transmission electron microscope for analysis of ultrastructure.
Statistical analysis
All data were expressed as the mean values with standard deviation (±SD) for three independent experiments. Differences were considered statistically significant at the level of *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs. control. Data analysis was performed by GraphPad Prism V. 8 using parametric unpaired two-tailed t test.
Results
Minimum inhibitory concentrations of tested disinfectants at selected pH values
The MIC of test disinfectants at the pH values of interest are represented in . H2O2 and PAA concentrations (8.2 and 0.3 µmol/L respectively) were selected to study the redox status inside cells, except for LAS, which was found to be more potent at pH 5.0 (10.2 µmol/L).
Analysis of the cell redox status and ultrastructure
PCO, total thiols and LMWT levels and enzymes specific activity were measured in the supernatants of bacterial cells homogenate after cells exposure for 30 min to MICs of the tested disinfectants, and to pH 8.2 (control for H2O2 and PAA treatments), pH 5.0 (control for LAS treatment) and pH 6.4 (primary control of unadjusted pH).
PCO assay
Protein oxidation (carbonylation) increased significantly at pH 8.2 and 5.0 (p = 0.0008 and 0.0021 respectively vs. the primary control, ). In , the PCO level was elevated with PAA and H2O2 treatments (both treatments had p < 0.0001). LAS MIC increased the PCO level (p = 0.0016 vs. control) ().
Figure 2. Effects of alkaline treatments (a) and LAS treatment (b) on protein carbonylation Effects of alkaline treatments on total thiols (c) and LMWT (d). Effects of LAS treatment on total thiols (e) and LMWT (f). Thiols concentration is expressed in nmol/mg protein.
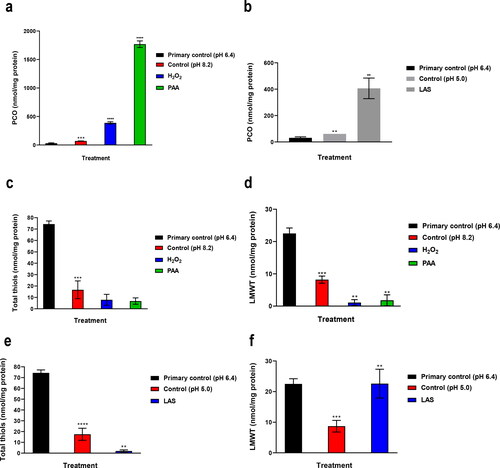
Table 1. Effect of pH alone on protein carbonylation.
Thiols assay
In , in comparison with pH 6.4 (primary control), exposed + hidden + LMWT level showed a higher decrease at pH 5.0 (p < 0.0001) than at pH 8.2 (p = 0.0003), while pH 8.2 was found to be more effective in decreasing the level of LMWT (p = 0.0003) than pH 5.0 (p = 0.0007).
Table 2. Effect of pH alone on total thiols.
The MIC of hydrogen peroxide and peracetic acid at pH 8.2 did not alter exposed + hidden + LMWT level (), while the LMWT level showed a significant decrease with hydrogen peroxide and PAA at pH 8.2 (p = 0.0010 and 0.0054 respectively vs. control) ().
LAS MIC oxidized hidden + exposed + LMWT (p = 0.0092 vs. control) (), and resulted in elevation of LMWT level (p = 0.0090 vs. control) ().
Catalase and SOD specific activities
As shown in , the SOD specific activity decreased significantly at pH 5.0 and 8.2 (p = 0.0001 and 0.0002 respectively vs. the primary control). The catalase specific activity was inhibited significantly at pH 5.0 (p < 0.0001 vs. the primary control), while increased markedly at pH 8.2 (p < 0.0001 vs. primary control).
Figure 3. Effect of pH alone (a), alkaline treatments (b) and LAS treatment (c) on SOD and catalase activities. Electron paramagnetic resonance spectra (d) for samples supernatants. Supernatants for EPR experiments were obtained after bacterial exposure to pH 6.4 (primary control); pH 8.2 (control for PAA, H2O2); pH 5.0 (control for LAS); H2O2; PAA; LAS. Each sample contained 10 µg/mL soluble proteins.
