 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Evidence suggests that the non-psychotropic phytocannabinoid cannabidiol (CBD) has anticancer activity on different cell lines and in animal models. The DNA-damaging drug epirubicin (EPI) is widely used for the treatment of malignant tumours. In this study we examined the effectiveness of CBD administered alone and in combination with EPI on HL-60, HL-60/Dox and MCF-7 cell-lines. We applied MTT reduction assay and the bioinformatics model of Chou-Talalay for experimental design of combination chemotherapy to evaluate the anticancer activity of several epirubicin-based combinations with CBD in various treatment regimens. In addition, we studied the efficacy of the co-treatment regimens to induce changes in two in-vitro model systems containing superoxide radical and 2-deoxyribose. We then evaluated the effectiveness of EPI plus CBD. The maximal growth cell inhibition ∼90% in leukemic and breast cancer cells was measured at the peak tested concentrations 2.5 EPI plus 25 CBD, µmol/L. The combination index (CI) value showed the presence of slightly expressed antagonism between EPI and CBD in most cases. The antiproliferative effect of the combination was reduced by only 8–10% vs. the inhibition induced by either EPI or CBD alone. We determined that the slight antagonism correlated positively to the registered dosage and decreased the extent of deoxyribose oxidative damage by using the enhanced chemiluminescent method with superoxide radicals. The therapeutic value of the combination of EPI plus CBD mainly consists of its high antiproliferative effect and the possibility to reduce the total dose on leukaemia-transformed cells by a factor of two.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1996270 .
Introduction
Cannabidiol (CBD) and CBD-containing products/preparations are widely used for various medical conditions in many European countries and in the United States [Citation1, Citation2]. At present, it is commonly accepted that CBD has antiproliferative, antiinvasive and antimetastatic properties in models of breast cancer, malignant brain tumours, lung cancer and multiple myeloma [Citation3–10]. It has been established that the anticancer properties of cannabinoids are associated with the G protein-coupled receptor 55 and others [Citation11, Citation12]; the vanilloid ion channels TRPV-1/2 [Citation13, Citation14]; and the cannabinoid receptors [Citation15]. Also, many signal molecules (Bid, Id-1) and pathways are involved in CBD’s anticancer activity [Citation11, Citation16]. Researchers suppose that a specific up- and down-regulation exists for increased production of ceramide and apoptosis.
Experimental in vitro data on combination treatments with CBD and cytostatics is limited, and often obtained by way of disparate methodical approaches. A synergistic effect of CBD with tamoxifen has been observed on MCF-7 and HL-60 cells [Citation17]; with paclitaxel on gastric and colon cancer cells [Citation18]; with temozolomide on glioma cell lines [Citation19]; with proteasome inhibitors Bortezomib, Carfilzonib [Citation10, Citation20]. In contrast to these data, Deng et al. [Citation21] have demonstrated the antagonism between CBD and BCNU and cisplatin (CDDP) on glioblastoma cells.
Hereby we have studied the tumor cell inhibiting effect of CBD alone and in combination with epirubicin at varying and fixed-ratio concentrations, as well as their potency to induce oxidative molecular damage.
Materials and methods
Drugs and chemicals
Cannabidiol (CBD) was obtained from Sigma-Aldrich (CAS No. 13956-29-1), and the substance was dissolved in 90% ethanol to sol. 1.0 mg/mL, immediately before use. Epirubicin (EPI) as Farmorubicin (fl. 2 mg/mL) was obtained from Pfizer and diluted in dimethyl sulfoxide (DMSO) to a final concentration of 0.1% to sol. 1.0 mg/mL.
MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromid] (CAS No. 298-93-1) was obtained from Genaxxon; xanthine oxidase (XO), xanthine (X), 2-D-deoxyribose and all other substances used in the oxidative assays (presented under the correlating figures) were obtained from Sigma-Aldrich.
Cell lines and culture conditions
The promyelocytic leukaemia HL-60 cell line and its corresponding multidrug resistant HL-60/Dox, and MCF-7 breast cancer cell line were obtained from DSMZ GmbH (German Collection of Microorganisms and Cell Cultures). Cell cultures were maintained within the exponential phase in a humidified atmosphere of 95% air, 5% CO2 at 37 °C. All cell lines were cultured in RPMI-1640 medium supplemented by 10% heat-inactivated foetal bovine serum, L-glutamine (final concentration 2.5 mmol/L) and antibiotics (100 IU/mL penicillin and 100 µg/mL streptomycin). Cell density was maintained between 105 and 106 cells/cm2.
