?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In recent decades, extensive research has been focused on the screening and investigating the antioxidant and anticancer activities of medicinal plants extracts or isolated natural product-based drugs. Therefore, the present study aimed to evaluate the phytochemical, antioxidant and in vitro anticancer activities of Bombax buonopozense extracts. Standard methods were used for the identification of alkaloids, tannins, flavonoids, coumarins, saponins, sterol, triterpenes and reducing compounds. Free-radical–scavenging activity was measured by the 2,2’-diphenyl-1-picrylhydrazyl (DPPH) free radical assay, and the cytotoxic potential was evaluated in the P815 murin lymphoblast-like mastocytoma cell line using the MTT assay. Regarding the antioxidant activity, our results showed a powerful in vitro free-radical scavenging activity of the ethanolic extract compared to the aqueous extracts, with IC50 values of 10 and 220 µg/mL, respectively. In the MTT assay, the ethanolic extract showed a moderate and dose-dependent association growth inhibition of P815 cells in a dose above of 200 µg/mL with IC50 of 74 µg/mL compared to cisplatin (IC50= 4 µg/mL). Our findings demonstrate that Bombax buonopozense ethanolic extract could be an effective antioxidant and promising antiproliferative agent.
Introduction
Herbal medicine is fast emerging as an alternative treatment to available synthetic drugs for cancer treatment as they are recognized to have less toxic side effects [Citation1,Citation2]. Therefore, herbal remedies have been widely attracting attention as a potential and prospective resource of treatment for several diseases across many civilizations [Citation3,Citation4]. As well known, numerous plant species have long been described to possess pharmacological activities and therapeutic value attributable to their phytochemical constituents such as alkaloids, flavonoids, terpenes, saponins, glycosides, tannins and steroids [Citation5–7]. In recent years, scientists have found that many tropical herbs have been scientifically reported to possess potent anticancer and anti-oxidant activities [Citation8,Citation9].
Bombax buonopozense is an important tropical medicinal tree widely distributed in Africa, with 40 m in height, tall buttresses (6 m), large conical spines, compound leaves with 5–9 leaflets, and branches organized in whorls [Citation10,Citation11]. In Nigeria for example, the floral part of Bombax buonopozense is used as a vegetable [Citation12]. Because of its therapeutic properties, the plant has been reported in the literature to possess enormous biological activities. Previous studies have reported its antimicrobial, antidiarrhoeic and antipyretic activities [Citation13–15]. Following the literature search, a rareness of scientific information regarding the biological activities of Bombax buonopozense was noted, especially the anticancer activity. Thus, the aims of the present study were to investigate the phytochemical profiles, the antioxidant and the in vitro anticancer activities of Bombax buonopozense stem bark extract. It is expected that the results established from this study will provide valuable insights into the pharmacological potential of B. buonopozense, therefore, validating and valorizing the traditional uses of this tropical plant.
Materials and methods
Plant collection
The bark of the stem of Bombax buonopozense, harvested in 2018 in Nkolbisson (the central region of Cameroon), was dried at room temperature and then crushed to obtain a fine powder.
Preparation of the extracts
As in numerous herbal preparations, water extraction was carried out by a decoction process [Citation16]. In the present study, the plant material extract was obtained by decoction and maceration using solvents of increasing polarity: hexane, ethyl acetate, ethanol and distilled water. Ten grams of bark powder was introduced into a 250 mL triple-necked flask, 100 mL of distilled water was added. The flask was surmounted by a cooler connected to a tap opened by a pipe. The mixture is maintained at a constant heating temperature for 1 h. After cooling, the mixture is filtered on Whatman paper, the filtrate obtained is displaced in the oven at a temperature of 55 °C, to give a yield of 8%. To do a comparative photochemical analysis of the different chemical groups of the bark powder extract, the maceration method with different polarities of solvents (ethanol, ethyl acetate and hexane) was chosen. In this extraction protocol, 25 g of the bark powder were subjected to maceration with magnetic stirring for 48 h in 125 mL of solvent. The mixture was filtered on a filter paper (Whatman), the filtrate was concentrated and dried with a rotary evaporator. The organic total extract was obtained and give a yield of 18%.
Phytochemical analysis
This qualitative test based on colour reactions and/or precipitation [Citation17], indicates different chemical groups and specific reagents ().The fluorescence characteristics were observed in visible light and using an ultraviolet (UV) lamp under long UV-light (315 to 400 nm) and short UV-light (100 to 280 nm).
Table 1. Specific reactions and phytochemical screening reagents.