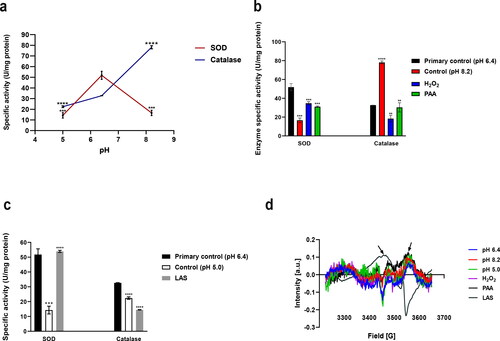
As presented in , in comparison to control (pH 8.2), bacteria treated with hydrogen peroxide showed a higher increase in the SOD specific activity (p = 0.0005) compared with peracetic acid treatment (p = 0.0006), on the contrary, hydrogen peroxide and peracetic acid were found to inhibit the catalase specific activity (p = 0.0021 and 0.0058 respectively).
LAS MIC resulted in activation of SOD (p < 0.0001), and inhibition of CAT specific activity (p < 0.0001), when compared to the control ().
Free radicals detection
Free radicals were detected in highly diluted supernatants of LAS and PAA only as depicted by arrows ().
Transmission electron microscopy
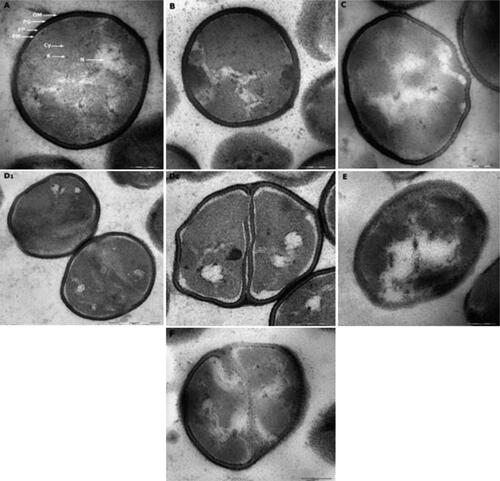
Transmission electron photomicrographs of P. fluorescens cells treated with the minimum inhibitory concentration of selected disinfectants for 30 min at early stationary growth phase are represented in .
Figure 4. P. fluorescens grown in TSB culture medium of pH 6.4 (primary control) presented no alterations in their features and even distribution of cytoplasmic structures (A). Cells exposed to alkaline conditions of pH 8.2 (control for PAA and H2O2) presented less dispersed, more confined nucleoid and electrodense structures accumulation (B). Cells exposed to acidic environment of pH 5.0 (control for LAS) (C) showed empty appearing vacuoles and deformed membranes. Cells treated with LAS showed corrugated membranes, empty vacuoles and black cytoplasmic condensations (D1, D2). Bacteria treated with PAA showed cytoplasmic vacuolization, protein accumulation, asymmetric and damaged membranes (E). Cells treated with H2O2 showed cytoplasmic vacuoles, distinct alteration of cell components with electrodense structures, and membranous bleb-like bulge indicating cell damage (F). OM, outer membrane; PG, peptidoglycan; PP, periplasm; PM, plasma membrane; N, nucleoid; Cy, cytoplasm; R, ribosomes. Scale bar = 200 nm (A, B, C, D2, E, F), 500 nm (D1).
Discussion
The effect of pH alone on ROS generation is not well investigated in prokaryotes. However in eukaryotic cells, it was found that the alkaline condition itself induces ROS production causing cell death [Citation30–35].
Our results supported similar studies that hydrogen peroxide is more potent on P. fluorescens in both acid and alkaline pH than at neutral conditions [Citation36–38]. Similar studies reported that alkaline H2O2 has a higher bleaching efficacy without an obvious surface erosion or modification [Citation39,Citation40], due to generation of perhydroxyl anion and active powerful hydroxyl radicals from hydrogen peroxide at alkaline pH [Citation41–43], but since H2O2 is more stable in acidic conditions, many products have an acidic pH to maintain H2O2 stability [Citation43].