Drug treatment
CBD only
At first, each cell type was treated with CBD in concentrations ranging from 6.25 to 100 µmol/L in order to define cell viability based on absorption detected by the MTT-reduction assay.
Combination study: median effect analysis
Cells from the HL-60, HL-60/Dox and MCF-7 cell lines were reset in fresh culture medium and aliquot into 96-well plates. CBD at concentrations of 6.25, 12.5 and 25 µmol/L and epirubicin at ten times lower concentrations were applied alone and in combination at varying, and fixed-ratio concentrations (EPI:CBD = 1:10). Antiproliferative activity was determined after 72 h exposure by means of MTT assay, and combination index (CI) values were calculated based on the median-effect (Fa) equation [Citation22, Citation23]. CI < 0.9 denotes synergism, CI = 0.9–1.10 denotes additive interaction and CI > 1.10 denotes antagonism.
Combination study: pre-treatment with CBD
The MCF-7 cells were treated with CBD for the initial 24 h, the medium containing the drug was removed and the cells were rinsed with phosphate buffered saline (PBS) and treated with EPI for 48 h. The combined effect was assessed after 72 h exposure (total).
Combination study: modulatory effect
The ability of CBD to modify the effectiveness of EPI was studied by comparative assessment of the ED50, ED75 and ED90 values of EPI in the absence or presence of CBD. Dose reduction index (DRI) values for each drug were calculated based on computer-simulated data on the concentration-effect dependencies associated with the median effect (Fa) obtained by the two drugs applied in different regimens. If the value is greater than 1, a favourable reduction of the dose is possible. The equi-effective concentrations of CBD, EPI and their combinations were also determined.
Cell proliferation and viability assay
The MTT-based method modified slightly by Konstantinov et al. was utilized [Citation24, Citation25]. Cells were seeded into 96-well plates at a density of 3 × 105 cells/cm2 for the leukemic cells and 1.5 × 105 for MCF-7 cells and treated with CBD and EPI alone and in combination. Cell viability was determined by adding MTT (10 mg/mL in PBS) and the absorbance was measured after 72 h treatment. Absorption was measured at 540 nm by an automated microtiter plate spectrophotometer (Labexim LMR-1, Lengau, Austria).
Deoxyribose degradation assay
The potency of the [EPI + CBD] combination to modulate ferrous-induced 2-deoxyribose degradation was assessed in accordance with Sadowska-Bartosz et al. [Citation26]. The reaction initiating agent FeCl2 (0.1 mmol/L) was added to the samples containing 2-deoxyribose (0.5 mmol/L) in the absence/presence of different concentrations of EPI applied alone and in combination with CBD at fixed-ratio concentrations 1:10. In the control sample both substances under investigation were omitted. All samples were incubated at 37 °C for 30 min. Then after adding 0.5 mL of 2.8% trichloroacetic acid and 0.5 mL of thiobarbituric acid the samples were heated in a boiling water bath at 100 °C for 20 min. All test tubes were centrifuged at 3000 rpm for 20 min (Centrifuge Janetzky K23). The absorbance at 532 nm was measured. The observed oxidative damage referred to as ‘molecular damage’ was presented as a percentage of the control sample.
Xanthine/xanthine oxidase assay
The level of superoxide anion radical generated in a xanthine/xanthine oxidase (X/XO) model system was determined after adding the combination EPI + CBD by way of luminol-dependent chemiluminescence (LDCL) [Citation27, Citation28]. First, 25.4 mU/mL xanthine oxidase was dissolved in 50 mmol/L PBS with pH = 7.45. A 3 mmol/L solution of xanthine was prepared by dissolving the substance in 0.1 mol/L NaOH and further diluting with bi-distilled water. Results were presented as a chemiluminometric scavenging index (CL-SI), calculated according to the formula:
where Isample and Icontrol are the integral intensities, measured in the presence and in the absence of the investigated substances.
Statistical analyses and software programs
All statistical analyses were performed using GraphPad Prism® 6.0 or MS Excel 2010. p < 0.05 was considered statistically significant. Differences between treatment and control groups were determined by one-way analysis of variance (ANOVA) with Bonferroni post-test. Calculations of CI, DRI and equieffective concentrations were provided on the basis of the median effect (Fa) by means of CompuSyn software (Biosoft, Cambridge, UK). Results from the combination treatment were analyzed against the drugs applied on their own in the same experiment. Data values were presented as means or in most cases with SDs from at least three independent experiments.
