Abstract
Gene drives are being used to enhance a DNA sequence’s likelihood of passing between generations via sexual reproduction. Gene drives can be deployed to manipulate natural populations. They can be used to suppress populations by reducing the number of individuals in a population or to modify populations. There are more than 3000 mosquito species in the world, some of which are vectors of diseases. Malaria is a typical disease whose vectors are mosquitoes. It affects mostly tropical countries. It kills many people annually, many of whom are children. Interventions currently in use, such as indoor residual spraying and mosquito nets, are proving insufficient to eradicate malaria. Gene drives can be used in different ways to control mosquito populations or to eliminate mosquito species, thereby reducing malaria cases and deaths. This can occur through population replacement or suppression. However, before the elimination of any mosquito species for malaria control, it is necessary to consider the effects of such an action. Additionally, there is a need to review the options available for the control of mosquitoes and to create awareness of the benefits and risks of such an action. This paper, therefore, looks at the role of mosquitoes in the environment, the methods of controlling mosquitoes and malaria and necessary considerations when using gene drives inter alia.
Introduction
The current increase in the use of genetic engineering tools requires us to carefully advance science while navigating uncertainty and aligning our research with public values. Science must be unpacked for the general populace to understand. This will reduce knowledge gaps between experts in the field and the general populace and help in holistic management of the risks associated with applications of the tools. Natural and synthetic (engineered) gene drives can propagate a set of genes throughout a population by biasing inheritance against Mendelian inheritance laws. Gene drives can make a particular DNA sequence or trait pass between generations via sexual reproduction with more than the natural 50% chance [Citation1]. They can achieve this by different methods, such as cutting a wild-type allele and copying the drive system in its place (homing) [Citation2]. A gene can therefore produce multiple copies of itself in a genome, disabling other genes to increase its inheritance odds. Consequently, gene drives can be used to suppress populations. This can be done by engineering traits such as male sterility that result in a population crash. Offspring will predominantly be female, thereby reducing the reproduction rates leading to a population crash. Some gene drives can be limited in time and space, while others are not. Global gene drives spread throughout all populations of a species connected by gene flow and persist [Citation3]. Gene drives such as Daisy drives can be limited in time and space. This gives some control over gene drives.
Natural gene drives occur naturally. They include bacteria of genus Wolbachia and transposable elements (jumping genes). The sterile insect technique relies on sterility being conferred by a transgene. It has been successfully used to control urban mosquito populations [Citation4]. Gene drives can be used to the same effect by conferring sterility in mosquito offspring. This can help reduce malaria cases and deaths. Consequently, gene drive-modified organisms can yield great benefits or harmful ecological changes. They offer a high-impact, cost-effective and durable method of controlling mosquito populations, other disease vectors and invasive species [Citation5]. Gene drive systems include the maternal effect dominant embryonic arrest system (MEDEA), homing-based drives using homing endonuclease genes (HEGs), underdominance or heterozygote inferiority drives, sex-linked meiotic drives and heritable microorganisms. Homing for gene knockouts is a simple mechanism of achieving drive. It is based on the activity of endonucleases. A homing drive can home into a critical gene whose disruption induces recessive sterility or lethality, thereby suppressing a population as the gene drive spreads. However, a homing-based drive can be removed from a population using a reversal drive targeting it [Citation6].
The novelty of recent gene drives resides in the use of the clustered regularly interspaced short palindromic repeats (CRISPR) technique, which not only allows gene editing with precision, speed and economy but also has the potential of ensuring that alterations made in wild organisms will persist in nature. CRISPR-Cas systems can be precisely used to alter DNA regions to yield a gene drive [Citation7]. The CRISPR gene drives make use of CRISPR’s DNA revision mechanism by copying engineered nucleotide sequences into homologous chromosomes, thereby guaranteeing the inheritance of edited genes in all offspring [Citation8]. CRISPR-based gene drives can spread genes particularly rapidly because their components can be tailored to replace alternative copies of a targeted gene. This will ensure that only desired versions of the genes are passed on to offspring. Self-propagating gene drives are designed so that they can always spread as long as there are wild organisms around. Self-exhausting gene drives lose their ability to spread over time. Self-propagating CRISPR-based gene drives can spread if a few organisms with gene drive elements are released into the wild [Citation1].
