 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Solanum anguivi Lam. is an ethnomedicinal plant. Local traditional practitioners believe that it reduces the risk of diabetes and atherosclerosis diseases. The present study was intended to conduct qualitative phytochemical analysis, determine the total flavonoid and phenolic contents, estimate the antioxidant capacity and antibacterial activities of the extracts of the fruits of this plant. The antioxidant activity was determined by analyzing the radical scavenging activity (RSA) using 2,2-diphenyl-1-picryhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP) assays. The antibacterial activities were determined by the agar well diffusion method. Qualitative phytochemical screening of the crude extracts obtained from the fruits of the plant indicated the presence of alkaloids, flavonoids, phenols, glycosides, steroids, terpenoids, saponins and tannins. The highest total phenolic and total flavonoid content were obtained in the ethanol extract of the fruits, followed by dichloromethane and n-hexane extract. The total phenolic content (in gallic acid equivalents, GAE) ranged from 113.3 to 202.72 mg GAE/g. The total flavonoid content (in catechin equivalent, CE) varied from 61.72 to 142.64 mg (CE)/g. All fruit extracts of S. anguivi exhibited antioxidant activity as revealed by DPPH and FRAP assays. The DPPH RSA (% inhibition) of the fruit extract varied from 35.11 to 80.13. The total phenolic and Flavonoid contents showed alinear correlation with RSA. Furthermore, all fruit extracts showed antibacterial activity against gram-positive and gram-negative bacteria varying from 12.5 to 16.75 mm. The result showed that the extracts of the plant exerted stronger bactericidal effect on gram-positive bacteria than on gram-negative bacteria.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1993087 .
Introduction
Since times immemorial, humans have used ethnobotanical species for medicine, food, shelter, and other purposes [Citation1]. Rural communities in many developing countries still rely on the use of traditional medicinal plants for the treatment of a variety of health problems. This is partly due to the lack of access to modern health care facilities for rural communities.
It has been recognized since ancient times that nature is a potential source of pharmacologically important drugs. This has resulted in the use of a large number of medicinal plants for treating various diseases. In fact, many modern medicaments are based on the traditional use of such drugs. In some Asian and African countries, up to 80% of the population rely on traditional medicine for their primary health care needs [Citation2]. The term traditional medicine refers to ‘the sum total of the knowledge, skills and practices based on the theories, beliefs and experiences indigenous to different cultures, whether explicable or not, used in the maintenance of health, as well as in the prevention, diagnosis, improvement or treatment of physical and mental illnesses’ [Citation3].
Plants secrete many organic bioactive compounds, known as secondary metabolites that do not have a direct role in the photosynthesis, growth and development of the plant but are crucial in ensuring survival of the plant by performing many important functions like protection from herbivores. The secondary metabolites are also known to have antioxidant, antifungal, antibiotic and antiviral activity, thus holding a significant position in boosting the plant’s defence system against any kind of pathogens [Citation4].
Solanum anguivi belongs to the plant family Solanacae and can be found as a wild plant in many places throughout the non-arid parts of Africa. The fruit of S. anguivi is used in folklore medicine for the treatment of high blood pressure, ulcer, nerve disorder and diabetes [Citation5–7]. The fruits of S. anguivi have also been reported to possess antioxidant activity in addition to bactericidal effects similar to some other species of Solanum genus [Citation8,Citation9]. The present study was aimed at the phytochemical screening of the fruits extracts of S. anguivi and some fractionated compounds, and investigating their antioxidant and antibacterial activities.
Materials and methods
Plant materials
The fresh fruits of S. anguivi were collected from Danigila, 477 km North-West of Addis Ababa, Ethiopia in August 2019. The plant was identified by a botanist at the Department of Biology, Bahir Dar University, Ethiopia.
Instruments
A pH meter (Heidolpunimax, England), electrical shaker, IR spectrometer (Jasco, FT-IR-6600, Japan), UV–Vis spectrophotometer (Cary 60 Agilent technologies China) were the instruments used in this study.
Chemicals and reagents
Solvents and reagents were used as received from the suppliers. N-hexane (Pentokey Organy India LTD); dichloromethane, ethanol, acetone, methanol, aluminum chloride, ethyl acetate (all from Carlo Erba SAS), Wagner’s reagent, sodium nitrite, potassium hexacyanoferrate (II), ascorbic acid, trichloroacetic acid, gallic acid, ferric chloride, petroleum ether, Mueller Hinton agar (all from Blulux Laboratories (P) Ltd.-120 001); dimethyl sulfoxide (DMSO) (Sisco Research Laboratory Pvt. Ltd), a standard antibiotic disc (Gentamicine µg/disc); Folin-Ciocalteu reagent, 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (Himedia, India) and catichine (Himedia, India) were among the analytical grade chemicals and reagents used in the study. Distilled water was used throughout the entire research work.
Sample preparation
The collected fruit samples were thoroughly washed with tap water to remove all the dust particles. The cleaned fruits were air-dried in the absence of light, ground into a fine powder and stored at room temperature in a plastic bag prior to use.
Extraction
The air-dried powdered fruit sample was successively extracted with n-hexane, dichloromethane and ethanol using the maceration technique for 48 h in each solvent. The solvent from each extract was removed using a rotary evaporator under reduced pressure. The resulting semi-dried mass of each extract was kept away from light until used for further experiments.