Testing for alkaloids
The characterization of alkaloids was carried out using the Dragendorff’s reagent. Each extract (10 mg) was dissolved in 2 mL of hydrochloric acid 5%. After mixing and filtering a few drops of Dragendorff’s reagents (Potassium Bismuth Iodide) were added to each. Formation of a red–orange precipitate indicated the presence of alkaloids.
Test for tannins (ferric chloride test)
Briefly, 2 mL of each extract were added to a few drops of ferric chloride aqueous solution 10% w/v. The formation of blackish blue or green-blackish colour indicated presence of catechol tannins.
Test for flavonoids
Flavonoids were characterized using Cyanidin reaction. A volume of 2 mL of each plant extract was evaporated to dryness. After cooling, 5 mL of diluted hydrochloric alcohol and a few magnesium turnings were added to each extract residue. Then, a few drops of isoamyl alcohol were added. A pink–orange, purple or red rose indicate the presence of flavonoids.
Test for coumarins
The presence of coumarins was determined using the Bornträger reaction. Spots of each dry extract were applied on the chromatographic plates using thin layer chromatography. Then, the plates were sprayed with 10% of potassium hydroxide and observed on a UV lamp (365 nm). Intense fluorescence indicates the presence of coumarins.
Testing for saponins
The content of saponins was measured by adding 10 mL of the plant extract to a test tube. After shaking vigorously for about 15 s and left to stand for 15 min. The formed foam height was measured. Foam greater than 10 mm in height indicated the presence of saponins (abundant).
Test for sterols and triterpenoids (Liebermann–Burchard’s test)
One milliliter of anhydrous acetic acid and a few drops of concentrated sulfuric acid were added to each extract. After 5 min a blue–green colour middle layer was indicative of sterols, but formation of a purple-colored ring indicated the presence of triterpenoids.
Free reducing sugar (Fehling’s test)
About 2 mL of each extract was added into 5 mL mixture of equal volumes of Fehling’s solutions A and B and heated in a water bath for 2 min. Formation of a brick-red precipitate was an indication of the presence of reducing sugars.
In vitro anticancer activity
Cytotoxicity was measured using MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-(2H) tetrazolium bromide) assay as described and modified by Tim Mosmann [Citation18]. Briefly, P815 (mouse DBA2 mastocytoma ATCC: TIB64) cancer cells (6.105) were seeded in each well containing 100 µL of the RPMI 1640 medium supplemented with 10% of fetal bovine serum (FBS) and 1% of penicillin and streptomycin in a 96-well plate. After exposure of cells to serial concentrations of tested product for 48 h at 37 °C and 5% CO2, 20 µL of MTT (5 mg/mL stock solution) were added and the plates were incubated for an additional 4 h in the same conditions, after that the plates were treated with a solution of HCl/isopropanol (24:1) to dissolve the blue intracellular formazan product. One hour later, the plates were read in a MicroELISA reader at dual wavelength of 540–630 nm. Cell lysis (%) was calculated for each concentration as: 100 X (1 – Abstreated/Absc), where Abstreated and Absc are the absorbance readings for the wells with and without product, respectively. All tests and analyses were run in duplicate and mean values recorded. Cisplatin was used as a positive control.
Antioxidant activity: DPPH radical scavenging assay
The 2,2′-diphenyl-1-picrylhydrazyl (DPPH) free radical assay was carried out to measure the free radical scavenging activity. Briefly, DPPH (100 μL, 0.2 mmol/L) solution was added to MeOH solution (200 μL) of the extract or standard compounds at various concentrations. The reaction mixture was shaken vigorously, and the absorbance of remaining DPPH was measured at 517 nm after 30 min in room temperature. Ascorbic acid (Vitamin C) was used as a standard drug. The scavenging activity of the extract against the stable DPPH* was calculated using the following equation:where AB is the absorption of blank sample, AA is the absorption of test sample.
The percentage of inhibition was calculated and the graph was plotted to determine the IC50 value.
Statistical analyses
The results were expressed as mean ± SD. The study was analyzed by using Student’s t-test. The data were presented as mean SEM (n = 3), and differences were considered statistically significant at a p < .05 level.
Results
Phytochemical analysis
The results obtained from the phytochemical assay are given in . This preliminary phytochemical screening of the stem bark extracts of Bombax buonopozense revealed the presence or absence of secondary metabolites. The qualitative analysis showed the presence of alkaloids, terpenes, sterols, flavonoids, tannins and saponins compounds in the various extracts with varying intensities. As shown in , the majority of these secondary metabolites are present in the ethanolic extract. The presence of flavonoids was noted essentially in the ethanolic extract ().
Table 2. Chemical groups in various extracts of stem bark of Bombax buonopozense.