Gene drives are clearly a possible solution for controlling mosquito populations and malaria [Citation9]. However, there are presently no clear pathways for their testing in malaria-endemic countries. This is partly due to the lack of well-characterized promoters that can be used for infection-relevant tissues and some regulatory hurdles [Citation10]. Additionally, the consequences of eliminating some mosquito species are not fully understood. There is therefore a need to assess, inter alia, the benefits of mosquitoes, the impacts of malaria and the potential effects of elimination of mosquito species using gene drives. In this paper, we discuss the methods that are used in mosquito control in malaria programs and how gene drives can be used to aid them in the control of mosquito populations. We also discuss the benefits of mosquitoes and their negative impacts on the environment.
Role of mosquitoes in the ecosystem and to human health
There are more than 3000 mosquito species in the world. These are grouped into 39 genera and 135 subgenera [Citation11]. Out of 460 different Anopheles species, 30 to 40 are vectors for the Plasmodium parasite responsible for malaria in different locations. Mosquitoes exist at the bottom of the food chain, have a role in the aquatic food chain and are necessary for maintaining a natural balance. Mosquito larvae are filter feeders. They feed on unicellular algae and tiny organic particles, clearing water bodies of debris [Citation12]. The larvae, in turn, serve as food for fish, frogs and tadpoles, among other aquatic organisms. The role of mosquitoes on the bottom of the food chain passes the larval stage. They are prey for birds, spiders, salamanders, lizards, turtles, dragonflies, swallows and bats. Mosquito eggs survive harsh weather. They hatch when the snow melts providing food for migratory birds in areas such as the Arctic tundra [Citation13]. Mosquitoes depend on nectar for energy. They act as pollinators, ensuring that mainly aquatic plants thrive. An example is the swamp orchid Habenaria obtusata and its specialized pollinators from the Aedes genus.
Mosquitoes are vectors of several viruses known as mosquito-borne viruses (moboviruses). These include yellow fever, West Nile virus [Citation14], dengue fever [Citation15], filariasis, Zikaflavivirus [Citation16], Chikungunya [Citation17] and other arboviruses [Citation18]. They also carry malaria-causing parasites in humans such as Plasmodium vivax, Plasmodium ovale, Plasmodium malariae and Plasmodium falciparum.
Malaria has ravaged the Sub-Saharan region for over 300,000 years, causing approximately 438,000 annual deaths [Citation19]. However, mosquitoes have important direct roles in human life. Their saliva has medicinal properties in cardiovascular disease and produces anticoagulant factors [Citation20]. It can therefore be used to develop anticlotting drugs such as clotting inhibitors and capillary dilators [Citation21]. Additionally, mosquito bites are associated with the modulation of the host immune response [Citation22]. Plasmodium-carrying mosquitoes are of increasing interest in cancer treatment. This is because infection with some species of Plasmodium was shown to help stimulate the immune system to better fight cancers such as hepatocellular carcinoma (HCC). HCC accounts for between 85 and 90% of primary liver cancers and is the third most common cause of cancer mortality worldwide.Under laboratory conditions, in experimental animals, Plasmodium yoelii 17XNL infection significantly suppresses Lewis lung cancer (LLC) cell growth. This occurs via the induction of innate and adaptive antitumor responses. Additionally, Plasmodium infection inhibits tumour angiogenesis [Citation23, Citation24]. Thus, although seemingly not important, mosquitoes that carry Plasmodium can help us find ways to treat cancer and other diseases. Understanding mosquitoes is, therefore, a potential source of treatment methods and drugs that can turn out into billion-dollar industries and save millions of lives.
Despite their potential usefulness to human health, a debate abounds about their ultimate importance. Some scientists are proposing the eradication of certain species of mosquitoes responsible for the transmission of diseases with the hope that there will be no significant negative effects on the environment. Among other risks, there is the potential of successor species rising to replace the eliminated species as disease vectors.
Methods of controlling mosquitoes and malaria
The methods for control and treatment of malaria have been relatively successful, as evidenced by decreasing malarial deaths [Citation19]. They include vector control through insecticide-treated mosquito bed nets and indoor residual spraying (IRS). These methods have decreased the presence of Anopheles mosquitoes and malaria cases [Citation25, Citation26]. However, the residual transmission of malaria from mosquito vectors that feed outdoors or early in the evening is still being reported.