Isolation of compounds
Of the crude S. guivi fruit extracts obtained using the aforementioned solvents, the dichloromethane extract showed a relatively better antibacterial activity. This extract was impregnated with silica gel and subjected to column chromatography to isolate compounds using acetone-petroleum ether mixtures (1% − 3%) as the eluent. The eluted fractions were analyzed by thin layer chromatography (TLC). The fractions having similar Rf (retention factor) values were pooled together and subjected to further chromatography to obtain the pure compounds as judged by TLC (single spot) and observation under ultraviolet (UV) light. This led to the isolation of three gummy compounds (labelled CM-1, CM-2 and CM-3), each exhibiting strong absorption bands in the infrared spectrum ascribable to carbonyl groups. Full characterization of these compounds, however, awaits the obtainment of additional requisite spectral data.
Qualitative phytochemical analysis
Test for terpenoids (Salkowski’s test)
The phytochemical analysis of n-hexane, dichloromethane and ethanol extracts of the fruit part of S. anguivi was performed as per literature reports [Citation10,Citation11]
Quantitative phytochemical determination
Determination of total phenolic content (TPC)
The total phenolic content of the crude extracts was determined by using the Folin-Ciocalteu method [Citation12]. A series of standard gallic acid solutions (10, 20, 30, 40 and 50 ppm) were prepared from a gallic acid stock solution. The plant extract containing 1 mL of each of these standard solutions was mixed with 5 mL of Folin-Ciocalteu reagent and allowed to stand for 6 min. Following the addition of 4 mL of 10% sodium carbonate solution to each mixture, absorbance was recorded after 30 min at 765 nm in a UV-visible spectrophotometer. The total phenolic content of the n-hexane, dichloromethane and ethanol extracts was calculated as gallic acid equivalents (mg GAE/g).
Determination of total flavonoid content (TFC)
Aluminum chloride complex forming assay was used to determine the total flavonoid content of the extracts. Catechin was used as a standard to make the calibration curve, and the flavonoid content was determined as catechin in equivalents [Citation13]. Standard catechin solutions (2.4, 4.7, 9.4, 18.7 and 37.4 mg/L were prepared. Distilled water (5 mL) was added to a mixture containing the plant extract and 1 mL of each of the catechin solutions. Finally, 0.3 mL of 5% sodium nitrite solution was added to each mixture. After allowing the mixture to stand for 5 min, 0.3 mL of 10% aluminum chloride solution was added and the mixture was allowed to stand for 6 min at room temperature. Then 2 mL of 1 mol/L sodium hydroxide solution was added followed by the addition of distilled water until the final volume reaches 10 mL. Finally, the absorbance of the reaction mixtures was recorded at 510 nm in a UV-visible spectrophotometer. The total flavonoid content of each extract was calculated as catechin equivalents (mg CE/g).
Antioxidant capacity assays
DPPH radical scavenging assay
The free-radical scavenging activities of the extracts and isolated compounds were determined by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging method [Citation14]. A fresh 0.004% solution of DPPH was prepared in ethanol. Standard solutions of 40, 60, 80 and 100 mg/L of ascorbic acid were used as a reference. To a separate solution of each extract and isolated compounds containing 4 mL of the ascorbic acid, 1 mL of DPPH solution was added. Each mixture was allowed to stand in darkness for 20 min. A control was prepared by mixing 2 mL of the DPPH solution and 3 mL of ethanol. Finally, the absorbance was measured and the percentage inhibition of DPPH by extracts and isolated compounds were calculated by using the following formula:
Where A is the absorbance of pure DPPH in oxidized form and B is the absorbance of sample taken after 20 min of reaction with DPPH.
Ferric reducing antioxidant power (FRAP) assay
The reducing power of the prepared extracts and isolated compounds were determined according to methods reported in the literature [Citation15]. Different concentrations (40, 60, 80 and 100 mg/L) of S. anguivi fruit extracts, isolated compounds and the same concentrations of ascorbic acid were prepared. From each concentration, 2.5 mL was taken and mixed with 2.5 mL phosphate buffer (pH = 6.6) and 2.5 mL of 1% potassium ferricyanide solution. The mixture was incubated at 50 °C for 20 min. Then, 2.5 mL of trichloroacetic acid (TCA) solution (10% w/v) was added to terminate the reaction. Then the solution was mixed with 2.5 mL of distilled water and 0.5 mL ferric chloride solution (0.1% w/v). Finally, absorbance was measured by using a UV-vis spectrophotometer against a blank solution.
Where Asample: Absorbance of sample, and Ablank: Absorbance of blank
Antimicrobial activity assays
Two gram-positive (Staphylococcus aureus, Streptococcus pyogenes) and gram-negative (Escherichia coli, Klebsiella pneumonia) bacteria with ATCC (American Type Culture Collection) type were used.
Preparation of test solutions
Test solutions (1000 mg/L) were prepared by dissolving 1 mg of each crude extract and isolated compounds in 1 mL of dimethyl sulfoxide (DMSO). A 500 and 250 ppm of isolated compounds were prepared by serial dilution method from 1000 ppm solution of isolated compounds.