Results
CBD alone
Three types of tumour cells were exposed to different concentrations (1–100 μmol/L) of CBD alone for 72 h. Cell proliferation inhibition was measured by the MTT-cell growth assay ().
Figure 1. Dose-response curves of human cell lines HL-60, HL-60/Dox and MCF-7 cells after 72 h exposure to CBD.
Note: Data are expressed as means in three independent experiments; SD have been omitted for clarity; CBD, cannabidiol.
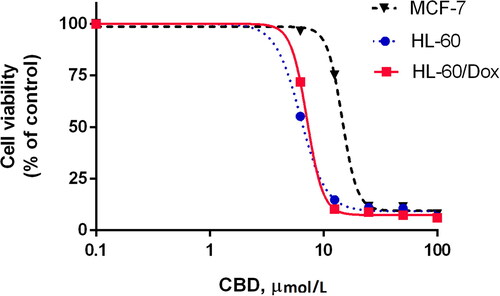
CBD showed antitumor properties within a wide range of concentrations: from 12.5 to 100 μmol/L for the leukemic cells, and above 25 μmol/L for MCF-7. The dose-response curves of CBD had relatively identical characteristics for the three types of tumour cell lines (). It seems that the sensitivity of leukemic cells is greater than that of breast cancer cells. Maximum effect (∼80–90% inhibition) was achieved at a wide range of concentrations which were 7 to 15 times greater than the IC50 of CBD. The IC50 values were equal to 6.62 μmol/L and 7.49 μmol/L for the sensitive and resistant leukemic cells, respectively, and 14.59 μmol/L for MCF-7 cells.
CBD inhibited the resistant HL-60/Dox cells in the same manner (with similar potency and effectiveness as the sensitive leukemic cells), and profoundly reduced the viability of the MDR-1 resistant cells at concentrations between 12.5 and 100 μmol/L. These results are indicative of no cross resistance between CBD and EPI.
Combination study: median tumour-cell inhibiting effect and drug–drug interaction
In these experiments, CBD and EPI were applied alone and in combination simultaneously or with pre-treatment at varying and fixed-ratio concentrations under the same experimental conditions ().
Figure 2. Cytotoxicity of CBD + EPI in leukemic cell lines HL-60 (a), HL-60/Dox (b), and breast cancer cells MCF-7: applied simultaneously (c) and in a drug sequence: CBD 24 h followed by EPI 48 h (d).
Note: Data are presented as tumor inhibition (T/C,%) ean ± SD *Legend: [
 ] CBD; [
] CBD; [ ] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.
] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.![Figure 2. Cytotoxicity of CBD + EPI in leukemic cell lines HL-60 (a), HL-60/Dox (b), and breast cancer cells MCF-7: applied simultaneously (c) and in a drug sequence: CBD 24 h followed by EPI 48 h (d).Note: Data are presented as tumor inhibition (T/C,%) ean ± SD *Legend: [Display full size] EPI; [Display full size] CBD; [Display full size] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.](/cms/asset/3205c245-d7ce-4663-bd01-15999c6c8fca/tbeq_a_1996270_f0002_b.jpg)
Effect on cell viability
Comparative analysis of the results for cell inhibition obtained on HL-60 leukaemia cells shows that only the sub-effective concentrations of CBD and EPI applied simultaneously exhibit an effect ∼50% which is greater than or equal to the effect of the drugs applied alone (). At the highest concentrations (2.5 EPI + 25 CBD, μmol/L) 77% inhibition was observed being 13–21% lower than that of the drugs applied alone. The multi-resistant HL-60/Dox cells did not respond to the treatment of EPI. In contrast, CBD successfully inhibited cell growth in a dose-dependent manner and achieved a maximum effect of 91% inhibition at 25 μmol/L – the same as the combination’s, supposedly due solely to CBD (). The results on MCF-7 cells () indicate that the combination treatment at low concentrations (0.625 EPI + 6.25 CBD, μmol/L) is not effective. The maximal dose-response of these cells (∼90% inhibition) was obtained at the highest tested concentrations (27.5 μmol/L in total), that being 4–8% more than the inhibition of the two drugs alone. As shown in , 77% inhibition was reached by pre-treatment with CBD (24 h) followed by EPI (48 h), meaning that there is no improvement in the effectiveness of the combination in comparison to the simultaneously applied drugs. These results suggest that such a drug sequence is not preferable as it diminished the effect of the two drugs applied simultaneously.