Malaria is treatable with antimalarial drugs. These decrease the number of parasites in the blood. However, insecticide and drug resistance to malaria drugs is being reported, reducing the impacts of the drugs. Both Anopheles mosquitoes and the Plasmodium parasites show resistance to the most commonly used chemical and pharmaceutical options used to fight malaria, such as pyrethroids. Additionally, artemisinin combination therapy (ACT) resistance has been reported and is spreading. ACTs are key to the treatment of P. falciparum malaria in the malaria-endemic world. The resistance is due to mutations in the PfKelch13 gene. This gene has multiple independent origins across the greater Mekong sub-region. This has motivated a regional malaria elimination agenda [Citation27]. Additionally, there are multiple mechanisms of insecticide resistance. These include changes to insecticide target molecules that make the insecticide fail to bind, mosquito behavioural changes that make them avoid insecticide contact, thickening of the insect’s cuticle making the insecticide fail to reach its target and detoxification of the insecticide before it reaches its target (metabolic resistance) [Citation28]. In the malaria vector An. Funestus pyrethroid resistance is mainly conferred by metabolic resistance associated with a major quantitative trait locus (QTL) at which two duplicated cytochrome P450 monooxygenases (CYP6P9a and CYP6P9b) are the main resistance genes [Citation29]. The development and geographical spread of resistance to insecticides mean that malaria eradication requires new tools in addition to those currently deployed.
Blood in a mosquito blood meal contains excess salts such as potassium chloride. These salts need to be excreted via the kidneys [Citation27]. A team of researchers from Vanderbilt University Medical Center and Ohio State University screened approximately 26,000 compounds for their ability to inhibit a potassium channel, Kir1, involved in urine production. Compound VU041 rapidly blocked Kir1 channel activity [Citation30]. VU041 is specific to mosquitoes. It does not affect any mammalian potassium channels tested. The team monitored mosquitoes to assess kidney function. They observed that when untreated mosquitoes consumed a blood meal, their abdominal diameter immediately doubled and then decreased over the next 24 h. In contrast, the abdominal diameters of mosquitoes treated with VU041 increased but did not decrease. This suggested the impairment of kidney function. The mosquitoes continued to increase in weight until they burst. Additionally, VU041 was found to reduce egg-laying after blood feeding, suggesting that it can be used to control mosquito populations. The team consequently developed a new class of insecticides that target the mosquito kidney.
Another strategy is to use Metarhizium anisopliae. This is a fungus that naturally attacks mosquitoes. It can be used as a mosquito-specific biopesticide [Citation31]. In this strategy, mosquitoes must acquire the fungus soon after becoming infected with the malaria parasite. Rather than developing fungi that rapidly kill the mosquito, the fungus is genetically modified to block Plasmodium development inside the mosquito. After invading a mosquito, transgenic fungi produce small molecules such as the human antimalarial antibody and a scorpion antimicrobial toxin [Citation30]. When mosquitoes that are heavily infected with P. falciparum are sprayed with transgenic fungi, they have significantly reduced parasite development. The transgenic fungus did not significantly affect mosquito survival compared to the wild-type fungus. Hence, it does not lead to rapid mosquito resistance when used in the field.
