Abstract
Xanthium strumarium L. (Asteraceae) is a commonly distributed weed and invasive species to Bulgarian flora. Previous reports focussed on the composition of stem and leaf essential oils and gave possible medicinal use of this species. The aim of this study was to obtain an essential oil by hydro-distillation from the fruits of X. strumarium growing in Bulgaria and to analyse its content. A highly accurate gas chromatography – Orbitrap® mass spectrometry was used, paired with database-assisted interpretation of the results. The essential oil consisted mainly of terpenes (52%) with sesquiterpenes isovalencenol (14.8%) and himachalol (14.9%) as the main constituents. For the first time 16 compounds were identified: five sesquiterpenes, one sterol, four alkanes, an ester and an acetal. Three sesquiterpenes and an ester were found novel for the fruits as well. In addition, three compounds were identified in the sample, but already known in leaf oil. The results of the study will serve as a basis for possible utilization of this invasive weed in Bulgaria.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1986426.
Introduction
Invasive plants are undesirable from a biodiversity point of view because they interfere with the native vegetation. These aggressive aliens over compete the local plants and even suppress the germination and early-stage development of their seedlings [Citation1, Citation2]. However, these plants may contain valuable bioactive compounds. Thus, their abundant biomass becomes an advantage because it is an inexpensive source for natural products.
Xanthium strumarium L. (Common cocklebur), Asteraceae, is an annual, widely distributed weed [Citation3, Citation4] originating from Central or South America [Citation5]. The plant height is 20–90 cm, its stems are erect, branched, often speckled with purple and have short white hairs scattered across the surface. Leaves are green, cauline, mostly alternate (proximal 2–6, sometimes opposite) with petiole, which are 5–20 cm long and 4–16 cm wide. The shape of blades are lanceolate, linear, ovate, orbicular-deltate or suborbicular, and both surfaces are hirtellous or strigose, usually with gland-dotted, margin entire or toothed. The capitula are discoid, whose female (proximal) or functionally male (distal) are in racemiform to spiciform arrays or borne singly (in axils). The female capitula are elliptic, 2–5 mm in diameter. Male capitula are saucer-shaped, 3–5 mm in diameter. The achenes are black, fusiform, obovoid, enclosed in the hardened involucre, with two hooked beaks and hooked bristles [Citation6]. This plant is often considered invasive, particularly in agricultural areas [Citation7], and in some parts of Bulgaria it is the most frequently recorded weed [Citation8]. Traditionally the plant has been used as an immunostimulant and a febrifuge as well as to cure dysentery, stomach diseases, diarrhoea, leucorrhoea and urinary diseases (diuretic properties), dental sourness and toothaches, paralysis, bleeding (astringent), small pox, leprosy, tuberculosis, eczema, skin disease, boils and pimples, chronic malaria, earache and strumous disease, insect bites, etc. [Citation9]. Contemporary pharmacological investigations reveal the potential anticancer activity of X. strumarium (due to the presence of sesquiterpene lactones). They also demonstrate the antitussive, antifungal, anti-inflammatory, antinociceptive, hypoglycaemic, antimitotic, antioxidant, CNS depressant activity of this plant, as well as its diuretic and antitrypanosomal effect [Citation9].
Essential oils are valuable natural products, used as raw materials in many fields such as perfumes, cosmetics, aromatherapy, spices, and nutrition [Citation10]. The essential oil of X. strumarium significantly inhibited the growth of gram-positive and gram-negative bacteria (most efficient against Staphylococcus aureus), as well as some fungi (Candida albicans and Aspergillus niger). Moreover, the common cocklebur’s essential oil showed scolicidal activity against Echinococcus granulosus [Citation11]. These data add to the possible pharmaceutical significance of the species.