Interaction between CBD and EPI
and represent the calculated CI values at varying and fixed-ratio concentrations. In most cases, CBD plus EPI exhibited antagonism on the tested leukemic and breast cancer cells (CI > 1.1). An exception was observed for HL-60/Dox-resistant cells at the highest concentrations of EPI (2.5 μmol/L) where the effect was assessed as additive (CIs from 0.88 to 0.97). CI values of EPI in combination with CBD at fixed-ratio concentrations of 1:10 also had a value greater than one in most cases. An exception was observed only at the highest total concentrations when the effect of the combination on HL-60/Dox was 90–95%; and at the lowest concentrations when the inhibition on HL-60 was less than 50% (CI values 0.9 to 1.10). In these cases, the effect observed was evaluated as additive. The results of the antagonistic interactions between CBD and EPI and additive effect in some cases were confirmed by isobolograms (not shown here).
Table 1. Combination index (CI) values of epirubicin in combination with varying concentrations of cannabidiol on three tumor cell lines*.
Table 2. Combination index (CI) values of epirubicin plus cannabidiol at fixed-ratio concentrations of 1:10 on three tumor cell lines*.
Dose modulating effect and DRI values
The DRI values of CBD on HL-60 and MCF-7 cells are greater than one for all treatment regimens (). But the concentration of EPI is an important factor for a more plausible effect, namely EPI needed to be ≤1.39 μmol/L on the HL-60 leukaemia cells, and ≥1.90 μmol/L on MCF-7 breast cancer cells (in these cases, DRIEPI > 1). It seems that the fixed-ratio combinations at a total dose of ∼20–26 μmol/L may achieve 80% inhibition when the drugs are applied simultaneously in an EPI-to-CBD ratio of 1:10.
Table 3. Dose reduction index (DRI) values of EPI and CBD at fixed-ratio concentrations of 1:10 on HL-60 and MCF-7 cells*.
The comparative analysis of the results for equi-effective concentrations of CBD, EPI and their combinations showed that 50% inhibition of HL-60 cell growth was achieved at combination concentrations two times smaller compared to those of the drugs applied alone. However, the same effect was observed neither on MCF-7 cells nor at 90% inhibition on both cell lines (no significant differences existed between the concentrations of CBD and EPI alone and in combination). These results indicate that CBD has a modulating effect only when applied on the sensitive HL-60 leukaemia cells; however, such effect is not observed in higher concentrations, and on MCF-7 cells.
Impact of CBD, EPI and their combination on oxidative damage of 2-deoxyribose
The model of 2-D-deoxyribose (Dr) – containing system with a standard iron-oxidizing agent was used to define the extent of oxidative molecular damage, induced by EPI and CBD, applied alone or simultaneously at the fixed-ratio concentrations of 1:10 (). Neither sample manifested a decrease in the absorbance values compared to the control, suggesting a lack of a protective effect. The samples containing CBD did not show an increase in the absorbance values compared to the controls, meaning CBD exerts an insignificant impact on the processes in the system. The effect of EPI and the combination EPI + CBD was dose-dependent: an elevated extent of molecular damage was observed when applied in higher concentrations. As shown in , the lowest concentrations were not effective: neither the drugs nor the CBD + EPI combination had a significant impact on the ferrous-induced oxidative degradation. The estimated molecular damage in the samples containing EPI + CBD at a total dose of 13.75 µmol/L and 27.5 µmol/L was, respectively 112% and 119% – significantly higher compared to the control group. The maximum deoxyribose damage was observed in the sample containing only EPI at a concentration of 2.5 µmol/L: it was 30% higher than that of the controls.
Figure 3. Degree of 2-deoxyribose (DR) oxidative damage in the absence or presence of different concentrations of EPI and CBD, applied by themselves or simultaneously at fixed-ratio concentrations of 1:10.
Note: Data are presented as percentage of molecular damage, mean ± SD. Groups were compared by one-way ANOVA with Bonferroni post hoc test. Asterisks indicate statistically significant differences versus the control: **p < 0.01, ***p < 0.001 [
 ] CBD; [
] CBD; [ ] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.
] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.![Figure 3. Degree of 2-deoxyribose (DR) oxidative damage in the absence or presence of different concentrations of EPI and CBD, applied by themselves or simultaneously at fixed-ratio concentrations of 1:10.Note: Data are presented as percentage of molecular damage, mean ± SD. Groups were compared by one-way ANOVA with Bonferroni post hoc test. Asterisks indicate statistically significant differences versus the control: **p < 0.01, ***p < 0.001 [Display full size] EPI; [Display full size] CBD; [Display full size] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.](/cms/asset/cc1183f1-2437-4506-bf30-2955e0716f49/tbeq_a_1996270_f0003_b.jpg)
Impact of the CBD + EPI combinations on the LDCL in the presence of X/XO-generated superoxide
The effect of the various CBD + EPI combinations on LDCL in the presence of the X/XO model system is shown in , presented as CL-SI (see section ‘Materials and Methods’). All five investigated combinations of EPI + CBD behaved as scavengers of X/XO-generated superoxide. Mean values of CL-SI ranged between 29.9% and 80.2%. Within the three sample groups, containing CBD at the same concentration (6.25 µmol/L), a statistically significant decrease of CL-SI was noted as the concentration of EPI increased to 5 µmol/L. It could be concluded that increasing the concentration of EPI resulted in diminished X/XO-generated superoxide levels. But no statistically significant difference was observed when comparing sample data with the same concentration of EPI (1.25 µmol/L) and different concentrations of CBD (6.25 µmol/L and 12.5 µmol/L). The combination of (2.5 EPI + 25 CBD, µmol/L) is associated with the greatest decrease (with ∼70%) in the CL-SI, which in turn corresponds to the greatest decrease in superoxide level, compared to the control. That may be due to: (1) direct scavenging of superoxide by CBD and/or EPI; or (2) interaction between CBD and/or EPI and the components of the X/XO-generating system.
Figure 4. Impact of the investigated combinations CBD + EPI on the luminol-dependent chemiluminescence in the presence of xanthine/xanthine oxidase-generated superoxide.
Note: Data are presented as means ± SD. Three parallel measurements were performed for each composition (n = 3), each representing an individual data point. The baseline was subtracted from both control and sample measurements: (1) Control measurement – 0.1 mL xanthine solution, 0.05 mL xanthine oxidase solution (2.9 mU), 0.1 mL luminol, 0.75 mL PBS; (2) Sample measurement – 0.1 mL xanthine solution, 0.05 mL xanthine oxidase solution (2.9 mU), 0.1 mL luminol, the necessary volumes of stock CBD and EPI solutions, PBS up to 1.0 mL; (3) Baseline sample measurement – 0.1 mL xanthine, 0.1 mL luminol, 0.8 mL PBS; [
![Figure 4. Impact of the investigated combinations CBD + EPI on the luminol-dependent chemiluminescence in the presence of xanthine/xanthine oxidase-generated superoxide.Note: Data are presented as means ± SD. Three parallel measurements were performed for each composition (n = 3), each representing an individual data point. The baseline was subtracted from both control and sample measurements: (1) Control measurement – 0.1 mL xanthine solution, 0.05 mL xanthine oxidase solution (2.9 mU), 0.1 mL luminol, 0.75 mL PBS; (2) Sample measurement – 0.1 mL xanthine solution, 0.05 mL xanthine oxidase solution (2.9 mU), 0.1 mL luminol, the necessary volumes of stock CBD and EPI solutions, PBS up to 1.0 mL; (3) Baseline sample measurement – 0.1 mL xanthine, 0.1 mL luminol, 0.8 mL PBS; [Display full size] EPI + CBD; EPI, epirubicin; CBD, cannabidiol.](/cms/asset/e8792fd7-372f-4e33-be7f-526872fe7d07/tbeq_a_1996270_f0004_c.jpg)
Discussion
Cannabidiol (CBD) as an investigational new drug is of great interest to many researchers, specifically regarding its pharmacological effects, mechanisms of action and possible applications for various diseases and disorders. Despite the great number of pre-clinical studies and clinical trials of CBD, many aspects of its mechanisms have not been sufficiently clarified.
Our study explored the antiproliferative effect of CBD and its combination with epirubicin on three tumour cell lines. We were also particularly interested in examining the potency of this drug combination in inducing oxidative changes.