Genetic engineering of the mosquito gut which reduces survival chances of the plasmodium can also help control mosquito populations and malaria [Citation29]. The mosquitoes will need to have new effector genes introduced. These can be expressed as anti-Plasmodium proteins within the mosquito gut, making the environment uninhabitable for Plasmodium. Fungi, viruses or bacterial symbionts that already infect mosquitoes and inhabit the mosquito gut can be used to introduce effector genes [Citation32]. Symbiotic gut bacteria can also be genetically modified to enable the introduction of effector genes. When the mosquito begins to express the anti-Plasmodium proteins, its gut becomes inhospitable to Plasmodium parasites. Additionally, genetic engineering can be used to alter the expression of mosquito anti-Plasmodium immune genes in a population with wild-type mosquitoes. Genetically modified Anopheles stephensi lines resistant to Plasmodium falciparum can be created. Resistance will be due to the up-regulation of mosquito immune genes in the midgut or fat body after a blood meal. This can be achieved using the carboxypeptidase (Cp) or vitellogenin (Vg) promoters [Citation27]. These strains will possess elevated anti-Plasmodium and antibacterial activities through either the immune-deficiency pathway–associated NF-κB transcription factor Rel2 or the Down syndrome cell-adhesion molecule (AgDscam) splice form AgDsPf [Citation33]. The genetically modified lines can be backcrossed with the original wild-type stock for five generations and be continually reared under the same conditions to ensure the same genetic and environmental background. Genetically modified mosquitoes will not have a fitness disadvantage. They will show reduced microbial loads in the midgut and reproductive organs. These changes result in a mating preference. Genetically modified males will prefer wild-type females. Wild-type males will prefer genetically modified females. These changes will foster the spread of the genetic modification in a mosquito population controlling mosquito populations [Citation30].
Another approach used in Aedes mosquito control to combat dengue is engineering male mosquitoes so that their offspring never mature. The offspring died before they were able to transmit dengue [Citation32]. This resulted in a wild mosquito population decrease of 80–95%. Hence, disease incidence can also consequently be reduced.
Potential of gene drives in eradicating mosquitoes and malaria
Mosquito population suppression results in fewer mosquitoes transmitting malaria. Gene drives can be characterized by the rate of spread, species specificity, fitness cost, susceptibility to resistance, removability and reversibility. Engineered gene drives can be divided into:
The modification drives designed to spread genomic changes and/or genetic payloads throughout a population (alteration drives).
Suppression drive designed to reduce or eliminate the population of its target organism. These often use methods such as the reduction of vectorial capacities.
Reversal gene drives which induce changes that undo a phenotypic alteration caused by the initial gene drive.
Immunising drives which prevent the spread of unwanted genes by pre-emptively altering genetic sequences to block the effects of precision drives [Citation34].
The envisioned goal for applying gene drives is to reduce or eliminate vector mosquito populations or to render them less competent to transmit pathogens. With a gene drive, not only is it possible to alter an organism’s gene, but it is also possible to insert in the genome the CRISPR copy-paste system, which includes the gRNA and Cas protein. This allows the gene alteration to self-replicate in subsequent generations [Citation9].
As an example, when an altered mosquito mates with a wild mosquito, the offspring receive an altered chromosome and a wild chromosome from each parent. The CRISPR system aquired from the altered parent will cut the wild gene inherited from the wild parent and duplicate the altered gene into the offspring’s genome along with the gene drive. The offspring, therefore, carry two copies of the modified gene, guaranteening its transmission to the next generation with a probability of up to 100%. At the point when a new generation of altered mosquitoes mates with the wild-type, the process will repeat itself. This permits the modification and gene drive to spread to the entire population. Gene drive appear to be a reliable mechanism for propagating altered genes. They can allow gene alterations to persist in nature and permanently change the target population and possibly an entire species. Computational modelling based on other gene drive systems suggests that the CRISPR/Cas9 system can be used to engineer gene drives so effectively that the release of low numbers of modified mosquitoes into the environment can result in the establishment of the genetic modification in the natural interbreeding population [Citation26].
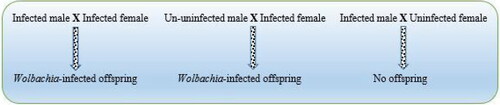
One way of applying gene drives in mosquito control is via the use of naturally occuring bacteria known as Wolbachia (). Wolbachia block the development of Plasmodium parasites in mosquitoes. They can be transmitted by an infected female insect to the offspring. Uninfected females that mate with infected males rarely produce viable eggs. This is a reproductive dead-end that gives infected females a reproductive advantage and helps the bacteria spread quickly. Wolbachia were successfully used in a field trial to control dengue and other mosquito-borne diseases [Citation33]. A strain of Wolbachia derived from another type of mosquito was introduced into A. stephensi embryos. The adult females mated with uninfected male mosquitoes after maturation to establish a Wolbachia infection. The infection lasted for 34 generations (the end of the study). Uninfected females rarely produced viable eggs with infected males. In addition, Wolbachia infection resulted in fewer malaria parasites in the mosquito midgut and salivary glands and caused the formation of reactive oxygen species, which inhibited parasite development.