Phytochemical investigations of X. strumarium are still not numerous but they report various secondary metabolites such as terpenes, flavonoids, phenolic acids, thiazinediones and sesquiterpene lactones [Citation9, Citation12]. Recently terpenes were reported cytotoxic against colon cancer [Citation13]. Common cocklebur contains also an essential oil, whose composition varies vastly depending on the plant parts used for the extraction (stems, leaves or fruits) and the origin of the material (reflecting the ecological specifics of the area). Two main groups of components are registered in the oil from vegetative parts: monoterpenes and sesquiterpenes. Main constituents of the oil are limonene, borneol, α-ionone, cis-β-guaiene, β-caryophyllene, carveol, p-cymene, etc. but their quantity also varies significantly [Citation11, Citation14–17]. There is only one study of the essential oil from the fruits with major constituents methyl linoleate, methyl oleate and methyl palmitate [Citation18]. The information on chemical composition of the fruit essential oil is limited; moreover, Bulgarian plants have not been studied. The aim of this study was to obtain essential oil from the fruits of X. strumarium collected from Bulgaria and to investigate its chemical composition by gas chromatography–mass spectrometry (GC-MS).
Materials and methods
Plant material
The fruits of X. strumarium were collected in 2019 from a locality in Kamen Bryag, Bulgaria. One of us (E. K) identified the plant and a voucher specimen was deposited in the Herbarium of the Faculty of Pharmacy at the Medical University of Sofia (FF-173/2019). The fruits were cleaned and crushed with a mechanical hammer.
Obtaining the essential oil
The chopped fruit (40 g) was extracted in a Clevenger-type apparatus (250 mL of water) for 4 h. The essential oil (0.3 mL) was separated, dried over anhydrous Na2SO4 and stored at −40 °C until analysis.
GC-MS analysis
The essential oil was diluted 1:10000 with hexane immediately before analysis. GC-MS was performed with an Exactive™ Orbitrap™ GC-MS (ThermoFisher Scientific, Germany) system operating at 70 eV, ion source temperature 230 °C, transfer capillary temperature 260 °C, with split injection (1 μL, 20:1 ratio) at 250 °C injector temperature. A capillary column with 5% phenyl residues/95% methyl polysiloxane (TraceGOLD TG-5SilMS GC Column 30 m x 0.25 mm x 0.25 µm, Thermo) was used. The oven temperature program was: initial at 60 °C for 5 min, increased to 300 °C (rate: 6 °C/min), maintained at 300 °C for 5 min. Helium was used as the carrier gas (flow rate: 1 mL/min). The EI ionization mode and full MS-SIM scan were used (resolution 600, AGC target 1e6, maximum IT 200 ms, and scan range of m/z 50-450). Data collection, peak processing and compound identification were performed with Xcalibur 4.2.28.14 (Thermo Scientific, Germany). The GC column was calibrated by a standard procedure [Citation19, Citation20] using a calibration mixture of n-alkanes (C8-C40 Alkanes Calibration Standard, Supelco, USA). Peaks were processed using GENESIS algorithm. The Kovats indexes and the relative percentage of constituents were calculated using the capabilities of the software. Each compound was identified based on a comparison of its mass spectral fragmentation in the positive ionization mode. The results were compared with the records in two databases. Both Wiley Registry 10 and NIST 2014 were used, and data was interpreted through software NIST MS Search v. 2.2. Criteria for identification were the coincidence of the results from both databases, the coincidence of the confidence intervals (statistical %) for the compound in question from both databases and the coincidence of the Kovats index of the substance to that in the literature [Citation19, Citation21, Citation22].
Results
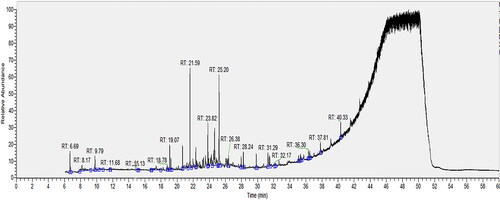
The hydro-distillation of X. strumarium fruits yielded 0.75% (w/w) light-yellow essential oil, based on the dry weight of the plant material. The oil was analysed by GC-MS, and 29 ingredients were identified, representing 99.99% of the total composition. The GC-MS chromatogram is given in and the constituents of the sample are listed by their retention time (tR) over the TG-5SilMS column in and .