On the other hand, it was demonstrated that the efficacy of H2O2 solutions is increased at more acidic pH ranges [Citation44], but the disadvantage is that bleaching at acidic conditions is found to be harmful to surfaces [Citation39,Citation40,Citation45–49]. It was also reported that bacterial resistance to H2O2 increases at low pH values, regardless of catalase activity [Citation50].
We have similarly reported that PAA is more potent at alkaline pH [Citation51], which could be due to the increased hydrolysis of peracetic acid at high pH values [Citation52], leading to formation of active peracetyl radicals responsible for peracetic acid activity. Other studies have demonstrated that PAA is more effective in acidic conditions, since the non-dissociated unionized form (CH3CO3H) is believed to be a more active form than the dissociated ionized form (CH3CO3−) [Citation53–60], while other investigations reported that PAA is more active at neutral pH ranges and the pH does not play a role in efficacy [Citation57,Citation61–63].
We found that pH plays a major role in increasing the efficacy of the tested oxidizing agents and anionic surfactant. As shown, H2O2 and PAA were found to be more potent at alkaline pH, while LAS was found to be more potent at acidic environment, which could result from the direct powerful harmful effects of each pH range on specific cellular biomolecules and ultrastructure, which could be the same targets for the actions of these disinfectants, and could partially explain the differential potencies and biological synergistic effects of pH and disinfectants. These direct biological effects of pH alone and disinfectants were further dissected in this research work. On the other hand, previous studies have proposed that alkaline and acidic conditions alter the efficacy of disinfecting agents mainly by influencing the stoichiometry of disinfectants [Citation41–43,Citation51–60].
Protein oxidation was reported by Kuiken [Citation64] at alkaline conditions, which could explain the efficacy of disinfectants i.e. H2O2 and PAA tested at alkaline pH. Our findings support some reports [Citation1,Citation53,Citation60,Citation65–85] that proteins are a main target for PAA, hydrogen peroxide and LAS action.
Our results are in agreement with many studies [Citation86–92] that acidic conditions significantly reduce total thiols, while alkaline environment mainly decreases LMWT levels. The higher decrease in total thiols at pH 5.0 could explain the efficacy of the anionic surfactant (LAS) tested at acidic pH, while the higher reduction in LMWT could increase the efficacy of H2O2 and PAA at alkaline pH.
Some research work [Citation53,Citation60,Citation72–76,Citation93] has reported oxidizing effects of H2O2 and PAA on thiols, which explain hydrogen peroxide and peracetic acid damaging effects via oxidation of reduced thiols. Similar studies [Citation83–85] suggested that total thiols are a main target for LAS in tested bacteria.
We suggest that low molecular weight thiols are not a reliable biomarker of cell damage imposed by anionic surfactants, since the tested surfactant was found to just increase the reactivity (not the actual concentration) of low molecular weight thiols, probably through formation of thiol-surfactant adduct which reacts with two DTNB molecules instead of one molecule as follows:
The minimum inhibitory concentration of hydrogen peroxide at pH 8.2 (284.1 ppm) was found to increase catalase specific activity when tested at pH 6.4 rather than enzyme inhibition caused at pH 8.2, which suggests that alkaline conditions contributed to the effect of hydrogen peroxide by impairing the antioxidant defense system (data not shown). We suggest that the increment in SOD specific activity and decrease in the catalase specific activity with the tested disinfectants could be a late stage of oxidative stress/damage [Citation94,Citation95].
As expected, pH alone was found to have variable effects on the antioxidant enzyme activities. The tested pH values could have direct effects on the ionization state of acidic or basic amino acids of proteins, e.g. enzymes, and hence the ionic bonds that help to determine the 3-D structure of the enzymes could be altered. This could lead to variable degrees of alterations in the enzyme activity. Moreover, alteration in pH could change the shape or charge properties of the substrate molecules, so that the enzyme affinity to substrate could increase or decrease. In general, enzymes have pH of optimum activity. However, the optimum pH is found to be enzyme and strain-specific [Citation96].
We also found that exposure of cells to the selected disinfectants at the tested pH values resulted in differential oxidative changes in proteins. Moreover, the tested disinfectants caused different antioxidant enzymes responses probably due to the variable effects on the transcriptional factors that control the bacterial genetic expression of the antioxidant enzymes. These effects on proteins and enzymes have been reported in past studies and could also be strain-specific [Citation1,Citation67–69].