Antioxidant activity
The ethanolic extract exhibited a better free-radical-scavenging activity, close to that of vitamin C, compared to the aqueous extract (). Overall, the comparison demonstrated that the ethanolic extracts exhibited powerful scavenging effects on DPPH radicals, while the aqueous extract had the lowest, with IC50 values of 10 and 220 µg/mL, respectively ().
Figure 1. In vitro DPPH radical-scavenging potential of aqueous and ethanolic extract of stem bark of Bombax buonopozense.
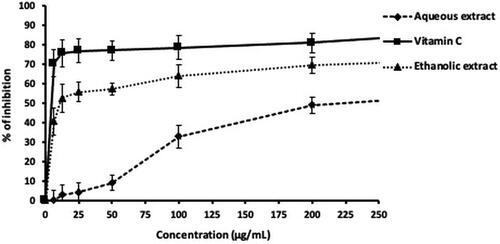
Table 3. IC50 values of ethanolic and aqueous extract of stem bark of Bombax buonopozense in relation to DPPH inhibition.
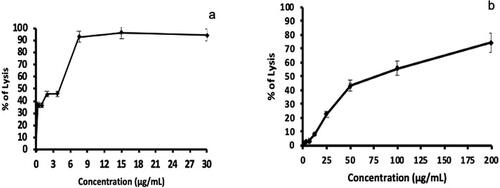
In vitro cytotoxic activity
The powerful free-radical-scavenging activity of the ethanolic extract, prompted us to investigate it’s in vitro cytotoxic potential in a cancer cell line. The cytotoxic activity of the extract was investigated through 3-(4) 5-Dimethyl-thiazol-Zyl) − 2,5 biphenyl tetrazolium bromide (MTT) assay. The results revealed an increase of cell lysis in a dose-dependent manner. The chemotherapy medication used to treat the P815 cancer cell line in this test is cisplatin; this agent presented powerful IC50= 4 µg/mL (); however, the ethanolic extract presented an IC50= 74 µg/mL (). As shown in , the lysis % showed a dose-dependent association with increasing of ethanolic extract concentration from 1 to 200 µg/mL.
Discussion
The phytochemical screening of Bombax buonopozense extracts revealed the presence of various secondary metabolites including alkaloids, tannins, flavonoids, saponins, sterol and triterpenes as shown in . Similarly, Akuodor et al. have shown that the methanolic extract of B. buonopozense obtained by maceration contained tannins, saponins, terpenes, steroids, flavonoids, alkaloids and carbohydrates compounds [Citation10]. Furthermore, a study on the root extract, demonstrated the presence of alkaloids, flavonoids, tannins, saponins, terpenoids, steroids phlo-batannins, anthraquinones and carbohydrates [Citation10]. The phytochemical groups detected are known to have medicinal properties. For example, alkaloids have been reported to exhibit various biological activities like, anti-inflammatory [Citation19], antimalarial [Citation20] and cytotoxic activities [Citation21]. Similarly, tannins, flavonoids and saponins derived from plants are known to possess many biological activities [Citation22–24].
DPPH radical is one of the routinely employed antioxidant assays, which reacts with suitable reducing agents losing color stoichometrically with the number of electrons consumed, which is measured spectrophotometrically at 540 nm. In the present study, both Bombax buonopozense extracts exhibited rich scavenging effects on DPPH as illustrated in and . However, the ethanolic extract showed stronger free-radical-scavenging activity compared to the aqueous extract, with IC50 values of 10 and 220 µg/mL, respectively (). Stronger radical scavenging effect could be attributed to the presence of flavonoids and higher amount of sterol and terpenoids of the ethanolic extract as shown in . The higher antioxidant activity of ethanolic extract could be attributed to flavonoid compounds and their hydrogen-donating ability. Studies have concluded that large plants which contain flavonoid compounds possess antioxidant activity [Citation25–28]. Furthermore, the presence of flavonoids and tannins in the plant extract plays an important role for their antioxidant property. Several plant components like tannins and flavonoids are responsible for showing antioxidant activity.
The important antioxidant activity of the ethanolic extract prompted us to study its in vitro cytotoxic capacity. It is well established that flavonoids present in the ethanolic extract are free-radical scavengers and prevent oxidative damage; they have powerful anticancer effect and also protect cells against all stages of carcinogenesis [Citation29–31]. To the best of our knowledge, B. buonopozense extracts have not been evaluated for anticancer properties. However, other biological activities of this plant have been studied such as antiulcer [Citation32], antihemorrhoids [Citation33], antidiarrheal [Citation34] and antimalarial [Citation35] activities. The previous study on the anti-ulcer activity of B. buonopozense leaf aqueous extract, showed a protective effect in the stomach against the necrotic damage of ethanol at a dose of 400 mg/kg [Citation32]. In the present study, the cytotoxic activity of the ethanolic extract was probably due to its alkaloids, tannins, sterol and triterpenoids contents. There is abundant evidence that a great number of plant extracts, contain such chemical compounds exhibiting anticancer properties [Citation36–41]. Interestingly, studies have reported no acute toxicity of B. buonopozense-treated mice at doses ranging from 10 to 5000 mg/kg; no mortality in mice after oral administration of the aqueous extract; and oral LD50 greater than 5000 mg/kg [Citation42,Citation43].
