Abstract
This study aimed to use different dose verification methods Treatment Planning System (TPS), thimble ionization chamber and two-dimensional matrix ionization chamber (MatriXX) to explore the compensating effects of several materials on high-energy electron beam irradiation. In this study, we positioned plexiglass panels, wet medical gauze, colloidal compensatory material and Vaseline compensator, all of the same thickness, flat on the surface of a MatriXX. The above four materials were placed on the surface of a thimble ionization chamber and irradiated with a linear accelerator. Under the same irradiation parameters, the compensation dose of of the tested materials was worked out by a dosimeter, which was connected at the thimble ionization chamber. Under vertical irradiation from the linear accelerator, the accelerator used the same irradiation conditions for the four materials, which were flat on the surface of the MatriXX, then determined the distribution of compensation doses by the soft system matching with the MatriXX. The results showed that the compensation dose of moist medical gauze was lower than the other three materials, the compensation dose of Plexiglass plate, colloidal compensatory material and vaseline compensation were approximate. The dose compensation effect of the vaseline compensation material made by our research group was close to the plexiglass plate and colloidal compensatory material used in radiotherapy in real life. Its physical and chemical properties are stable, easy to manufacture and store, and economical and practical.
Introduction
Since the early 1950s, high-energy electron beams have been used in radiotherapy [Citation1–3], mainly for irradiating superficial lymph node metastases, skin haemangioma, scar tissue, etc. In the treatment of superficial and eccentric lesions, high-energy electron beams have advantages that high-energy X-rays do not have [Citation4]. When single-field irradiation with electron beams is used, the maximum dose area falls on the diseased tissue, and the tissues and organs behind the target area receive just a minimal dose. However, when the diseased tissue is on the skin surface, and its thickness is less than the built-up thickness of the high-energy electron beam, the maximum dose area of the electron beam falls behind the diseased tissue, and the irradiation dose to the diseased tissue on the surface is relatively low and unevenly distributed. Therefore, it is necessary to place some substance on the surface of the diseased tissue for tissue compensation, that is, to play a structural role so that the maximum dose area covers the entire diseased tissue.
The traditional tissue compensation materials include a plexiglass plate, moist medical gauze and colloidal compensator. In this study, we also added the Vaseline compensator made by our research team. The aim of this study was to measure the four compensation materials of the same thickness through three dose verification methods: TPS (Treatment Planning System) [Citation5], thimble ionization chamber [Citation6] and two-dimensional matrix ionization chamber, MatriXX, and analyse and compare their similarities and differences from the perspective of dosimetry to provide a specific theoretical basis for the wide application of high-energy electron beam irradiation.
Materials and methods
Materials
The main equipment for this research included GE LightSpeed VCT 64-row CT scanner, Philips classic planning system Pinnale9 (TPS), Varian CLINAC 23E Linear Accelerator, thimble ionization chamber, PTW dosimeter, two-dimensional air ionization chamber matrix MatriXX. A plexiglass plate, moist medical gauze (a thin layer of medical gauze dipped in distilled water and stacked into a cuboid with a bottom area of 15 × 15 cm), colloid compensator (purchased from a medical device company) and Vaseline compensator (made of white medical Vaseline shaped into a cuboid with a bottom area of 15 × 15 cm), all with a thickness of 0.5 cm.