Male killing strains of Wolbachia can be used for population suppression. However, they have a moderate rate of spread, the resistance allele generation rate is unknown and they cannot be reversed.It must be noted that Wolbachia do not pass consistently from mother to offspring in Anopheles mosquitoes. This has to be considered when designing gene drives for the control of malaria.
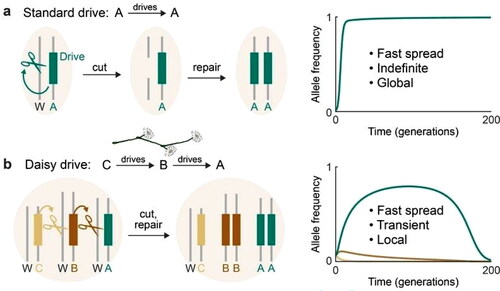
Genetic alteration of an entire species without causing any temporal or geophysical limitations to their spread can be achieved by the use of standard drives (). For instance, Kyrou et al. [Citation35] demonstrated a method of engineering a construct targeting the Agdsx gene that led to a ‘total population collapse’ in caged mosquitoes. Daisy drive systems, also known as split drives, instead contain split up portions of CRISPR that require sequential action for the drive to operate: element A drives element B, which drives element C and so on until the final element, which does not drive any others [Citation36]. This gives a spatial and temporal limitation to Daisy drives.
Comparison of self-propagating and daisy-chain gene drive systems. (A) Self-propagating gene drives distort inheritance in a self-propagating manner by converting wild-type (W) alleles to drive alleles in heterozygous germline cells. (B) A daisydrive system consists of a linear chain of serially dependent, unlinked drive elements; in this example, A, B and C are on separate chromosomes. Elements at the base of the chain cannot drive and are successively lost over time via natural selection, limiting the overall spread of the drive [Citation36].
Although the theoretical results are promising, existing technological limitations prevent the safe application of daisy drive elements. For example, there is a risk of converting a linear daisy drive chain into a self-sustaining gene drive ‘necklace’ that may spread all over the world. This might occur if one or more gRNAs within an upstream element of the chain move into any downstream element as a result of recombination [Citation36, Citation37].
The designing of HEGs may aid the manipulation of populations by targeting other suitable genes, such as genes that decrease lifespan, to bias sex ratios, to hinder host-seeking, to obstruct pathogen development, or to hinder the capacity of the modified organism to act as a vector for pathogens. In mosquitoes, a synthetic version of the homing reaction was first demonstrated in Aedes gambiae [Citation38] using a homing endonuclease from yeast. Conceptually, the simplest use for homing is to produce a population-wide gene knockout. Modelling shows that if the knockout phenotype is recessive and if the homing reaction is confined to the germline, it is possible for a homing endonuclease that causes lethality or sterility to increase in frequency in a population, potentially suppressing the population as it does so [Citation39]. Another possibility is to disrupt malaria transmission by targeting genes needed for the Plasmodium parasite to invade into, develop within, or exit out of the mosquito vector. One review lists 38 genes that, when knocked down, show some reduction in oocyst number or sporozoite count [Citation40] and some of these genes may be suitable for this approach. For the homing reaction to lead to preferential inheritance of the enzyme construct, the enzyme must be expressed in the germline. Thus far, promoters used for this in Anopheles have been from the B2-tubulin gene [Citation41] and the vasa gene [Citation42].