Free radicals detected in highly diluted supernatants of LAS and PAA only could explain the higher efficacy of LAS followed by PAA in comparison with H2O2. In addition, organic radicals generated from peracetic acid generally have a long half-life, so they are proposed to be more effective in cell penetration and the bacterial control effects [Citation93,Citation97,Citation98].
We demonstrated that each treatment presents distinct alterations and disruption in cell morphology and cytoplasmic structures, resulting in the bacterial control effects of tested bleaching agents and surfactant against P. fluorescens. These effects of the selected disinfectants on cell ultrastructure of P. fluorescens are of a great interest, since they were not studied in past research work.
Conclusions
Our findings suggested that the selected disinfectants may indirectly induce ROS production in the bacterial cell causing oxidation of thiols, which initially increases antioxidant enzymes activities, but with sustained exposure to minimum inhibitory concentrations, they eventually oxidatively inhibit antioxidant enzymes. This may result in accumulation of H2O2 and therefore highly potent •OH generation via the Fenton reaction, which may increase protein carbonyls (PCO) levels, and further deplete thiols that explain late stage of oxidative stress.
The antioxidant enzymes may not be considered reliable biomarkers of oxidative stress, because their activity may be altered solely after an acute exposure to disinfectants and then return to normal levels. However, oxidative damage products, i.e. protein carbonyls and oxidized thiols, seem to be useful biomarkers, since damage products can persist even after the stress factor ceases, suggesting the reliability of protein carbonylation and thiol oxidation in determining oxidative stress.
Since DNA is considered the last target for xenobiotics when used in high doses, we suggest future investigations to determine the DNA damage products, e.g. 8-oxoguanine (8-oxoG), which could be increased in the tested bacterial strain exposed to the minimum inhibitory concentrations (and sublethal concentrations) of selected bleaching agents and surfactant.
Importantly, we suggest further studies to understand the effects of pH on the relationship/ratio between concentration of unionized forms of the tested disinfectants that cross the biological membrane and the degree of availability of the active species of these chemical agents in situ that exert the intrinsic bacterial control effects against P. fluorescens. Understanding this relationship could allow the development of highly efficient disinfectants.
Author’s contributions
Osama H. Bekhet conceived and designed the study, performed the laboratory work and statistical analysis and wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Acknowledgement
This work was performed at University of Siena and Procter & Gamble Newcastle Innovation Centre (P&G NIC).
Availability of data and materials
Data and materials related to this study are available upon reasonable request.
Disclosure statement
Osama H. Bekhet declares that he has no conflict of interest.
Data availability statement
All data that support the findings reported in this manuscript are available from the author upon reasonable request.
Additional information
Funding
References
- Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3(1):3–8.
- Sies H. Damage to plasmid DNA by singlet oxygen and its protection. Mutat Res. 1993;299(3–4):183–191.
- Sies H, Menck CF. Singlet oxygen induced DNA damage. Mutat Res. 1992;275(3–6):367–375.
- Dizdaroglu M. Measurement of radiation-induced damage to DNA at the molecular level. Int J Radiat Biol. 1992;61(2):175–183.
- González-Flecha B, Demple B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270(23):13681–13687.
- Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37(45):15835–15841.
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128.
- Fucci L, Oliver CN, Coon MJ, et al. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983;80(6):1521–1525.
- Stadtman ER. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325.
- Niederhoffer EC, Naranjo CM, Bradley KL, et al. Control of Escherichia coli superoxide dismutase (Soda and Sodb) genes by the ferric uptake regulation (Fur) locus. J Bacteriol. 1990;172(4):1930–1938.
- Compan I, Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993;175(6):1687–1696.
- Benov LT, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269(41):25310–25314.
- Finn GJ, Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975;123(2):570–579.
- von Ossowski I, Mulvey MR, Leco PA, et al. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol. 1991;173(2):514–520.
- Kim G, Weiss SJ, Levine RL. Methionine oxidation and reduction in proteins. Biochim Biophys Acta. 2014;1840(2):901–905.