Agar diffusion method
The disk diffusion method with Muller Hinton agar was used for evaluating the antimicrobial activity. Using a sterile cotton swab, the fresh culture of bacteria strains was swabbed on the surface of sterile agar plates. Each plant extract of the test solution was poured on the surface of agar plates inoculated with culture bacteria in Petri dishes by using 5 mm diameter sterile discs (Whatman No 3 paper). The standard antibiotic disc (gentamycin, 30 µg/disc) served as the positive antibacterial control and the DMSO solution was used for the negative control. Then the plates were incubated for 24 h at 37 °C. The antibacterial activities of crude extracts and isolated compounds at different concentrations (1000, 500 and 250 mg/L) were determined. After 24 h, the zone of inhibition was observed and recorded in millimetres. The tests were performed in triplicates for each microorganism evaluated and the final results were presented as arithmetic average with standard deviation (Mean ± Std). Antibacterial activity was recorded if the zone of inhibition was greater than 6 mm [Citation16].
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) values of isolated compounds were determined by using the broth dilution method. Each isolated compound was individually prepared in four test tubes having different concentrations (125, 62.5, 31.25 and 15.625 mg/L) using serial dilution. Test tube 1 was filled with 5 mL of an equal amount of Muller Hinton broth and isolated compounds. Then 2.5 mL of solutions from test tube 1 was transferred to test tube 2 and diluted to 5 mL with Muller Hinton broth. This procedure was repeated for the next two test tubes and each test tube was filled with 1 mL Muller Hinton broth including bacterial suspension. The resulting mixtures were incubated at 37 °C for 24 h. The concentration that exhibited turbidity was taken as an indication of the growth of bacteria, and the lowest concentration at which the medium remained clear (no growth of bacteria) was recorded as the relative minimum inhibitory concentration [Citation17].
Results
Qualitative preliminary phytochemical analysis
In the present study, the phytochemical screening test of fruit extracts of S. anguivi revealed the presence of saponins, glycosides, alkaloids, tannins, phenols, flavonoids, steroids and terpenenoids as shown in .
Table 1. Preliminary phytochemical screening of fruit extracts of S. anguivi.
Isolation of compounds/fractionation
Three compounds (labelled CM-1, CM-2, and CM-3) were isolated from dichloromethane fruit extracts of S. anguivi. There was a single spot on TLC when visualized under UV-Vis light (λmax 254 and 365 nm) by the different solvent systems.
Compound-1
A light yellow gummy compound (16 mg) was isolated from dichloromethane fruit extracts of S. anguivi and eluted by 30% petroleum ether in acetone and labelled as CM-1. Preliminary qualitative phytochemical test of compound-1 gave only a positive result for flavonoids. The UV-visible spectrum of compound-1 showed two absorption maxima at 238 nm and 336 nm. The flavonoids spectrum typically consists of two absorption maxima at the ranges 240–285 nm (band II) and 300–550 nm (band I) in the UV-visible spectrum. Methylation of glycoside groups (especially of 3, 5, 7 and 4 hydroxyls) causes band shifts to a shorter wavelength [Citation18]. The absorption maxima of compound-1 were present in the range of the flavonoid spectrum in the UV-visible range, indicating that compound-1 is a flavonoid. Some important IR results of compound-1 are summarized in and compared with the previously IR result of flavonoid C-glycoside.
Table 2. IR result of Compound-1 and previous IR result of glycoside flavonoids.
Compound-2
A greenish gummy compound (12 mg) labelled as CM-2 was isolated and eluted by 70% petroleum ether in mixtures of ethyl acetate-acetone (70:15:15). The TLC profile of compound-2 (CM-2) indicated one single spot in different solvent systems. Preliminary qualitative phytochemical test of compound-2 gave only a positive result for tannins.
The UV-visible spectrum of compound-2 had an absorption maximum (λmax) at 246 nm in the UV-Vis region spectrum. Tannins have absorption maxima in the range of 205 nm to 280 nm in the Ultraviolet-visible spectrum [Citation20]. The IR result of compound-2 is summarized in and compared with the previous IR results of tannins.
Table 3. IR result of compound-2 and previous IR results of tannin compounds.
Compound-3
This compound was eluted by 60% petroleum ether in a mixture of acetone and dichloromethane (60:20:20) combinations. It was 15 mg reddish-brown gummy compound and had a single spot on TLC when visualized under UV-Vis light (λmax 254 and 365 nm) by a different solvent system. Qualitative phytochemical screening test of compound-3 gave only a positive result for flavonoid compounds.
The UV-visible spectrum of compound-3 showed two peaks at 251 and 325 nm in the UV-visible spectrum region. The peaks at λmax of 251 nm and another peak at 325 nm were found in the absorption spectrum of flavonoids.
The IR data result of compound-3 showed that a band at 3404 cm−1 implies the presence of hydroxyl (-OH) stretching and at 2925 indicates CH2 stretching. An intensive and sharp peak at 1612 cm−1 is characteristic of –C = O stretching or aromatic compounds and a sharp peak at 1458 cm−1 is characteristic of aromatic compounds. The peak at 1255 cm−1 also confirms the presence of O-C stretching. The IR results of compound-3 (CM-3) were in agreement with the IR result reported before for characterization of flavonoid compounds [Citation23]. shows the IR result for compound 3 compared with previously reported IR results of flavonoids.
Table 4. IR result of compound-3 and previous IR results of flavonoid compounds.