The spread (‘knock-in’) of a novel ‘cargo’ gene through a population can be achived through the use of a homing reaction. Cargo can be an effector gene that disturbs parasite transmission through the mosquito. More than 28 effector genes could interfere with malaria parasite transmission [Citation29]. These include genes for antimicrobial peptides, single-chain antibodies, immune system activators and peptides that bind to mosquito proteins in the midgut or salivary glands. Because malaria is transmitted only by female mosquitoes, a population-wide distortion of the sex ratio towards males would have a direct impact on reducing malaria transmission. In Aedes mosquitoes, there is a naturally occurring driving Y chromosome that in some crosses leads to more than 90% male progeny. A proof-of-principle demonstration of this route to drive in A. gambiae was reported by Galizi et al. [Citation43]. They showed that expression of an engineered variant of the PpoI nuclease from a slime mould, expressed during spermatogenesis using the B2-tubulin promoter sequences, led in some lines to males producing 95% male offspring. Other gene drive systems have been proposed for spreading an effector through pest populations, including chromosomal rearrangements that display underdominance and various combinations of toxins and antidotes that mimic underdominance systems, MEDEA systems or variants thereof [Citation44]. In a MEDEA system, the progeny of heterozygous females dies unless they inherit the MEDEA element. These sorts of drivers are ‘weaker’ than those based on homing or driving sex chromosomes [Citation39]. However, when a single mosquito species that transmit malaria parasites are eliminated, there is a possibility of a successor species arising to take its place. This new species will start transmitting malaria parasites.
Transposable elements are linked to a genetic payload, which can increase the frequency of the transposable element and genetic payload in the genome of a target organism and eventually in the population. However, they often have transposition rates that are too low to be usable. Transposable elements are unpredictable. Control over integration sites of transposable elements is too low and they are difficult to mobilize after integration [Citation45].Therefore, with current knowledge and technology, it is not advisable to use transposons to control mosquito populations.
Sex-linked meiotic drives have a low resistance allele generation rate. They can be reversed and may not be removable with wild-type. However, they can be inactivated. This can be achieved by a suppressor using a second-generation drive ‘reversal’ element [Citation7]. Sex-linked meiotic drives can suppress or eliminate populations. This can bring about unanticipated ecological ramifications. Sex-linked meiotic drives have a moderate rate of spread. They can result in the extinction of species. Consequently, they can be used to suppress or eliminate mosquito species that transmit malaria parasites.
Supernumerary B chromosomes can act as vehicles to carry payload genes. This is because they are inherited at rates greater than Mendelian rates and can express transcripts. However, they are poorly understood, making their engineering difficult. This makes them unfavourable for mosquito control [Citation46].
The Killer–Rescue system uses a toxin and an antidote gene that are at separate loci. It is a hypothetical threshold-dependent gene drive system. The inverse MEDEA system depends on a toxin that becomes active in the zygote, except if the zygote receives a maternally delivered antidote. Unlike MEDEA, the Merea system uses a recessive antidote to the maternal toxin. In the Semele system there is a paternal semen-based toxin and a maternally delivered antidote. The population crash in the Medusa system is a result of a pair of sex-linked toxins and antidotes. In the future, RNA-guided nucleases may contribute to the development of each of these systems in mosquito species, leading to greater control of malaria [Citation9].
There is a range of genetic approaches that have potential to help manage mosquito populations, which feed indoors or outdoors during daytime or night-time. Different approaches could work in various settings, e.g. hypo- or holo-endemic, urban or rural [Citation39]. They offer area-wide control and consequently, protection regardless of a person’s age, financial status or level of education. Conversely, gene drives should be compatible and coupled with other disease control initiatives, both current and under development. Gene drives can be relatively easy to deliver and deploy, with little or no change required in people’s behaviour. They will potentially be highly cost-effective. These key features motivate the continued development of gene drive driven approaches to malaria control [Citation5].
Although gene drives can be useful in malaria control, there is a need to employ strategies to control the spread of genetically modified mosquitoes after release. This will help prevent unintended ecological effects and keep trust in scientists and science [Citation6]. This is because experiments and deterministic models have shown that drive resistance can result from mutations that block cutting by the CRISPR nuclease. The effects of this phenomenon are not always certain. However, this is not a major impediment to the invasion of unintended populations. However, Genetically modified mosquitoes can cross international borders, even from isolated islands. Thus, there is a need to develop ‘local’, sensitive methods of monitoring population genetics, intrinsically self-exhausting gene drive systems and strategies for countering self-propagating drive systems as well as removing all engineered genes from wild populations [Citation1]. Several other promising gene drive systems have thus far only been advanced at the theoretical level.
There is also an unknown likelihood of unauthorized releases of self-propagating gene drive engineered species. This is affected by species, application, containment strategies, economic motivations, drive development stages, geography and the caution of scientists. However, the possibility of consequently having serious negative ecological consequences given the high likelihood of spread to most populations of the target species is low. This is because gene drive systems are typically predicted to be transient. Thus, there might be a reduced need for social backlash from the unauthorized spread of self-propagating gene drives [Citation1, Citation47].