- Levine RL, Mosoni L, Berlett BS, et al. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93(26):15036–15040.
- Wirth SM, Lowry GV, Tilton RD. Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved silver. Environ Sci Technol. 2012;46(22):12687–12696.
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (Mic) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) . Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect. 2000;6(9):509–515.
- Jakubowski W, Walkowiak B. Resistance of oxidative stress in biofilm and planktonic cells. Braz Arch Biol Technol. 2015;58(2):300–308.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol. 1990;186:464–478.
- Levine RL, Williams JA, Stadtman ER, et al. Carbonyl assays for determination of oxidatively modified proteins. Meth Enzymol. 1994;233:346–357.
- Dalle-Donne I, Rossi R, Giustarini D, et al. Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic Biol Med. 2001;31(9):1075–1083.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77.
- Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126.
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140.
- Jakubowski W, Biliński T, Bartosz G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic Biol Med. 2000;28(5):659–664.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287.
- Cutaia M, Kroczynski J, Tollefson K. pH-dependent oxidant production following inhibition of the mitochondrial electron transport chain in pulmonary endothelial cells. Endothelium. 2002;9(2):109–121.
- Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies?Arch Biochem Biophys. 2008;476(2):107–112.
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352.
- Sakihama Y, Cohen MF, Grace SC, et al. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177(1):67–80.
- Maeta K, Nomura W, Takatsume Y, et al. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl Environ Microbiol. 2007;73(2):572–580.
- Majima HJ, Oberley TD, Furukawa K, et al. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998;273(14):8217–8224.
- Curran HR, Evans FR, Leviton A. The sporicidal action of hydrogen peroxide and the use of crystalline catalase to dissipate residual peroxide. J Bacteriol. 1940;40(3):423–434.
- Hamiltong A. Chemical models and mechanisms for oxygenases. In: Hayaishi O, editor. Molecular mechanisms of oxygen activation. New York: Academic Press; 1974. p. 405–451.
- Sawyer DT. Oxygen: inorganic chemistry. In: King E, editor. Encyclopedia of inorganic chemistry. Vol. 6. Chichester: Wiley & Sons; 1994. p. 2951.
- Xu B, Li Q, Wang Y. Effects of pH values of hydrogen peroxide bleaching agents on enamel surface properties. Oper Dent. 2011;36(5):554–562.
- Young N, Fairley P, Mohan V, et al. A study of hydrogen peroxide chemistry and photochemistry in tea stain solution with relevance to clinical tooth whitening. J Dent. 2012;40(Suppl 2):e11–e16.
- Brooks RE, Moore SB. Alkaline hydrogen peroxide bleaching of cellulose. Cellulose. 2000;7(3):263–286.
- Gould JM. Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol Bioeng. 1985;27(3):225–231.
- Torres CRG, Crastechini E, Feitosa FA, et al. Influence of pH on the effectiveness of hydrogen peroxide whitening. Oper Dent. 2014;39(6):E261–E268.
- Raffellini S, Guerrero S, Alzamora SM. Effect of hydrogen peroxide concentration and pH on inactivation kinetics of Escherichia coli: E. Coli inactivation by hydrogen peroxide at different ph values. J Food Saf. 2008;28(4):514–533.
- Cavalli V, Arrais C. A G, Giannini M, et al. High-concentrated carbamide peroxide bleaching agents effects on enamel surface. J Oral Rehabil. 2004;31(2):155–159.
- Jiang T, Ma X, Wang Y, et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008;13(1):014019.
- Bistey T, Nagy IP, Simó A, et al. In vitro FT-IR study of the effects of hydrogen peroxide on superficial tooth enamel. J Dent. 2007;35(4):325–330.
- Attin T, Müller T, Patyk A, et al. Influence of different bleaching systems on fracture toughness and hardness of enamel. Oper Dent. 2004;29(2):188–195.
- Rodrigues JA, Marchi GM, Ambrosano GMB, et al. Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design. Dent Mater. 2005;21(11):1059–1067.
- Jackett PS, Aber VR, Lowrie DB. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978;104(1):37–45.
- Envirotech. The use of peracetic acid at increased pH levels. Patent Application (US2012/024461 A1). 2012.