Table 1. Identified compounds in the essential oil of X. strumarium fruit.
After comparing the obtained information with the Wiley Registry and NIST databases () and considering the Kovats indexes of the components () [Citation19, Citation21, Citation22], it was noted that the essential oil was composed mainly of terpenes 52.36%, and all the other constituents represented 47.64% of the sample (as a sum). The sesquiterpenes isovalencenol (14.89%) and himachalol (14.98%) were identified as the main compounds.
Sixteen compounds were identified for the first time in an essential oil from this species: sesquiterpenes, humulene-1,2-epoxide (5.51%), modephene (3.56%), α-isocomene (1.73%), α-bisabolol oxide (1.23%) and acetic acid, 10-hydroxymethyl-6-methyl-12,13-dioxa-tricyclo[7.3.1.0(1,6)] tridec-5-yl ester (0.23%); a sterol − 3-hydroxyspirost-8-en-11-one (0.80%); four alkanes, 2-hydroxy-1,1,10-trimethyl-6,9-epidioxydecalin (1.78%), 2-(7-tert-butoxyheptyl)-5-methoxy-2-cyclopenten-1-one (5.58%); 3-ethyl-5-(2′-ethylbutyl)octadecane (0.89%), 1β,12,12-trimethyl-7,11-dioxapentacycloeicos-13-en-20-ol-8-one (6.63%); an ester, (8Z)-7-methyl-8-tetradecenyl acetate (0.64%) and an acetal, 1-methylene-2-(4,4-diethoxybutyl)cyclopropane (3.64%), as presented in
The only germacrane, (1 R,7S)-germacra-4(15),5,10(14)-trien-1β-ol, was found in 2.29%, while the ester E-8-methyl-9-tetradecen-1-ol acetate was a minor component − 1.79%. Humulene was detected in high amount (2.91%), unlike the quantity of limonene, which was only 1.97%. In addition, the sesquiterpene epi-cubebol was found in small amount (0.55%) in fruit essential oil. Two phthalic acid derivatives: phthalic acid, butyl tridec-2-yn-1-yl ester (2.26%) and phthalic acid, butyl 4-isopropylphenyl ester (2.27%), were identified in the studied sample as well. Notheworthy, the phenol 2,6-di-tert-butyl-p-cresol was in significant quantity in Bulgarian fruit essential oil (13.75%), which could be assumed unique to date.
Our study showed that Bulgarian essential oil from fruits had predominantly sesquiterpenes (52%), followed by hydrocarbons (20%), and in addition contained some monoterpenes (limonene) and diterpenes (methyl ester of gibberiline A9, ).
Discussion
The main sesquiterpenes isovalencenol and himachalol identified in the essential oil were not found in fruits before [Citation14, Citation18]. Ghahari et al. [Citation18] reported that the main components in fruit essential oil were the methyl esters of fatty acids and terpenes were as trace ingredients only. Our study gave additional information on the phytochemical variation of the essential oil’s content. In the sample several components were identified, similar to those already reported in leaf essential oil of different origin. A derivative similar to the identified germacrane (see ), germacene D, was reported previously in trace amounts − 0.05% [Citation11] and 0.75% [Citation17], respectively. El-Gawad et al. [Citation17] proved another phytol derivative (E-phytol acetate) in the leaves in a low quantity (0.49%) proving that the phytol found in the sample is a minor constituent. The humulene content of the fruit essential oil was rather different to leaves, where its content was reported to be very low − 0.6% [Citation11] and 1.1% [Citation15]. Limonene quantity was found low in the fruit oil, while a very high percentage was found in the leaves before − 35.0% [Citation14], 20.3% [Citation11] and 24.7% [Citation15]. Only Parveen et al. [Citation16] reported a quantity of this monoterpene close to 5.66%. The sesquiterpene epi-cubebol was found in small amount in this essential oil, and it was reported before in the leaves (0.2%) [Citation11]. The presence of phthalates in the volatile oil of X. strumarium is an already known fact; though these compounds were reported in extracts from other representatives of the genus as well [Citation23]. The large quantity of phenol 2,6-di-tert-butyl-p-cresol was a novelty, although it was previously found in leaves as trace amounts [Citation6, Citation24]. On