Quantitative analysis for total phenolic and total flavonoid contents
Total phenolic content
Phenolic compounds, especially phenolic acids, play an important role in the overall radical scavenging ability [Citation25]. The total phenolic content of each extract was determined using the Folin-Ciocalteu’s reagent in terms of gallic acid equivalent. The regression equation of the calibration curve of the gallic acid standard for the present study was y = 0.00112x–0.0067 with R2 of 0.9994. The absorbance readings and calibration curve of the standard solutions are shown in Appendix 1 (Online Supplemental Material). The total phenolic content of each extract of fruits of S. anguivi was determined using this regression equation and the results are depicted in .
Table 5. Total phenolic content of each extract.
Total flavonoid content
Aluminum chloride complex forming assay was used to determine the total flavonoid content of the extracts. Catechin was used as standard and flavonoid content was determined as catechin equivalent (mg CE/g) [Citation26]. The total flavonoid content (TFC) of each fruit extract was determined as catechin equivalent (mg CE/g) dry sample. To perform the calculations of total flavonoid content, a standard curve is needed which is obtained from a series of different catechin concentrations. The calibration curve was constructed by plotting absorbance versus concentration of catechin as shown in Appendix 2 (Online Supplemental Material). A straight line with an equation of y = 0.0076x + 0.0669 and a coefficient of determination (R2) of 0.9968 was obtained as shown in Appendix 2 (Online Supplemental Material). Using this regression equation, the total flavonoid content was determined at the given absorbance values and the results obtained are depicted in .
Table 6. Total flavonoid content of the extract.
Antioxidant capacity assay
DPPH radical scavenging activity
DPPH accepts a hydrogen atom from antioxidants thereby getting reduced. As a result, the absorbance decreases, as the radical changes from DPPH to the DPPH-H form. The degree of discoloration indicates the scavenging potential of the antioxidant compounds or extracts in terms of hydrogen and electron-donating ability [Citation27].
The purple colour of DPPH is changed when mixed with the crude extracts and each of the compounds isolated from the extract (the colour change depends on the antioxidant ability), indicating the scavenging of the radicals by antioxidants. This demonstrates that the antioxidants found in the extracts and the isolated compounds quench the free radicals. In the present study, the percentage of inhibition was determined () and to evaluate the antioxidant activity of the extracts and isolated compounds with the ability to inhibit free radicals. The absorbance of each extract and isolated compounds at each concentration is shown in Appendix 3 (Online Supplemental Material).
Figure 1. Inhibition % of DPPH free radical by ascorbic acid (AA), n-hexane extract (HE), DCM extract (DCME), ethanol extract (EE), compound-1 (CM-1), compound-2 (CM-2) and compound-3 (CM-3).
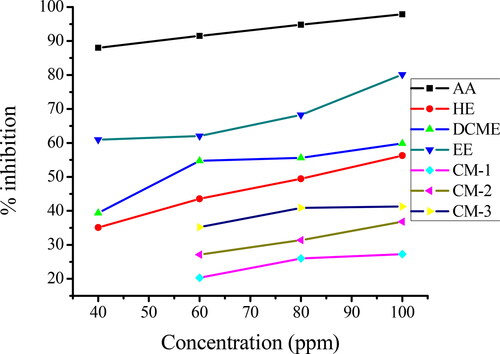
Table 7. Inhibition (%) at each concentration of ascorbic acid, extracts and isolated compounds.
Ferric reducing antioxidant power (FRAP) assay
The reducing power of fruit extracts of S. anguivi and isolated compounds were evaluated by the transformation of Fe3+ to Fe2+ through electron transfer ability which serves as a significant indicator of its antioxidant capacity. Like the antioxidant activity measured by the DPPH assay, the reducing power increased with increasing concentration of the extract. When potassium ferricyanide reacts with ferric chloride in the presence of antioxidants, potassium ferrocyanide and ferrous chloride are found as a product. The presence of antioxidants causes the conversion of the Fe3+/ferricyanide complex to the ferrous form.
The reducing power of fruit extracts of S. anguivi and isolated compounds were expressed as absorbance vs. concentration (40, 60, 80 and 100 mg/L) and the result are shown and . The absorbance readings at each concentration of the extracts are depicted in Appendix 4 (Online Supplemental Material).
Figure 2. Percent (%) reducing power of ascorbic acid (AA), n-hexane extract (HE), DCM extract (DCME), ethanol extract (EE), compound-1 (CM-1), compound-2 (CM-2) and compound-3 (CM-3).
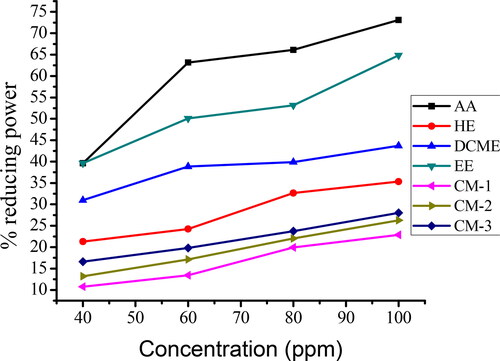
Table 8. Percent (%) reducing power of each extract and isolated compounds.
Antibacterial activity
Antibacterial activities of crude extracts of fruits of S. anguivi
Antibacterial activities of fruit crude extracts of S. anguivi obtained by using n-hexane, dichloromethane and ethanol as the extracting solvents were evaluated by using Agar well diffusion method. Two gram-negative bacteria (Escherichia coli and Klebsiella pneumonia) and two gram-positive bacteria (Staphylococcus aureus and Streptococcus pyrogens) were used in the Agar well diffusion method.