Treatment planning system (TPS)
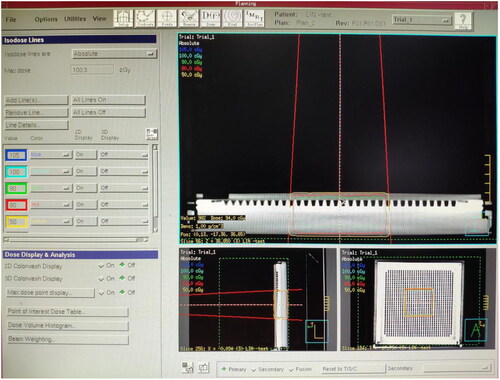
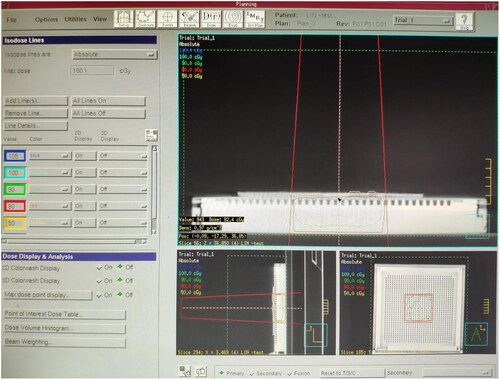
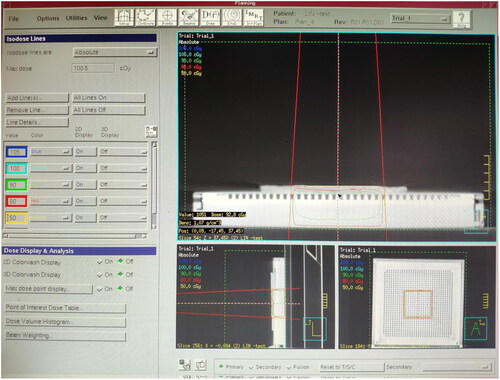
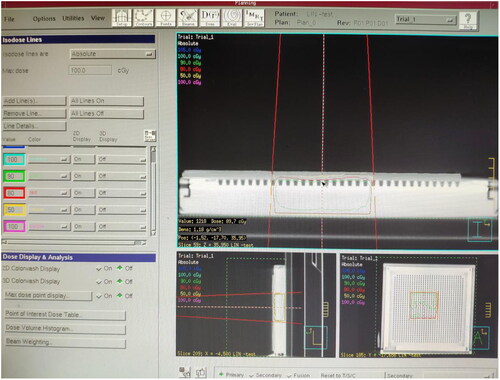
The plexiglass plate, moist medical gauze, colloid compensator and Vaseline compensator of the same thickness were laid onto the MatriXX surface. A CT machine was used to scan them separately and transfer the CT image to the TPS. The following can be obtained from the TPS, the CT value of the plexiglass plate: −84 HU, electron density: 0.998 g/cm3. The CT value of moist medical gauze: +28 HU, electron density: 1.029 g/cm3. The CT value of colloid compensator: −84 HU, electron density: 0.998 g/cm3. The CT value of Vaseline compensator: −103 HU, electron density: 0.942 g/cm3. The irradiation parameters were set at 6 MeV electron beam, linear accelerator hops 100 Mu (100 cGY), with the ray perpendicular to the irradiation surface, irradiation field 10 × 10 cm and source skin distance 100 cm. The dose at any point on the MatriXX surface can be obtained through the TPS calculation under the compensation of different materials. The dose distribution on the MatriXX surface under the compensation of the plexiglass plate is shown in ; the wet medical gauze is shown in ; the colloidal compensator TPS is shown in ; the Vaseline compensator is shown in . The compensation dose of each material was randomly selected at five points on the MatriXX surface. After setting the irradiation parameters, the surface dose, the dose at the maximum dose point and the depth of the maximum dose point of MatriXX can be obtained by calculating the treatment planning system under the compensation effect of different materials.
Thimble ionization chamber
The plexiglass plate, moist medical gauze, colloid compensator and Vaseline compensator of the same thickness were laid on the surface of the thimble ionization chamber and irradiated with a linear accelerator. The irradiation conditions were 6 MeV high-energy electron beam, linear accelerator irradiation hops 100 Mu (100 cGY), irradiation field 10 × 10 cm, with the ray perpendicular to the irradiation surface, and source skin distance of 100 cm. Through the dosimeter connected to the thimble ionization chamber, compensated dose readings of various materials were obtained, and five measurements were made for each material.
MatriXX
The above four materials were laid flat on the surface of the MatriXX and irradiated with a linear accelerator. The irradiation conditions were 6 MeV high-energy electron beam, linear accelerator irradiation hops 100 Mu/100 cGY, with the ray perpendicular to the irradiation surface, irradiation field 10 × 10 cm and source skin distance 100 cm. The compensation dose of the four materials was collected, displayed and analyzed in real time through a dedicated computer connected to MatriXX, and five measurements were made for each material.