The initial experiments evaluated the activity of CBD alone based on MTT-reduction assay. The results obtained on the HL-60, HL-60/Dox and MCF-7 cell lines confirmed previous data reported by other investigators evaluating the anticancer activity of CBD in various in vitro and animal tumour models [Citation3–10]. The comparative analysis of the kinetic dose-response curves proves a greater sensitivity of leukemic cells to CBD compared to estrogen-positive MCF-7 breast cancer cells. This may be due to the higher expression of CB2 receptors in leukaemia-transformed cells or to differences in the intrinsic pathways of intracellular signalling as shown by Scott et al. [Citation29]. In our study we evaluated that CBD hinders the development of HL-60/Dox-resistant cells containing the MRP1- and MDR1-gene, and affects the other two investigated cell lines (HL-60 and MCF-7) in a similar manner. That means that CBD might be effectively administered to cancer patients that have resistance to anthracyclines or other cytostatic agents. It is interesting that the dose-dependent inhibitory effect of CBD has some specific characteristics: the lowest concentrations are non-effective, but the concentrations that are 7 to 15 times greater than the IC50 values are very and equally effective (∼86–89% inhibition) within a wide range of concentrations (from 25 to 100 μmol/L). At such concentrations the cytostatic effect of CBD transforms into cytotoxic effect. Our results in this aspect correspond to data by other researchers about differences between antiproliferative and cell-killing effects of the phytocannabinoids (CANNs) [Citation29, Citation30]. In addition, a clinical trial on brain tumours shows better effectiveness of CANNs and CBD at higher doses [Citation31], as it was observed in our experiments.
As a second step, we evaluate the anticancer activity of EPI-based combinations with CBD using the bioinformatic model of Chou-Talalay [Citation22, Citation23]. It is well known that the purpose of combining drugs in a therapeutic regimen is to achieve a synergistic effect. The results from our combination study indicate an antagonistic interaction between CBD and EPI at a significant number of treatment schedules (CI > 1). However, no major loss of the overall drug effectiveness has been observed. The combination treatment of HL-60 leukaemia cells lowered the tumour cell inhibition by ∼10–20% in comparison to CBD and EPI alone. The simultaneously applied drugs at higher concentrations never fell under 77% of inhibition. The antiproliferative effect of the combination on the MCF-7 breast cancer cells at the highest tested concentrations was approximately equal to that of CBD and EPI alone, despite the antagonism between the two drugs (CIs > 1). We could estimate treatment regimens with 2.5 μmol/L EPI and 10 times higher CBD concentration as highly effective, but they are not evaluated as synergistic according to the classical understanding. Our results correspond to data reported by Deng et al. demonstrating an antagonism between CBD and the DNA-damaging agents BCNU and CDDP on glioblastoma cells [Citation21]. The results on MCF-7 with CBD pre-treatment (24 h) followed by EPI (48 h) are similar to those with simultaneously applied agents, meaning that this drug sequence cannot be preferred when it comes to optimizing treatment schedules. In addition, we consider that CBD at the tested concentrations applied for a short time does not improve the intracellular influx of EPI, as it is obtained for CANNs at the highest doses and for a long duration [Citation32, Citation33]. Other authors demonstrate higher cytotoxicity of CANNs when applied in the ‘recovery’ phase of the cell cycle, i.e. CANNs use after chemotherapy [Citation29, Citation30]. It seems that the drug sequence in the combination treatment regimen with CBD and other CANNs plays a crucial role in a possible negative interaction with classical cytostatics.
Combination treatments can be assessed as clinically attractive not only because of an overall increase of anticancer activity, but also because of a possibility for reduction of the doses of individual drugs. It is well known that a major problem of EPI is cardiotoxicity in high-dose chemotherapy [Citation34]. Studying the results on HL-60 leukaemia cells demonstrates a possibility to use a lower concentration of EPI (see and ) when it is combined with a wide range of CBD concentrations (DRIs > 1). This proposition is confirmed by the calculated ED50 value of the combination with a fixed-ratio concentration of 1:10 reducing the ED50 of EPI and CBD alone by a factor of two. A similar reduction was not observed on the MCF-7 cells and for combinations reaching 75% and 90% inhibition.
Table 4. Equieffective concentrations of EPI, CBD and their combination at fixed-ratio concentrations of 1:10 after 72 h exposure of HL-60 and MCF-7 cells, calculated using computer-simulated dose-response relationship*.
In recent years, a hypothesis posits the importance of reactive oxygen species (ROS) for the anticancer activity of CBD, associated with autophagy and ferroptosis [Citation4, Citation6, Citation35, Citation36]. In this relation, we defined the impact of CBD + EPI combinations on oxidative damage of 2-deoxyribose and in the presence of X/XO-generated superoxide.