The dichloromethane crude extracts exhibited better antibacterial activity followed by ethanol and n-hexane crude extract (). The dichloromethane crude extract showed maximum antibacterial activity followed by ethanol and n-hexane crude extracts against gram-positive bacteria (Staphylococcus aureus and Streptococcus pyogenes). The antibacterial activity of crude extracts of fruits of S. anguivi on Staphylococcus aureus was observed in the range between 12.5 to 16.75 mm inhibition zones. The highest inhibition zone (16.75 mm) was displayed by dichloromethane crude extract, while the least activity was observed with the n-hexane extracts (12.5 mm inhibition zone). All the crude extracts exhibited more potent bactericidal effects toward the gram-positive bacteria Staphylococcus aureus.
Table 9. Zones of inhibition of crude extracts of fruits of S. anguivi against gram-positive and gram negative-bacteria.
The crude extracts of fruits of S. anguivi also showed antibacterial activity against gram-negative bacteria (Escherichia coli and Klebsiella pneumonia). The ethanol crude extract exhibited better antibacterial activity (14.5 mm) inhibition zone followed by dichloromethane extract (14.25 mm inhibition zone) against Klebsiella pneumonia. As shown in , maximum activity was exhibited by dichloromethane extract (14.5 mm inhibition zone) followed by ethanol extract (13.5 mm inhibition zone) and n-hexane crude extract exhibited the least inhibitory activity (11.25 mm) against Escherichia coli. Of the gram-negative bacteria, Escherichia coli was more susceptible compared to Klebsiella pneumonia to n-hexane and dichloromethane extracts, and Klebsiella pneumonia was more susceptible to the ethanol extract.
Antibacterial activities of isolated compounds
Isolated compounds (CM-1, CM-2, and CM-3) of dichloromethane crude extract of fruits of S. anguivi exhibited antibacterial activity at different concentrations. All isolated compounds showed antibacterial activity against gram-negative and gram-positive bacteria. CM-2 and CM-3 showed comparable antibacterial activity and CM-1 exhibited less pontent antibacterial activity against gram-positive and gram-negative bacteria as shown in and . The isolated compounds displayed an inhibition zone against Staphylococcus aureus ranging between 10.25 and 13.5 mm as shown in . The largest inhibition zone was observed with CM-3, followed by CM-2, and CM-1 had the smallest inhibition zone. The isolated compounds also showed antibacterial activity against Streptococcus pyogenes and the inhibition zone ranged from 9.5 to 11.5 mm. The larger inhibition zone was observed with CM-2 and the smallest one, with CM-1. Compound CM-2 and compound CM-3 showed stronger antibacterial activity against Streptococcus pyogenes. The result also showed that the isolated compounds exhibited antibacterial activity against gram-negative bacteria (Klebsiella pneumonia and Escherichia coli) in different concentrations (). The isolated compounds produced an inhibition zone ranging from 9.25 to 11.25 mm in diameter against Klebsiella pneumonia. The highest activity was displayed by CM-3 and the least potent activity was observed with CM-1. The maximum antibacterial activity was shown by CM-3, which produced an 11.5 mm inhibition zone, followed by CM-2 (11 mm inhibition zone), and the least potent activity was shown by CM-1 (9.55 mm) inhibition zone against Escherichia coli bacteria.
Table 10. Zones of inhibition of isolated compounds from DCM extracts of fruits of S. anguivi against gram-positive bacteria.
Table 11. Zones of inhibition of isolated compounds from DCM extracts of fruits of S. anguivi against gram-negative bacteria.
Minimum inhibitory concentration determination (MIC) of isolated compounds
Minimum inhibitory concentration (MIC) refers to the lowest concentration of isolated compounds that will inhibit the visible growth of bacteria after 48 h of incubation. The MIC values of isolated compounds were determined at different concentrations. The concentration that exhibited turbidity was taken as an indication of growth of bacteria and the lowest concentration at which the medium remained clear (no growth of bacteria) was recorded as the relative minimum inhibitory concentration [Citation17]. In this study, the antibacterial activities of isolated compounds of dichloromethane crude extract of fruits of S. anguivi were evaluated at four different concentrations (125, 62.5, 31.25 and, 15.625 ppm) and the results are shown in . All isolated compounds showed MIC value of 31.25 mg/L against S. aurues and S. pyogenes. Compound-1 and compound-2 showed MIC value at 31.25 mg/L and compound-1 showed MIC value at 62.5 mg/L against gram-negative bacteria (E. coli), whereas K. pneumonia showed resistance to the antibiotics at lower concentration and had MIC value at 62.5 mg/L in all isolated compounds.
Table 12. MIC of isolated fractions.
Discussion
Qualitative preliminary phytochemical analysis
The result showed that the dichloromethane and ethanol extracts contained more bioactive compounds as compared to the n-hexane extract. It is due to their greater polar nature than the n-hexane extract. A previous study reported that steroids, flavonoids, alkaloids and saponins were present in ethanol and diethyl ether-chloroform extracts of fruits of S. anguivi [Citation28].