Statistical analysis
In this study, SPSS 20.0 statistical software was used for data processing, and measurement data were expressed as mean values with standard deviation ¯x ± s). Counting data were expressed in percentage (%). The normality test used the W test. The F test was used to test the homogeneity of variance. The comparison between multiple groups that obey the normal distribution used a one-way analysis of variance. The post hoc test was LSD (least significant difference). The comparison between groups that do not follow the normal distribution used a nonparametric test. A chi-square test was used for counting data. p < 0.05 indicates that the difference is statistically significant.
Results
The surface dose, maximum dose point dose and maximum dose point depth of the MatriXX under compensation of different materials
In this study, the plexiglass plate (), moist medical gauze (), colloid compensator () and Vaseline compensator () of the same thickness were placed on the MatriXX surface. After setting the irradiation parameters, through the calculation of the treatment planning system, the surface dose, maximum dose point dose and maximum dose point depth of the MatriXX can be obtained under the compensation effect of different materials. For example, TPS shows the dose distribution on the MatriXX surface (i.e. beam incidence surface) under the compensation of the plexiglass plate. TPS shows the dose distribution on the MatriXX surface under the compensation of the moist medical gauze. TPS shows the dose distribution on the MatriXX surface under the compensation of the colloid compensator. TPS shows the dose distribution on the MatriXX surface under the compensation of the Vaseline compensator. The data are presented in greater details in .
Table 1. Original data of dose compensation effect of four materials obtained by different methods (unit: cGy).
TPS test results of four different materials
For the three dose verification methods, the analysis of variance of completely randomised design data was used, as shown in . It was found that when the TPS method was used for detection, after placing four different materials on the surface of the MatriXX, there was a statistical difference in the overall measured dose. The Bonferroni method was further used for pairwise comparison, and it was found that there were statistically significant differences between the moist medical gauze group and the other three groups (P < 0.05), while there was no statistically significant difference between the three groups of the plexiglass plate, colloid compensator and Vaseline compensator (P > 0.05). The detection results of the thimble ionization chamber and the MatriXX method were consistent with the detection results of the TPS method. The compensation effect of the three materials of plexiglass plate, colloid compensator and Vaseline compensator on 6 MeV high-energy electron beam was similar. The compensation effect of moist medical gauze was quite different from that of the other three materials. The compensation effect of moist medical gauze was lower than that of the plexiglass plate, colloid compensator and Vaseline compensator ().
Table 2. Absorbed dose of different materials (cGy).
Discussion
The 6MeV electron beam of the Varian 23EX linear accelerator used in this study was measured by a three-dimensional water tank during the annual inspection: on the central axis, 100% dose depth R100 was 15.2 mm, 80% dose depth R80 was 22.2 mm, 50% dose depth R50 was 26.33 mm, electron beam range Rp was 32.3 mm. The thickness of the four compensation materials in this study were all 5mm. The depth from the surface to the depth of R100 was the dose built-up area, and the depth from R100 to R85 was the high-dose plateau area, which was also the treatment area. The dose below the R85 depth would drop sharply, which was the dose drop area. The inherent thickness of the MatriXX detection equipment and the thimble ionization chamber was about 3 mm, 3 mm < 5 mm < 15.2 mm < 22.2 mm, the material could be effectively compensated and built.
Radiotherapy Treatment Planning System (TPS) is a device that simulates a recommended radiotherapy treatment by modeling the radioactive source and the patient [Citation5–7] . The system uses one or several specialized algorithms to calculate the distribution of the absorbed dose in the patient. The computer algorithm used by TPS in this study is: Adaptive Convole. It can acquire CT images, perform image fusion, contour editing, image processing, and three-dimensional display [8–10], edit beam current, calculate dose, evaluate radiotherapy plan, and read out the dose of any point and surface. The work flow is relatively long, and CT scan, image conduction, target area setting, and computer calculation are required first. The thimble ionization chamber is mainly used for the daily monitoring of the linear accelerator by the physicist. It is mainly used for point dose measurement and high-energy X-ray detection. It can be used to measure directly, but the amount of ray information collected is relatively simple, and low-energy electron beams are generally detected by flat-panel ionization chambers. (IAEA TRS-398) Two-dimensional air ionization chamber matrix MatriXX can collect, display and analyze radiation in real time. It can compare and analyze the dose distribution curves and isodose lines in the X and Y directions, and the data can be summed, differed, multiplied, divided, and Gamma analyzed. It is often used for the dosimetry verification of IMRT patients before treatment [12–15]. The use of MatriXX to verify the irradiation field in quality assurance can simplify the measurement procedure and improve the efficiency of QA. The dose can be measured at any point or surface. It can be used for direct measurement, two-dimensional measurement [16–19].