The results from the deoxyribose assay followed the basic characteristics of the specific tendency of the antiproliferative effect of the drugs and their combinations, namely lack of effect on deoxyribose (Dr) damage in the concentrations where cell viability assays denoted effect near the criteria for activity, and maximal Dr-damaging effect exerted by EPI when applied by itself at the maximum tested concentration followed by a combination treatment (2.5 EPI + 25 CBD, μmol/L). This assay is characterized by certain complexity due to the fact that products of multiple simultaneous processes in the system compete for the same oxidizable substrate: (1) Fe(II) induced degradation under aerobic conditions associated with the auto-oxidation of Fe2+ leading to formation of superoxide [Citation26]; (2) the anthracycline used in the system has been proven to be capable of generating oxygen free radicals after being mixed with Fe (II) [Citation37]; and (3) the Fe(III) generated during (1) and (2) could exert direct degradation effects on the deoxyribose molecules [Citation38] or (4) form complexes with epirubicin after self-reduction mechanisms of intermolecular electron transfer associated with the oxidation of the a-ketol group as well as subsequent formation of hydrogen peroxide [Citation39]. The latter generates the extremely reactive hydroxyl radical after interaction with Fe(II).
In the X/XO model system a significant decrease of CL-SI value was observed when increasing the CBD + EPI concentrations to 27.5 µmol/L total. The observed effect could not possibly be attributed to EPI only. Despite the fact that anthracyclines (though not specifically epirubicin) have been found to be substrates of XO [Citation40], it seems that the presence of 25 µmol/L CBD dramatically lowers CL-SI. A study by Atalay et al. has demonstrated that CBD decreases the activity of XO in UV-irradiated keratinocytes [Citation41]. Also, a modulation effect of CBD on the activity of NADPH oxidase (NOX1 and NOX4) and other ROS scavenging enzymes has been described [Citation41, Citation42]. In this regard, we suggest that CBD impairs XO activity and may be a cause of the observed decrease of CL-SI. The results concerning the scavenging activity of CBD in the X/XO model system correspond with the proposed antagonism between the two drugs.
Data from both radical-generating model systems fully support earlier quantitative estimates of the antiproliferative activity of EPI-based combinations with CBD, applied simultaneously at varying and fixed-ratio concentrations.
Conclusions
The present study supports the idea that the non-psychotropic cannabinoid CBD can be used by itself or in combination as an agent for cancer chemotherapy. Our investigations on sensitive tumour cell lines HL-60 and MCF-7 provided evidence of high cell inhibiting activity of CBD by itself and in combination with epirubicin. The effectiveness of CBD on the resistant HL-60/Dox cell line reveals its effective application as an agent on its own on tumours with MDR1- and MRP1- resistance gene. The combination study with epirubicin and CBD reveals a great potential to achieve high antiproliferative activity on sensitive tumour cells despite a slightly expressed antagonism between the two drugs, when applied simultaneously. Data from the deoxyribose and X/XO model systems confirm that altered ROS generation and modified cell redox status could be among the important factors associated with negative modulation of the drugs’ inhibiting effect. Further investigations are needed to better understand the interactions between CBD and DNA-damaging agents and to develop the best strategy for effective treatments of different malignant tumours.
Disclosure of interest
No potential conflict of interest was reported by the authors
.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Kirilov B, Zhelyazkova M, Petkova-Gueorguieva E, et al. Regulation and marketing of cannabidiol-containing products in European countries. Pharmacists’ knowledge in Bulgaria. Biotechnol Biotechnol Equip. 2020;34(1):1158–1165.
- Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3(1):152–161.
- McAllister SD, Murase R, Christian RT, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129(1):37–47.
- Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–1172.
- Elbaz M, Nasser MW, Ravi J, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of cannabidiol in breast cancer. Mol Oncol. 2015;9(4):906–919.
- Massi P, Vaccani A, Bianchessi S, et al. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63(17):2057–2066.
- Russo EB. Cannabis therapeutics and the future of neurology. Front Integr Neurosci. 2018;12:51–51.https://doi.org/10.3389/fnint.2018.00051
- Ramer R, Bublitz K, Freimuth N, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. Faseb J. 2012;26(4):1535–1548.
- Ramer R, Heinemann K, Merkord J, et al. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther. 2013;12(1):69–82.
- Morelli MB, Offidani M, Alesiani F, et al. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int J Cancer. 2014;134(11):2534–2546.