Isolation of compounds/fractionation
shows that the present IR result of compound-1 was compatible with the IR result of flavonoid C glycoside reported by [Citation19]. Therefore, by comparing the IR and UV-vis result of compound-1 to previously reported results of glycoside flavonoid and the qualitative phytochemical test in this study, compound-1 is believed to be a class of flavonoids.
shows that the present result was in agreement with the previous report about tannin. The bands at 1720 and 1700 cm−1 underline the presence of carbonyl groups that are particularly hydrolysable tannins. The hydrolysable tannins always present the –C = O stretching signal between 1720 and 1700 cm−1 [Citation22]. By comparing the IR result of compound-2 with the previous IR result of tannin and observing the qualitative phytochemical test result, compound-2 may be a class of tannin. reveals that the IR data in this study agreed with the previous IR data reports for flavonoid compounds. By comparing the IR and UV-visible spectra of compound-3 with previous IR and UV-visible spectra of flavonoids and by observing the qualitative phytochemical test results in this study, compound-3 might be a class of flavonoid compounds.
Total phenolic content
As presented in , the total phenolics content in the crude extracts of fruit samples of S. anguivi ranged from 113.3 to 202.72 mg GAE/g of dried weight sample. The highest total phenolic content was obtained from ethanol extract (202.72 mg GAE/g) followed by dichloromethane extract (170 mg GAE/g) and the lowest value was recorded in the n-hexane extract (113.63 mg GAE/g) of dry weight. This is in agreement with the expectation that phenolics, being polar compounds, are more efficiently extracted by polar solvents. A previous study reported that fruits of S. anguivi showed total phenolic content of 161 and 45 mg GAE/g of dry weight by ethanol and mixtures of diethyl ether-chloroform respectively [Citation28].
Total flavonoid content
shows the total flavonoid content (TFC) of crude extracts of fruits of S. anguivi ranged from 61.72 to 142.64 mg CE/g. The highest total flavonoid content was obtained in the ethanol extract (142.64), followed by the medium polarity dichloromethane extract (120.8) and the least total flavonoid content was recorded in the n-hexane crude extract (61.72) mg CE (catechin equivalent)/g of dried weight as shown in . The result revealed that the amount of flavonoid extracts from the plant depends on the polarity of the solvent. According to a literature report, the total flavonoid content of extracts of fruits of S. anguivi obtained using ethanol and diethyl ether-chloroform mixture as the extracting solvents was, respectively, 58.75 and 15.9 mg QE/g of dried weight, respectively [Citation28].
DPPH radical scavenging activity
Apparently, the antioxidants in the extracts were able to quench DPPH free radical, which absorbs at a higher wavelength than the control, a solution with all reagents but not the extracts of S. anguivi, and the absorbance decreased. The highest antioxidant activity was obtained for the ethanol extract, and the n-hexane extract showed the least antioxidant activity (). The activity of scavenging free radicals is based on the hydrogen or electron-donating ability of phytochemicals extracted by each solvent. The higher antioxidant activity of ethanol and dichloromethane crude extract can be ascribed to the higher concentrations of phytochemicals, presumable flavonoids and phenolics, contained therein relative to those found in the n-hexane crude extract. Flavonoids and phenolic compounds are known to quench free radicals via donation of hydrogen atom electron. The quantitative determination of total phenolic content and total flavonoid content of crude extracts of fruits of S. anguivi correlated linearly with the antioxidant activity in the present study. The results in also showed that the isolated compounds have good antioxidant activity, with compound CM-3 exhibiting better antioxidant activity. All isolated compounds exhibited less potent antioxidant activity than the crude extracts of fruits of S. anguivi suggesting a possible synergy among the various phytochemicals present in the crude extracts. All crude extracts of fruits of S. anguivi and isolated compounds showed less antioxidant activity than the standard (ascorbic acid). Inhibition of DPPH radical increases with increasing concentration of plant extracts and isolated compounds as shown in and . The present results are in close agreement with a literature report in which the antioxidant activity of ethanol and diethyl ether-chloroform extracts of S. anguivi fruits, recorded in terms of radical scavenging activity, ranged from 64.00 to 85.50% and 14.10–40.00%, respectively [Citation28]. Likewise, the antioxidant activity of crude extract obtained from a mixture of fruits of S. aunnum and S. anguivi using hexane-acetone and ethanol as the extracting solvents varied from 37 to 70% and 65–87%, respectively [Citation5].
Ferric reducing antioxidant power (FRAP) assay
The ethanol extracts of S. anguivi fruit exhibited the highest reducing power and the n-hexane extract showed the least reducing power ( and ). Of the isolated compounds, compound CM-3 showed the highest reducing power and compound CM-1 showed the least reducing power, indicating that all isolated compounds showed less reducing power (lower antioxidant activity) than the crude extracts. All crude extracts and isolated compounds exhibited less reducing power than the standard ascorbic acid used in the present study. The antioxidant property of the crude extracts as well as the isolated compounds in the FRAP assay can be traced to electron-donating capability of the constituent phytochemicals [Citation7]. The higher antioxidant activity of ethanol and dichloromethane extracts correlated linearly with their respective phenolics and flavonoids content.