Conclusions
Gene drives have raised hopes as a potential means of achieving control over the spread of malaria. However, this must be approached with caution and environmental consideration. There is a need to develop local capacity on gene drives in areas where they are to be used. This will help improve their acceptance and uptake. Wide consultations should be conducted before any species is eliminated using gene drives. Additionally, educational programs should be widely conducted to ensure everyone understands the technology, its benefits, advantages and disadvantages. Any elimination of a species should therefore be supported by a deep, wide and critical analysis of costs and benefits inter alia. This can help strike a balance between controlling disease and protecting the environment.
Conflict of interest declaration
The authors have no financial or other association with persons or organizations that could have inappropriate influence on this paper or bias the contents of this research article.
Data availability
Data sharing not applicable – no new data generated.
Author contributions
All authors contributed sections of the paper, assisted in compilation of the sections and correction of the article.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Noble C, Adlam B, Church GM, et al. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife. 2018;7:e33423.
- Esvelt KM, Smidler AL, Catteruccia F, et al. Concerning RNA-guided gene drives for the alteration of wild populations. eLife. 2014;3:e03401.
- Beaghton A, Beaghton PJ, Burt A. Gene drive through a landscape: reaction-diffusion models of population suppression and elimination by a sex ratio distorter. Theor Popul Biol. 2016;108:51–69.
- Nolan T. Control of malaria-transmitting mosquitoes using gene drives. Philos Trans R Soc Lond B Biol Sci. 2021;376(1818):20190803. https://doi.org/10.1098/rstb.2019.0803
- James S, Collins FH, Welkhoff PA, et al. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in Sub-Saharan Africa: recommendations of a Scientific Working Group. Am J Trop Med Hyg. 2018;98(6_Suppl):1–49.
- Marshall JM, Buchman A, Sanchez HM, et al. Overcoming evolved resistance to populationsuppressing homing-based gene drives. Sci Rep. 2017;7(1):3776.
- Champer J, Buchman A, Akbari OS. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet. 2016;17(3):146–159.
- Hayirli TC, Martelli PF. Gene drives as a response to infection and resistance. Infect Drug Resist. 2019;12:229–234.
- Hammond AM, Galizi R. Gene drives to fight malaria: current state and future directions. Pathog Glob Health. 2017;111(8):412–423.
- Hoermann A, Tapanelli S, Capriotti P, et al. Converting endogenous genes of the malaria mosquito into simple non-autonomous gene drives for population replacement. eLife. 2021;10:e58791.
- Crans WJ. A classification system for mosquito life cycles: life cycle types for mosquitoes of the northeastern United States. 2004. Available from: https://pdfs.semanticscholar.org/0a2d/3db372987ef00d954a52ca42ae0ed94002e5.pdf.
- Waldbauer G. The handy bug answer book. Visible Ink Press; 1998. Available from: http://agris.fao.org/agris-search/search.do?recordID=US201300067714. Accessed on 25 October 2021.
- Lundkvist E, Landin J, Jackson M, et al. Diving beetles (Dytiscidae) as predators of mosquito larvae (Culicidae) in field experiments and in laboratory tests of prey preference. Bull Entomol Res. 2003;93(3):219–226.
- Hubalek J, Halouzka J. West Nile fever – a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5(5):643–650.
- Rueda LM. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue virus transmission. Zootaxa. 2004;589(1):1–60. https://doi.org/10.11646/zootaxa.589.1.1.
- Kindhauser MK, Allen T, Frank V, et al. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94(9):675–686C.
- Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372(13):1231–1239.
- Gubler DJ. Human arbovirus infections worldwide. Ann N Y Acad Sci. 2006;951(1):13–24.
- Cartolovni A. Teilhard de Chardin’s oeuvre within an ongoing discussion of a gene drive release for public health reasons. Life Sci Soc Policy. 2017;13(1):18. https://doi.org/10.1186/s40504-017-0064-8.
- Stark KR, James AA. Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito, Aedes aegypti. J Biol Chem. 1998;273(33):20802–20809.