Conclusions
This is the first report reporting cytotoxic and antioxidant activities of Bombax buonopozense extracts. The ethanolic extract was an effective scavenger and promising cytotoxic agent. The presence of alkaloids, sterol/terpenes and especially flavonoids may explain these biological activities of the ethanolic extract compared to other extracts. Taken together, our results provide the basis to carry out further in-depth research to characterize the compounds and to clarify the molecular mechanisms underlying the observed biological activities.
Author’s contributions
Conceptualization, A.Z., M.K, A.H and H.A.M.; Methodology, H.A and M.T., Formal Analysis, M.K, A.H, M.L, H.A.M and A.Z., writing-original draft preparation, A.H and M.T; writing-review and editing A.Z, S.L, S.Z, M.T and H.A.M. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data that support the findings reported in this study are available from the corresponding author (M.T) upon reasonable request.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Tilaoui M, Mouse HA, Jaafari A, et al. Comparative phytochemical analysis of essential oils from different biological parts of Artemisia herba Alba and their cytotoxic effect on cancer cells. PLoS One. 2015;10(7):e0131799.
- Welz AN, Emberger-Klein A, Menrad K. Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complement Altern Med. 2018;18(1):92.
- Ahmad Khan MS, Ahmad I. Herbal medicine: current trends and future prospects. In: New look to phytomedicine. Chapter 1. Cambridge (MA): Academic Press; 2019.
- Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007;4(S1):40–47.
- Mollova S, Fidan H, Antonova D, et al. Chemical composition and antimicrobial and antioxidant activity of Helichrysum italicum (roth) G. Don subspecies essential oils. Turk J Agric For. 2020;44(4):371–378.
- Özcan FŞ, Özcan N, Us AA, et al. Comparison of extraction methods optimised by RSM for extraction of some bioactives from liquorice samples. Turk J Agric For. 2020;44(1):24–38.
- Shad AA, Ahmad S, Ullah R, et al. Phytochemical and biological activities of four wild medicinal plants. Sci World J. 2014;2014:1–7.
- García-Beltrán JM, Mansour AT, Alsaqufi AS, et al. Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata L.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish Shellfish Immunol. 2020;106:44–55.
- Zyad A, Tilaoui M, Jaafari A, et al. More insights into the pharmacological effects of artemisinin. Phytother Res. 2018;32(2):216–229.
- Akuodor GC, Essien AD, Ibrahim JA, et al. Phytochemical and antimicrobial properties of the methanolic extracts of Bombax buonopozense leaf and root. Asian J Med Sci. 2012;2(3):190–194.
- Essien AD, Essiet GA, Akuodor GC, et al. Pharmacological evaluation of the aqueous stem bark extract of Bombax buonopozense in the relief of pain and fever. Afr J Pharm Pharmacol. 2016;10(5):59–65.
- Mann A, Gbate M, Umar AN. Medicinal and economic plants of nupeland. Bida, Nigeria: Jube Evans Books and Publications; 2003.
- Akuodor GC, Muazzam I, Usman-Idris M, et al. Evaluation of the antidiarrheal activity of methanol leaf extract of Bombax buonopozense in rats. Ibnosina J Med Biomed Sci. 2011;3(1):15–20.
- Akuodor GC, Usman MI, Ibrahim JA, et al. Anti-nociceptive, anti-inflammatory and antipyretic effects of the methanolic extract of Bombax buonopozense leaves in rats and mice. Afr J Biotechnol. 2011;10(16):3191–3196.
- Mann A, Salawu FB, Abdulrauf I. Antimicrobial activity of Bombax buonopozense P. Beauv.(bombacaceae) edible floral extracts. Eur J Sci Res. 2011;48(4):627–630.
- Lebri M, Bahi C, Fofie NBY, et al. Analyse phytochimique et évaluation de la toxicité aiguë par voie orale chez des rats de l’extrait total aqueux des feuilles de abrus precatorius linn (fabaceae). Int J Bio Chem Sci. 2015;9(3):1470–1476.