- Xu C, Long X, Du J, et al. A critical reinvestigation of the TAED-activated peroxide system for low-temperature bleaching of cotton. Carbohydr Polym. 2013;92(1):249–253.
- Kitis M. Disinfection of wastewater with peracetic acid: a review. Environ Int. 2004;30(1):47–55.
- Colgan S, Gehr R. Disinfection. Water Environ Technol. 2001;13:29–33.
- Gehr R, Cochrane D, French M. 2002. Peracetic acid as a disinfectant for municipal wastewaters: encouraging performance results from physicochemical as well as biological effluents. Proceedings of the US Water Environment Federation Disinfection Conference, Chicago, Illinois.
- Baldry MGC, French MS. Activity of peracetic acid against sewage indicator organisms. Water Sci Technol. 1989a;21(12):1747–1749.
- Sanchez-Ruiz C, Martinez-Royano ST-MI. An evaluation of the efficiency and impact of raw wastewater disinfection with peracetic acid prior to ocean discharge. Water Sci Technol. 1995;32:159–166.
- Tutumi M, Imamura K, Hatano S, et al. Antimicrobial action of peracetic acid. J Food Hyg Soc Jpn. 1974;15(2):116–120.
- Baldry MGC, French MS, Slater D. The activity of peracetic acid on sewage indicator bacteria and viruses. Water Sci Technol. 1991;24(2):353–357.
- Block SS. Peroxygen compounds. In: Block SS, editor. Disinfection, sterilization, and preservation. 4th ed. Philadelphia (PA): Lea & Febiger; 1991. p. 167–181.
- Lenntech. Disinfectants peracetic acid; [accessed 2020 Apr 19]. Available from: https://www.lenntech.com/processes/disinfection/chemical/disinfectants-peracetic-acid.htm
- Seiner N, Corp D, Nj A. Evaluation of peracetic acid as an environmentally safe alternative for hypochlorite. Textile Chem Color. 1995;27:29–32.
- Shen X, Sheng L, Gao H, et al. Enhanced efficacy of peroxyacetic acid against Listeria monocytogenes on fresh apples at elevated temperature. Front Microbiol. 2019;10, 119. doi: 10.3389/fmicb.2019.01196.
- Kuiken KA, Lyman CM, Hale F, et al. Factors which influence the stability of tryptophan during the hydrolysis of proteins in alkaline solution. J Biol Chem. 1947;171(2):551–560.
- Xu Y-B, Xu J-X, Chen J-L, et al. Antioxidative responses of Pseudomonas fluorescens YZ2 to simultaneous exposure of Zn and Cefradine. Ecotoxicology. 2015;24(7–8):1788–1797.
- Ganie SA, Haq E, Hamid A, et al. Long dose exposure of hydrogen peroxide (H2O2) in albino rats and effect of Podophyllum hexandrum on oxidative stress. Eur Rev Med Pharmacol Sci. 2011;15(8):906–915.
- Finnegan M, Linley E, Denyer SP, et al. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother. 2010;65(10):2108–2115.
- Small DA, Chang W, Toghrol F, et al. Comparative global transcription analysis of sodium hypochlorite, peracetic acid, and hydrogen peroxide on Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2007;76(5):1093–1105.
- Semchyshyn H, Bagnyukova T, Storey K, et al. Hydrogen peroxide increases the activities of soxRS regulon enzymes and the levels of oxidized proteins and lipids in Escherichia coli. Cell Biol Int. 2005;29(11):898–902.
- Juven BJ, Pierson MD. Antibacterial effects of hydrogen peroxide and methods for its detection and quantitation. J Food Prot. 1996;59(11):1233–1241.
- Liochev SI. The mechanism of ‘Fenton-like’ reactions and their importance for biological systems. A biologist’s view. Met Ions Biol Syst. 1999;36:1–39.
- Baldry MGC. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J Appl Bacteriol. 1983;54(3):417–423.