- Pyszniak M, Tabarkiewicz J, Łuszczki JJ. Endocannabinoid system as a regulator of tumor cell malignancy – biological pathways and clinical significance. Oncol Targets Ther. 2016;9(9):4323–4336.
- Laun AS, Song ZH. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun. 2017;490(1):17–21.
- Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131–1141.
- Neumann-Raizel H, Shilo A, Lev S, et al. 2-APB and CBD-mediated targeting of charged cytotoxic compounds into tumor cells suggests the involvement of TRPV2 channels. Front Pharmacol. 2019;10:1198.
- Straiker A, Dvorakova M, Zimmowitch A, et al. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol. 2018;94(1):743–748. https://doi.org/10.1124/mol.118.111864
- Bih CI, Chen T, Bazelot M, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699–730.
- Nathan I, Vogel Z, Uppalapati LN, et al. 2018. Compositions comprising cannabidiol and second therapeutic agents for the treatment of cancer. U.S. Patent and Trademark Office. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=US232561348
- Aviello G, Romano B, Borrelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl). 2012;90(8):925–934.
- Torres S, Lorente M, Rodriguez-Fornes F, et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10(1):90–103.
- Strong T, Rauvolfova J, Jackson E, et al. Synergistic effect of cannabidiol with conventional chemotherapy treatment. Blood. 2018;132:1(Supplement 1):5382–5382.
- Deng L, Ng L, Ozawa T, et al. Quantitative analyses of synergistic responses between cannabidiol and DNA-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J Pharmacol Exp Ther. 2017;360(1):215–224.
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446.
- Chou TC, Talalay P. Quantitive analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Konstantinov SM, Eibl H, Berger MR. BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br J Haematol. 1999;107(2):365–374.
- Sadowska-Bartosz I, Galiniak S, Bartosz G. Modification of the deoxyribose test to detect strong iron binding. Acta Biochim Pol. 2017;64(1):195–198.
- Hadjimitova V, Traykov T, Mileva M, et al. Effect of some psychotropic drugs on luminol-dependent chemiluminescence induced by O2-, *OH, HOCl. Z Naturforsch C J Biosci. 2002;57(11–12):1066–1071.
- Radi R, Rubbo H, Thomson L, et al. Luminol chemiluminescence using xanthine and hypoxanthine as xanthine oxidase substrates. Free Radic Biol Med. 1990;8(2):121–126.
- Scott KA, Shah S, Dalgleish AG, et al. Enhancing the activity of cannabidiol and other cannabinoids in vitro through modifications to drug combinations and treatment schedules. Anticancer Res. 2013;33(10):4373–4380.
- Scott KA, Dalgleish AG, Liu WM. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int J Oncol. 2017;51(1):369–377.
- Likar R, Nahler G. The use of cannabis in supportive care and treatment of brain tumor. Neurooncol Pract. 2017;4(3):151–160.
- Holland ML, Panetta JA, Hoskins JM, et al. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71(8):1146–1154.
- Molnár J, Szabó D, Pusztai R, et al. Membrane associated antitumor effects of crocine-, ginsenoside- and cannabinoid derivates. Anticancer Res. 2000;20(2A):861–867.
- BC Cancer Agency. Epirubicin monongraph. Vancouver (BC): BC Cancer Agency; 2017.
- Solinas M, Massi P, Cinquina V, et al. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One. 2013;8(10):e76918.
- De Petrocellis L, Ligresti A, Schiano Moriello A, et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol. 2013;168(1):79–102.
- Grankvist K, Henriksson R. Doxorubicin and epirubicin iron-induced generation of free radicals in vitro. A comparative study. Biosci Rep. 1987;7(8):653–658.
- Genaro-Mattos TC, Dalvi LT, Oliveira RG, et al. Reevaluation of the 2-deoxyribose assay for determination of free radical formation. Biochim Biophys Acta. 2009;1790(12):1636–1642.
- Malisza KL, Hasinoff BB. Production of hydroxyl radical by iron(III)-anthraquinone complexes through self-reduction and through reductive activation by the xanthine oxidase/hypoxanthine system. Arch Biochem Biophys. 1995;321(1):51–60.
- Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact. 2000;129(1–2):195–208.
- Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2019;9(1):21.
- Gómez CT, Lairion F, Repetto M, et al. Cannabidiol (CBD) alters the functionality of neutrophils (PMN). Implications in the refractory epilepsy treatment. Pharmaceuticals. 2021;14(3):220.