Plexiglass plate, moist medical gauze and colloidal compensator are commonly used as electron beam radiation field dose compensation materials in practical radiotherapy at present, but there is no literature report on this research. In this study, three dose verification methods were used to compare the compensation effect of four materials on electron beam field dose. Among them, the compensation effect of plexiglass plate, colloidal compensator and vaseline compensation was similar, and the compensation effect of moist medical gauze was weaker than that of the other three materials.
Plexiglass, also known as acrylic, chemically known as polymethyl methacrylate, is an important thermoplastic developed relatively early [Citation7]. It has good transparency, chemical stability and weather resistance. In tumour radiotherapy, it is mainly used as the load-bearing and fixed carrier of low melting point lead block and used to compensate superficial tumour radiotherapy. The disadvantage is that the texture is hard, and there is no flexibility. When the skin’s surface is uneven, it cannot be completely moulded to the uneven surface, and it cannot give a good dose compensation effect.
As an alternative, a thin layer of medical gauze is dipped in distilled water, laid flat and stacked to form a certain thickness. Its advantage is that the material is easy to get, simple to manufacture, easy to shape and economical. The disadvantage is that the material is unstable, water is easy to volatilise and the texture is soft and easy to deform. The number of chemical molecules and atomic numbers are difficult to verify.
A colloid compensator is primarily made of polypropylene (PP) material, and the radiotherapy physicist measures its compensation dose. Medical equipment companies generally provide it. The price is expensive, the specifications are single, and the compensation material cut into a particular shape cannot be reused, increasing the department’s expenses and the patients’ economic burden.
The main component of the Vaseline Compensator in this study is white medical Vaseline. Vaseline is a colourless, odourless, non-fluorescent and transparent oily liquid obtained by deep refining of petroleum lubricating oil distillate and does not contain any additives, moisture and mechanical impurities. It is soluble in organic solvents such as ether, chloroform, gasoline and benzene, and insoluble in water. It has good chemical stability and oxidation resistance and is easy to get, simple to manufacture, economical and practical. It can be reused and can be moulded into various thicknesses and shapes. It fits well when there are bumps on the surface of the skin. Vaseline can keep the skin moist, protect the skin tissue at the wound site in the best condition and accelerate the skin’s repairability.
This study shows that the compensation effect of the moist medical gauze is relatively poor. The compensation effect of the Vaseline compensator was similar to that of the plexiglass plate and colloid compensator. The moist medical gauze should not be used in the daily electron beam treatment. Vaseline compensator can be used instead of a plexiglass plate and colloid compensator, but the dosage must be verified before use.
Different dose verification methods have advantages and disadvantages. Plexiglass plate, wet medical gauze and colloid compensator are commonly used electron beam dose compensating materials in radiotherapy, but little research in this field has been reported. The physical and chemical properties and practicality of various compensation materials have advantages and disadvantages, and the compensation effects are different. This research has some limitations. First, this study was not a randomised controlled experiment, and there was no blinding method, so there was still a certain risk of bias. Second, the results of this study showed that the compensation effect of the Vaseline compensator made by the research team is similar to that of the plexiglass and colloid compensator in the actual practice of radiotherapy, but the specific mechanism of action is not yet clear, and further research is needed.
Conclusions
The compensation effect of the Vaseline compensator made by the research team was similar to that of the plexiglass and colloid compensator in the actual work of radiotherapy. It has stable physical and chemical properties, is easy to manufacture and store, and is economical and convenient.
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Chaudhary N, Singh A, Aswal DK, et al. High energy electron beam induced improved thermoelectric properties of PEDOT:PSS films. Polymer. 2020;202:122645.