- Houghton P, Raman A. Laboratory handbook for the fractionation of natural extracts. London UK: Springer; 2012.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Liu Q-L, Chen A-H, Tang J-Y, et al. A new indole alkaloid with anti-inflammatory activity from Nauclea officinalis. Nat Prod Res. 2017;31(18):2107–2112.
- Benelli G, Maggi F, Petrelli R, et al. Not ordinary antimalarial drugs: Madagascar plant decoctions potentiating the chloroquine action against Plasmodium parasites. Ind Crops Prod. 2017;103:19–38.
- Sun N, Han Y. Cytotoxic isoquinoline alkaloids from the roots of Thalictrum foliolosum. J Asian Nat Prod Res. 2021;23(1):1–8.
- El Hazzam K, Hafsa J, Sobeh M, et al. An insight into saponins from quinoa (Chenopodium quinoa Willd): a review. Molecules. 2020;25(5):1059.
- Hussain G, Huang J, Rasul A, et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: an updated review. Molecules. 2019;24(12):2213.
- Jucá MM, Cysne Filho FMS, de Almeida JC, et al. Flavonoids: biological activities and therapeutic potential. Nat Prod Res. 2020;34(5):692–705.
- Afshar FH, Delazar A, Nazemiyeh H, et al. Comparison of the total phenol, flavonoid contents and antioxidant activity of methanolic extracts of Artemisia spicigera and A. splendens growing in Iran. Pharm Sci. 2019;18(3):165–170.
- Harassi Y, Tilaoui M, Idir A, et al. Phytochemical analysis, cytotoxic and antioxidant activities of myrtus communis essential oil from Morocco. J Complement Integr Med. 2019;16(3):1–8.
- Kaurinovic B, Vastag D. Flavonoids and phenolic acids as potential natural antioxidants. In: Antioxidants. London UK: IntechOpen; 2019. p. 1–20.
- Ruvanthika PN, Manikandan S. A study on antioxidant activity, phenol, and flavonoid content of seedpod of Nelumbo nucifera Gaertn. Drug Invent Today. 2019;11(4):835–840.
- Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013:1–16.
- Okwu DE. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci. 2005;1(4):375–381.
- Nwagba CA, Ezugwu CO, Eze CC, et al. Anti-ulcer activity of Bombax buonopozense P. Beauv. aqueous leaf extract (Fam: Bombacaceae). J Appl Pharm Sci. 2013;3(2):139–142.
- Soladoye MO, Adetayo MO, Chukwuma EC, et al. Ethnobotanical survey of plants used in the treatment of haemorrhoids in South-Western Nigeria. Ann Biol Res. 2010;1(4):1–15.
- Christain AG, Idris-Usman M, Megwas AU, et al. Evaluation of antidiarrhoeal activity of ethanol leaf extract of Bombax buonopozense in different animal models. Ibnosina J Med Biomed Sci. 2010;3(1):15–20.
- Asase A, Oppong-Mensah G. Traditional antimalarial phytotherapy remedies in herbal markets in Southern Ghana. J Ethnopharmacol. 2009;126(3):492–499.
- Wang Y, Gao Y, Ding H, et al. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017;218:152–158.
- Adamski Z, Blythe LL, Milella L, et al. Biological activities of alkaloids: from toxicology to pharmacology. Toxins (Basel). 2020;12(4):210.
- Bona NP, Pedra NS, Azambuja JH, et al. Tannic acid elicits selective antitumoral activity in vitro and inhibits cancer cell growth in a preclinical model of glioblastoma multiforme. Metab Brain Dis. 2020;35(2):283–293.
- López-Huerta FA, Delgado G. Totaianes, a new type of triterpenes (comments on the article “antiproliferative activity and energy calculations of a new triterpene isolated from the palm tree Acrocomia totai”). Nat Prod Res. 2020; (34)1–4.
- Randhir A, Laird DW, Maker G, et al. Microalgae: a potential sustainable commercial source of sterols. Algal Res. 2020;46:101772.
- Singh R, Madan J, Rao H. Antiulcer activity of black pepper against absolute ethanol induced gastric mucosal damage in mice. Pharmacogn Mag. 2008;4(15):232.
- Iwuanyanwu TC, Akuodor GC, Essien AD, et al. Evaluation of antimalarial potential of aqueous stem bark extract of Bombax buonopozense P. Beauv. (Bombacaceae). East J Med. 2012;17(2):72.
- Akuodor GC, Mbah CC, Megwas UA, et al. In vivo antimalarial activity of methanol leaf extract of Bombax buonopozense in mice infected with Plasmodium berghei. Int J Bio Chem Sci. 2012;5(5):1790–1796.