the other hand, the general composition of the investigated essential oil was rather different compared to others. There are several reports to describe the components of the essential oil from X. strumarium leaves collected from Pakistan, Iran, Egypt and Brazil. Those oils were all rich in different classes of volatiles, especially monoterpenes and sesquiterpenes. Esmaeili et al. [Citation15] and Sharifi-Rad et al. [Citation11] both reported that the essential oil from leaves of the Iranian species was rich in mono- and sesquiterpenes, as two main classes of components, with 46% and 47%, respectively, with the addition of diterpenes and hydrocarbons. Parveen et al. [Citation16] also described that monoterpenes were major components (55%), followed by sesquiterpenes (26%), with the addition of diterpenes and hydrocarbons in the essential oil from Pakistan. Studies of the composition of the essential oil from leaves of Egyptian plants were close to those of the Brazilian ones, in which sesquiterpenes were the main component (72%). The Egyptian oil was similar to the Pakistani and Iranian ones, in which monoterpenes (25%) were the second major components, followed by hydrocarbons and diterpenes [Citation17]. The gibberillin content of the sample was not surprising, since gibberilins act as phytohormones, regulating the vegetative phases, incl. flowering and fructification [Citation25]. However, the essential oil from the leaves of Brazilian species was found to include other sesquiterpenes as the main components (88%) with the addition of monoterpenes (4%), but excluding diterpenes and hydrocarbons [Citation26]. Our findings were more consistent to these from Asia and North Africa, than to the Brazilian report. Previous reports were inconsistent as was the main phytochemical group [Citation11, Citation18, Citation24, Citation26, Citation27].
Conclusions
The essential oil from the fruits of X. strumarium collected in Bulgaria was obtained and studied by GC-MS for the first time. It contained mainly sesquiterpenes (isovalencenol and himachalol), followed by hydrocarbons, mono- and diterpenes. Sixteen new ingredients were reported for the fruits essential oil. These findings could serve as the basis for commercial utilization of this invasive plant as a cheap source of pharmaceutically important compounds.
Acknowledgements
The authors are grateful for the financial support from Ministry of Education and Science of the Republic of Bulgaria through contract № DO1-217/30.11.2018 (BioActiveMed), agreement №DO1-358/17.12.2020.
Disclosure Statement
The authors have no competing interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, IK, upon reasonable request.
Additional information
Funding
References
- Schaefer M. Handbook of alien species in Europe, DAISIE. Dordrecht/Heidelberg: Springer; 2009. p. 398.
- Ullah R, Khan N. Xanthium strumarium L. an alien invasive species in Khyber Pakhtunkhwa (Pakistan): a tool for biomonitoring and environmental risk assessment of heavy metal pollutants. Arab J Sci Eng. 2021. DOI:10.1007/s13369-021-05839-6.
- Holm LG, Plucknett DL, Pancho JV, et al. The world’s worst weeds. Distribution and biology. Honolulu, Hawaii USA: University press of Hawaii; 1977.
- Holm L, Pancho JV, Herberger JP, et al. A geographical atlas of world weeds. New York, Chichester, Brisbane, Toronto UK: John Wiley and Sons; 1979.
- Löve D, Dansereau P. Biosystematic studies on Xanthium: Taxonomic appraisal and ecological status. Can J Bot. 1959;37(2):173–208. ]. Available from:
- Fan W, Fan L, Peng C, et al. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: a review. Molecules [Internet. 2019;24(2):359. ]. Available from: https://www.mdpi.com/1420-3049/24/2/359
- Forest SK. Invasive species compendium. Xanthium strumarium. 2018. Available from: https://www.cabi.org/isc/datasheet/56864#CA0B0975-4853-4688-B6E5-BBFA0D6265C9.
- Aneva IY, Zhelev P, Stoyanov SS, et al. Alien species as a part of plant composition in the periphery of agricultural fields. Acta Zool Bulg Suppl. 2018;11:173–176.