- Lee SJ, Lee YB, Lim YR, et al. High energy electron beam stimulated nanowelding of silver nanowire networks encapsulated with graphene for flexible and transparent electrodes. Sci Rep. 2019;9(1):9376.
- Jingu K, Tanabe T, Nemoto K, et al. Intraoperative radiotherapy for pancreatic cancer: 30-year experience in a single institution in Japan. Int J Radiat Oncol Biol Phys. 2012;83(4):e507–e511.
- Barkshire I, Karduck P, Rehbach WP, et al. High-spatial-resolution low-energy electron beam X-ray microanalysis. Mikrochim Acta. 2000;132(2–4):113–128.
- Shen C, Nguyen D, Chen L, et al. Operating a treatment planning system using a deep-reinforcement learning-based virtual treatment planner for prostate cancer intensity-modulated radiation therapy treatment planning. Med Phys. 2020;47(6):2329–2336.
- Patel NP, Majumdar B, Vijayan V. Comparative dosimetry of GammaMed plus high-dose rate Ir brachytherapy source. J Med Phys. 2010;35(3):137–143.
- Babo S, Ferreira JL, Ramos AM, et al. Characterization and long-term stability of historical PMMA: impact of additives and acrylic sheet industrial production processes. Polymers (Basel). 2020;12(10):2198.
- Kishida Y , Ikushima H , Sasaki M , et al. Use of a diagnostic positron emission tomography-computed tomography system for planning radiotherapy positioning: distortion of the tabletop. Japan J Radiol. 2010;28(2):143.
- Denis E , Guedon J P , Beaumont S , et al. Discrete and continuous description of a three dimensional scene for quality control of radiotherapy treatment planning systems[C]// Conference on Medical Imaging 2006: Physics of Medical Imaging pt.3 20060212-16 San Diego,CA(US). IRCCyN/IVC -UMR CNRS 6597, Image & Video Communication, Ecole polytechnique de l’Universite de Nantes, La Chantrerie, BP 50609, 44306 Nantes Cedex 3 -France, 2006.
- Hasani M , Farhood B , Ghorbani M , et al. Effect of computed tomography number-relative electron density conversion curve on the calculation of radiotherapy dose and evaluation of Monaco radiotherapy treatment planning system. Aust Phys Eng Sci Med / supported by the Aust Coll Phys Sci Med Aust Assoc Phys Sci Med. 2019;42(2):489–502.
- Macapinlac H A . Clinical applications of positron emission tomography/computed tomography treatment planning. Seminars in nuclear medicine, 2008, 38(2):137.
- Luciant, D, Wolfsberger, et al. Angular dose dependency of MatriXX TM and its calibration. J Appl Clin Med Phys. 2010.
- Han Z , Ng S K , Bhagwat M S , et al. Evaluation of MatriXX for IMRT and VMAT dose verifications in peripheral dose regions. Medical Physics, 2010;37(7):3704–3714.
- Yu M , Nelson N , Gladstone D . Comparisons of measured doses using ion chamber and Matrixx for IMRT-QA. Med Phys. 2008;35(6):2744–2744.
- Widodo P , Pawiro S A , Susila I P . Dosimetry for Intensity Modulated Radiotherapy (IMRT) Technique Using Ion Chamber Matrix (MatriXX-FFF) with Back Projection Method (Usual 6MV and 10MV). J Phys Conf Ser, 2020;1505:012015.
- Inal A , Ismail Hakki Sarpün. Dosimetric evaluation of phantoms including metal objects with high atomic number for use in intensity modulated radiation therapy. Rad Environ Biophys. 2020;59(3):503–510.
- Liura S , Pawiro S A . Comparison of gamma index passing rate in several treatment planning system algorithms. Atom Indonesia, 2020;46(2):77.
- Lan N T , Viet H D , Tai D T , et al. Dosimetric comparison of intensity-modulated radiation therapy (IMRT) and field-in-field (FIF) technique for head-and-neck cancer. J Radiother Pract. 2020:1–6.
- Sresty N N M , Raju A , Reddy B , et al. Evaluation and validation of IBA I’MatriXX array for patient-specific quality assurance of TomoTherapy. J Med Phys. 2019;44(3):222.