Antibacterial activity
The present study showed that all the fruit extracts of S. anguivi possessed significant antibacterial activity, which provides possible rationalization to the traditional use of this plant for the treatment of health problems like ulcer. The fruit extracts of S. anguivi had better activity toward gram-positive bacteria compared to gram-negative bacteria. Generally, gram-negative bacteria are more resistant to antimicrobial agents compared with gram-positive bacteria because they have a phospholipid membrane carrying the structural lipopolysaccharide impermeable to antibacterial substances [Citation29]. All crude extracts showed a weaker inhibition zone than that of gentamycin (standard antibiotic) used as a positive control in this study. Phytochemical groups () extracted in each solvent are responsible for these antibacterial activities. Alkaloids, flavonoids, phenolics and other secondary metabolites have antibacterial activity. Plants with alkaloids have been determined to exhibit antibacterial properties and are used in medicines for reducing headache and fever [Citation30]. The antibacterial activity of Solanum species is well known and probably caused by the alkaloids [Citation31]. The maximum antibacterial activity shown by the dichloromethane crude extract in the present study can be due to the alkaloids () detected in this extract. As reported elsewhere [Citation32], the dichloromethane leaf extract of Solanum lycocarpum was more active than the ethanol extract against eight tested bacteria (including the bacteria used in the present study). Other Solanum species extracts also exhibit antibacterial activity. Ethanol extracts of Solanum torvum were active against Staphylococcus aureus, Escherichia coli and Klebsiella and had an inhibition zone of 15,12,and 16 mm, respectively [Citation33]. The methanol, acetone and n-hexane extracts of seven Solanum species (S. nigrum, S. villosum, S. torvum, S. surratense, S. sysimbrifolium, S. diphyllum, and S. incanum) showed significant antibacterial properties against S. aureus, B. subtilis, P. vulgaris, S. typhi and E. coli, and the inhibition zone ranged from 7 to 16.66 mm [Citation34].
Generally, the present study showed that the antibacterial activities of all isolated compounds decreased as the concentration decreased. This result indicates that the isolated compounds were bacteria static at higher concentrations. The least antibacterial activity was found at lower concentration and the highest antibacterial activity was found at higher concentration in all isolated compounds and all bacteria.
Minimum inhibitory concentration (MIC) of isolated compounds
Compound-2 and compound-3 showed inhibition of bacteria at a lower concentration. As per reports in the literature, the ethanol extract from different parts of Solanum nigrum shows MIC values at 12, 11 and 12 mg/L on Staphylococcus aureus, Escherichia coli and Klebsiella pneumonia, respectively [Citation33, Citation35] and another study also reported that ethanol extracts of Solanum surattense gave the MIC value of 25–100 mg/L against all the tested bacteria (including all the tested bacteria in the present study) [Citation36], which was comparable with the MIC value of the isolated compounds of dichloromethane crude extract of fruits of S. anguivi.
Conclusions
Qualitative phytochemical screening test of n hexane, dichloromethane and ethanol extracts of fruits of S. anguivi revealed the presence of flavonoids, alkaloids, phenols, glycosides, steroids, terpenoids, saponins and tannins. Three gummy compounds were isolated from the dichloromethane extract that have a single spot as checked by TLC. The ethanol crude extract apparently contained most of the flavonoid and phenolic compounds followed by the dichloromethane extract. Alkaloids were detected only in the latter extract. All the crude extracts and isolated compounds possessed antioxidant activity as evidenced by DPPH and FRAP assays. The highest antioxidant activity was exhibited by the ethanol extract followed by the dichloromethane and n-hexane extracts. Compound-3 exhibited better antioxidant activity followed by compound-2. This may be due to the ability of phytochemicals to donate hydrogen atoms or electrons. Antibacterial effects of fruit extracts of S. anguivi showed different degrees of inhibition zone against both gram-positive and gram-negative bacteria. The dichloromethane extract showed better antibacterial activity relative to the ethanol and n-hexane extracts. Compound-3 and compound-2 showed better antibacterial activity than compound-1. Generally, the crude extracts showed higher antioxidant and antibacterial activities than the isolated compounds of the dichloromethane extract, reflecting the likelihood of synergistic effects among the various phytochemicals in the crude extracts. The results from this study support the use of S. anguivi as a medicinal plant in the form of fruit extracts.
Supplemental Material
Download MS Word (49.5 KB)Supplemental Material
Download MS Word (49.2 KB)Supplemental Material
Download MS Word (36.9 KB)Supplemental Material
Download MS Word (36.6 KB)Data availability statement
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Disclosure statement
The authors declare that there is no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Bahadur S, Khan MS, Shah M, et al. Traditional usage of medicinal plants among the local communities of Peshawar valley, Pakistan. Acta Ecol Sin. 2020;40(1):1–29.
- Orbán-Gyapai O, Liktor-Busa E, Kúsz N, et al. Antibacterial screening of Rumex species native to the Carpathian Basin and bioactivity-guided isolation of compounds from Rumex aquaticus. Fitoterapia. 2017;118:101–106.
- Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide research trends on medicinal plants. IJERPH. 2020;17(10):3376.
- Badyal S, Singh H, Kumar Yadav A, et al. Plant secondary metabolites and their uses. Plant Arch. 2020;20(2):3336–3340.
- Daramola B. Preliminary investigation on antioxidant interactions between bioactive components of Solanum anguivi and Capsicum annuum. J Food Sci Technol. 2018;55(9):3827–3832.
- Elekofehinti OO, Kamdem JP, Meinerz DF, et al. Saponin from the fruit of Solanum anguivi protects against oxidative damage mediated by Fe2+ and sodium nitroprusside in rat brain synaptosome P2 fraction. Arch Pharm Res. 2015;38(7):1–7.
- Elekofehinti OO, Kamdem JP, Bolingon AA, et al. African eggplant (Solanum anguivi Lam.) fruit with bioactive polyphenolic compounds exerts in vitro antioxidant properties and inhibits Ca2+-induced mitochondrial swelling. Asian Pacif J Tropic Biomed. 2013;3(10):757–766.