- Kozuharova E, Ionkova I, Spadaro V. Xanthium strumarium - a potential cheap resource of plant substances for medicinal use. Flora Mediterr. 2019;29:93–102.
- Hammami S, Jmii H, Mokni RE, et al. Essential oil composition, antioxidant, cytotoxic and antiviral activities of teucrium pseudochamaepitys growing spontaneously in Tunisia. Molecules. 2015;20(11):20426–20433. Available from: https://www.mdpi.com/1420-3049/20/11/19707
- Sharifi-Rad J, Hoseini-Alfatemi SM, Sharifi-Rad M, et al. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules. 2015;20(4):7034–7047. Available from: https://www.mdpi.com/1420-3049/20/4/7034
- Yen PH, Hoang NH, Trang DT, et al. A new thiazinedione glycoside from the fruits of Xanthium strumarium L. Nat Prod Commun. 2021;16(7):1934578X2110320. Available from.
- Li L, Liu P, Xie Y, Liu, Y., Chen, Z., Geng, Y., & Zhang, L. Xanthatin inhibits human Colon cancer cells progression via mTOR signaling mediated energy metabolism alteration. Drug Dev Res. 2021. 1–12. doi:10.1002/ddr.21850
- Habibi Z, Laleh A, Masoudi S, et al. Composition of the essential oil of Xanthium brasilicum vellozo from Iran. J Essent Oil Res. 2004;16(1):31–32. Available from:
- Esmaeili A, Rustaiyan A, Akbari MT, et al. Composition of the essential oils of Xanthium strumarium L. and cetaurea solstitialis L. from Iran. J Essent Oil Res. 2006;18(4):427–429.
- Parveen Z, Mazhar S, Siddique S, et al. Chemical composition and antifungal activity of essential oil from Xanthium strumarium L. leaves. Indian J Pharm Sci. 2017;79(2):316–321.
- El-Gawad AA, Elshamy A, El Gendy AE-N, et al. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: evidence from chemometrics analysis. Molecules. 2019;24(3):584. Available from. https://www.mdpi.com/1420-3049/24/3/584
- Ghahari S, Alinezhad H, Nematzadeh GA, et al. Biochemical composition, antioxidant and biological activities of the essential oil and fruit extract of Xanthium strumarium Linn. from Northern Iran. J Agric Sci Technol. 2017;19(7):1603–1616. Available from: http://jast.modares.ac.ir/article-23-6103-en.html
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Vol. 456. Carol Stream, IL: Allured Publishing Corporation; 2007.
- Rimayi C, Odusanya D, Mtunzi F, et al. Alternative calibration techniques for counteracting the matrix effects in GC-MS-SPE pesticide residue analysis - a statistical approach . Chemosphere. 2015;118:35–43.
- Babushok VI, Zenkevich IG. Retention indices for most frequently reported essential oil compounds in GC. Chroma. 2009;69(3-4):257–269. Available from:
- Babushok VI, Linstrom PJ, Zenkevich IG. Retention indices for frequently reported compounds of plant essential oils. J Phys Chem Ref Data. 2011;40(4):43101. Available from:
- Han T, Zhang H, Li H, et al. Composition of supercritical fluid extracts of some Xanthium species from China. Chem Nat Compd. 2008;44(6):814–816. Available from:
- Kamboj A, Saluja AK. Phytopharmacological review of Xanthium strumarium L. (cocklebur). Int J Green Pharm. 2010;4(3):129.
- Shininger TL. The regulation of cambial division and secondary xylem differentiation in xanthium by auxins and gibberellin. Plant Physiol. 1971;47(3):417–422.
- Scherer R, Wagner R, Meireles MAA, et al. Biological activity and chemical composition of hydrodistilled and supercritical extracts of Xanthium strumarium L. leaves. J Essent Oil Res. 2010;22(5):424–429. Available from:
- Mancianti F, Ebani VV. Biological activity of essential oils. Molecules [Internet. 2020;25(3):678. Available from: https://www.mdpi.com/1420-3049/25/3/678