- Marjani A. 2010. The effects of subacute exposure of peracetic acid on lipid peroxidation and hepatic enzymes in wistar rats. OMJ [Internet] [cited 2020 Apr 15]; Available from: http://www.omjournal.org/fultext_PDF.aspx?DetailsID=14&type=fultext
- Baldry MGC, Fraser JAL. Disinfection with peroxygens. Crit Rep Appl Chem. 1988;22:91–116.
- Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11(4):147–157.
- Kerkaert B, Mestdagh F, Cucu T, et al. Hypochlorous and peracetic acid induced oxidation of dairy proteins. J Agric Food Chem. 2011;59(3):907–914.
- Fraser JA, Godfree AF, Jones F. Use of peracetic acid in operational sewage sludge disposal to pasture. Water Sci Technol. 1985;17(4–5):451–466.
- Lazarova V, Janex ML, Fiksdal L, et al. Advanced wastewater disinfection technologies: short and long term efficiency. Water Sci Technol. 1998;38(12):109–117.
- Antonelli M, Rossi S, Mezzanotte V, et al. Secondary effluent disinfection: PAA long term efficiency. Environ Sci Technol. 2006;40(15):4771–4775.
- Jifa W, Zhiming Y, Xiuxian S, et al. Comparative researches on effects of sodium dodecylbenzene sulfonate and sodium dodecyl sulfate upon Lateolabrax japonicus biomarker system. Environ Toxicol Pharmacol. 2005;20(3):465–470.
- Messina CM, Faggio C, Laudicella VA, et al. Effect of sodium dodecyl sulfate (SDS) on stress response in the Mediterranean mussel (Mytilus galloprovincialis): regulatory volume decrease (Rvd) and modulation of biochemical markers related to oxidative stress. Aquat Toxicol. 2014;157:94–100.
- Abel PD. Toxicity of synthetic detergents to fish and aquatic invertebrates. J Fish Biol. 1974;6(3):279–298.
- Gloxhuber C, Künstler K, editors. Anionic surfactants: biochemistry, toxicology, dermatology. 2nd ed., rev. expanded. New York: M. Dekker; 1992.
- Geuther R. The plasma membrane: dynamic perspectives, genetics and pathology (Heidelberger Science Library vol. 18). Xi, 186 s., 27 abb., 24 tab. London-new york-heidelberg-berlin 1972: the English University Press Ltd. Und Springer Verlag. Dm 18,30. Z Allg Mikrobiol. 1974;14(2):175–175.
- Baker Z, Harrison RW, Miller BF. Action of synthetic detergents on the metabolism of bacteria. J Exp Med. 1941;73(2):249–271.
- Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med. 2015;80:148–157.
- Winther JR, Thorpe C. Quantification of thiols and disulfides. Biochim Biophys Acta. 2014;1840(2):838–846.
- Nagy P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid Redox Signal. 2013;18(13):1623–1641.
- Halprin KM, Ohkawara A. The measurement of glutathione in human epidermis using glutathione reductase. J Invest Dermatol. 1967;48(2):149–152.
- Stark AA, Arad A, Siskindovich S, et al. Effect of pH on mutagenesis by thiols in Salmonella typhimurium TA102. Mutat Res. 1989;224(1):89–94.
- Halliwell B. 1982. The toxic effects of oxygen on plant tissues. In: Oberley LW ,editor. Superoxide dismutase. Vol. 1. Boca Raton (FL): CRC Press. pp. 89–123.
- Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid Redox Signal. 2011;14(6):1065–1077.
- Block SS. 2001. Peroxygen compounds. In: Block SS, editor. Disinfection, sterilization, and preservation. Philadelphia (PA): Lippincott Williams & Wilkins. p. 185–204.
- Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257(10):5751–5754.
- de Oliveira MR. Vitamin A and retinoids as mitochondrial toxicants. Oxid Med Cell Longev. 2015;2015:140267–140213.
- Brooklyn College. The effect of pH on enzyme actvity; [accessed 21 Oct 2019]. Available from: http://academic.brooklyn.cuny.edu/biology/bio4fv/page/ph_and_.htm
- Clapp PA, Davies MJ, French MS, et al. The bactericidal action of peroxides; an E.P.R. spin-trapping study. Free Radic Res. 1994;21(3):147–167.
- Koivunen J, Heinonen-Tanski H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005;39(8):1519–1526.