- Rajalakshmi A, Jayachitra A. Antioxidant, antibacterial effects of solanine isolated from Solanum nigrum and its cytotoxic activity on the HEP-2 and AGS cell lines. Int J Pharm Sci Res. 2017;8:2932–2939.
- Pasdaran A, Pasdaran A, Mamedov N. Antibacterial and antioxidant activities of the volatile composition of the flower and fruit of Solanum sisymbriifolium (Litchi Tomato). Pharm Sci. 2017;23(1):66–71.
- Banu KS, Cathrine L. General techniques involved in phytochemical analysis. Int J Adv Res Chem Sci. 2015;2(4):25–32.
- Adeyanju O, Olutayo OO, Michael A, et al. Preliminary phytochemical and antimicrobial screening of the leaf extract Pilostigma reticulatum (dc) Hochst. Afr J Pure Appl Chem. 2011;5(3):43–46.
- Odabasoglu F, Aslan A, Cakir A, et al. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res. 2004;18(11):938–941.
- Chantiratikul P, Meechai P, Nakbanpotec W. Antioxidant activities and phenolic contents of extracts from Salvinia molesta and Eichornia crassipes. Res J Biol Sci. 2009;4(10):1113–1117.
- Shirazi OU, Khattak MM, Shukri NA, et al. Determination of total phenolic, flavonoid content and free radical scavenging activities of common herbs and spices. J Pharmacogn Phytochem. 2014;3(3):104–108.
- Oyaizu M. Studies on products of browning reaction. Jpn J Nutr Diet. 1986;44(6):307–315.
- Nascimento GGF, Locatelli J, Freitas PC, et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31(4):247–256.
- Pattewar SV, Patil DN, Dahikar S. Antimicrobial potential of extract from leaves of Kalanchoe pinnata. Int J Pharm Sci Res. 2013;4(12):4577.
- Ewais EA, Abd El-Maboud MM, Elhaw MH, et al. Phytochemical studies on Lycium schweinfurthii var. schweinfurthii (Solanaceae) and isolation of five flavonoids from leaves. J Med Plant Stud. 2016;4:288–300.
- Yang D, Xie H, Jia X, et al. Flavonoid C-glycosides from star fruit and their antioxidant activity. J Funct Foods. 2015;16:204–210.
- Ukoha P, Ejikeme P, Maju C. Tannins of the testa of anacardium occidentale (cashew) and husk of Arachis Hypogaea (groundnut): characterization and potential applications. J Am Leather Chem Assoc. 2010;105(7):242–249.
- Pantoja-Castro MA, González-Rodríguez H. Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Revist Latinoamericana Química. 2011;39(3):107–112.
- Tondi G, Petutschnigg A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind Crops Prod. 2015;65:422–428.
- Yoo HS, Lee JS, Kim CY, et al. Flavonoids of Crotalaria sessiliflora. Arch Pharm Res. 2004;27(5):544–546.
- El-Sayed Z, Hassan W. Polymethoxylated flavones from Solanum abutiloides, grown in Egypt (Solanaceae). Zagazig. J. Pharm. Sci. 2006;15:53–59.
- Zieliński H, Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48(6):2008–2016.
- C, M., P, Nakbanpotec W. Antioxidant activities and phenolic contents of extracts from salviniamolesta and eichorniacrassipes. J Biol Sci. 2009;4:1113–1117.
- Arulpriya P, Lalitha P, Hemalatha S. Antioxidant activities of the extracts of the aerial roots of Pothos aurea (Linden ex Andre). Der Pharma Chemica. 2010;2(6):84–89.
- Daramola B. Effects of extraction solvent, morphological parts and ripening stage on antioxidative activity of Solanum anguivi fruit. Int Food Res J. 2015;22(2):644–650.
- Patrone JB, Stein DC. Effect of gonococcal lipooligosaccharide variation on human monocytic cytokine profile. BMC Microbiol. 2007;7(1):7–15.
- Patra JK, Mohanta YK. Antimicrobial compounds from mangrove plants: a pharmaceutical prospective. Chin J Integr Med. 2014;20(4):311–320.
- Al-Fatimi M, Wurster M, Schröder G, et al. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol. 2007;111(3):657–666.
- da Costa GAF, Morais MG, Saldanha AA, et al. Antioxidant, antibacterial, cytotoxic, and anti-inflammatory potential of the leaves of Solanum lycocarpum A. St. Hil. (Solanaceae). Evid Based Complement Alternat Med. 2015;2015:315987.
- Sivapriya M, Dinesha R, Harsha R, et al. Antibacterial activity of different extracts of sundakai (Solanum torvum) fruit coat. Int J Biol Chem. 2011;5(1):1–5.
- Singh P, Singh P, Singh A, et al. In vitro evaluation of phytochemical and antibacterial activity of wild species of Solanum L. IOSR J Biotechnol Biochem. 2019;5(1):81–87.
- Gbadamosi I, Afolayan A. In vitro anti-radical activities of extracts of Solanum nigrum (L.) from South Africa. J App Bioscience. 2016;98:9240–9251.
- Sheeba E. Antibacterial activity of Solanum surattense. Burm F. Kathmandu Univ J Sci Eng Technol. 2010;6(1):1–4.