- Ha YR, Oh SR, Seo ES, et al. Detection of heparin in the salivary gland and midgut of Aedes togoi. Korean J Parasitol. 2014;52(2):183–188.
- Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102(5):400–408.
- Chen L, He Z, Qin L, et al. Antitumor effect of malaria parasite infection in a murine lewis lung cancer model through induction of innate and adaptive immunity. PLos One. 2011;6(9):e24407.
- Yang Y, Liu Q, Lu J, et al. Exosomes from Plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis. 2017;6(6):e351–e351.
- Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211.
- Eckhoff PA, Wenger EA, Godfray CJ, et al. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc Natl Acad Sci USA. 2017;114(2):E255–E264.
- Ménard D, Khim N, Beghain J, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374(25):2453–2464.
- Barnes KG, Weedall GD, Ndula M, et al. Genomic footprints of selective sweeps from metabolic resistance to pyrethroids in African malaria vectors are driven by scale up of insecticide-based vector control. PLoS Genet. 2017;13(2):e1006539. https://doi.org/10.1371/journal.pgen.1006539.
- Wang S, Jacobs-Lorena M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013;31(3):185–193.
- Pike A, Dong Y, Dizaji NB, et al. Changes in the microbiota cause genetically modified anopheles to spread in a population. Science. 2017;357(6358):1396–1399.
- Lovett B, Bilgo E, Milogo SA, et al. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science. 2019;364(6443):894–897.
- Carvalho DO, McKemey AR, Garziera L, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9(7):e0003864.
- Swale DR, Engers DW, Bollinger SR, et al. An insecticide resistance-breaking mosquitocide targeting inward rectifier potassium channels in vectors of Zika virus and malaria. Sci Rep. 2016;6:36954.
- European Network of Scientists for Social and Environmental Responsibility. Gene drives: a report on their science, applications, social aspects, ethics and regulations. Dressel H, editor. 2019. p. 34 [cited 2021 Oct 7]. https://www.Gene-Drive-Report-2019-WEB.pdf.
- Kyrou K, Hammond AM, Galizi R, et al. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol. 2018;36(11):1062–1066.
- Noble C, Min J, Olejarz J, et al. Daisy-chain gene drives for the alteration of local populations. Proc Natl Acad Sci USA. 2019;116(17):8275–8282.
- Scudellari M. Self-destructing mosquitoes and sterilized rodents: the promise of gene drives. Nature. 2019;571(7764):160–162.
- Deredec A, Burt A, Godfray HCJ. The population genetics of using homing endonuclease genes in vector and pest management. Genetics. 2008;179(4):2013–2026.
- Burt A, Coulibaly M, Crisanti A, et al. Gene drive to reduce malaria transmission in Sub-Saharan Africa. J Responsible Innov. 2018;5(sup1):S66–S80.
- Sreenivasamurthy SK, Dey G, Ramu M, et al. A compendium of molecules involved in vector pathogen interactions pertaining to malaria. Malar J. 2013;12(216):216.
- Catteruccia F, Benton JP, Crisanti A. An anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23(11):1414–1417.
- Papathanos PA, Windbichler N, Menichelli M, et al. The vasa regulatory region mediates germline expresiion and maternal transmission of proteins in malaria mosquito Anopheles gambiae: a versatile tool for genetic control strategies. BMC Mol Biol. 2009;10(65):65.
- Galizi R, Doyle LA, Menichelli M, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat Commun. 2014;5:3977. https://doi.org/10.1038/ncomms4977.
- Marshall JM, Akbari OS. Gene drive strategies for population replacement. In: Adelman ZN, editor. Genetic control of malaria and dengue. London (UK): Academic Press; 2016. p. 169–200.
- Tu Z, Li S. Mobile genetic elements of malaria vectors and other mosquitoes. 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6186/
- Benetta ED, Akbari OS, Ferree PM. Sequence expression of supernumerary B chromosomes: function or fluff? Genes. 2019;10(2):123. Accessed on 25 October 2021.
- Funk C, Rainie L. 2015. Public and scientists’ views on science and society. https://www.researchgate.net/publication/279513537_Public_and_Scientists’_Views_on_Science_and_Society. Accessed on 25 October 2021.