Abstract
Cataract is a leading cause of reversible blindness. Secondary cataract is the result of migration, proliferation, and epithelial-mesenchymal transformation (EMT) of lens epithelial cells (LECs), induced by transforming growth factor β (TGFβ). The extracellular signal-regulated kinases 1 and 2 (ERK 1/2) and casein kinase 2 (CK2) are important for fundamental cellular processes. This study aimed to evaluate the role of CK2 in the EMT of LECs. Primary LECs cultures were obtained from age-related and type 2 diabetes-induced cataracts. Diabetic LECs were capable of spontaneous in vitro differentiation. Treatment of LECs with TGFβ2-stimulated ERK1/2 activity, which correlated with the expressed α-smooth muscle actin (α-SMA). siRNA-mediated attenuation of the endogenous catalytic subunit (α) of CK2-inhibited ERK1/2 activity and decreased α-SMA expression. This study shows evidence that the function of ERK1/2 is regulated by the constitutively active heterotetrameric protein kinase CK2 in this cellular phenotype. This study enriches the knowledge on LECs physiology in normal and in pathological conditions.
Introduction
Cataract, a leading cause for reversible blindness worldwide, represents any opacification of the lens. It is a multifactorial disease associated with many risk factors: genetic factors, age-related degenerative changes, traumatic, inflammatory, and degenerative eye diseases, metabolic disorders (diabetes, etc.), exposure to ultraviolet radiation, and so on. Age-related cataract occurs in patients over 50 years of age and is not associated with previous mechanical, chemical, or radiation trauma [Citation1]. Cataract among type 2 diabetes (T2D) patients is more common and appears at an earlier age, when compared with nondiabetics [Citation2].
The opacification of the posterior lens capsule (PCO) after extracapsular cataract extraction, known as a secondary cataract, is the result of proliferation, migration, and transformation of residual lens epithelial cells (LECs) from the anterior lens capsule [Citation3]. Increased expression of growth factors and cytokines leads to proliferation and migration of residual cells to the posterior lens capsule, where they undergo epithelial-mesenchymal transformation (EMT) [Citation4]. Clinically, there are two morphological types of secondary cataracts: fibrous and pearly. Fibrosis is caused by the proliferation and migration of cells that undergo EMT and fibrous metaplasia. The pearl type is caused by an abnormal attempt by LECs located at the equator of the lens to differentiate into crystalline expressing lens fibres forming Elschnig pearls and a Soemmering ring [Citation4]. Several regulatory factors play a major role in the formation of secondary cataract. Residual cells do not need exocrine growth factors for their growth but synthesize autocrine ones [Citation5]. Among them are transforming growth factor β (TGF-β), fibroblast growth factor 2, hepatocyte growth factor, interleukin 1 and 6, and epithelial growth factor. TGF-β, a multifunctional cytokine involved in embryogenesis and tissue homeostasis, is one of the main inducers of EMT [Citation6]. Their role in the proliferation, migration, and differentiation of cells has been demonstrated in vitro. The main pathological process associated with the formation of secondary cataract is EMT. LECs transform into myofibroblasts [Citation7], α-smooth muscle actin (α-SMA) is a key factor in cell fibrosis and a specific marker of myofibroblasts and EMT. The change in the structure of the actin cytoskeleton and the induction of α-SMA are the basis of the contractility of transdifferentiated cells, their migration and invasion [Citation8].
The extracellular signal-regulated kinases 1 and 2 (ERK 1/2) and mitogen-activated protein kinase (MAPK) have an important role in fundamental cellular processes like differentiation, proliferation, cell cycle, and apoptosis. Protein kinase, casein kinase (CK2), phosphorylates ERK and regulates its nuclear activity [Citation9]. CK2 is an active enzyme, phosphorylating a large variety of proteins, maintaining the cell morphology, and cell polarity [Citation10]. It is a serine/threonine kinase, most commonly detected as a tetramer, consisting of two catalytic subunits (α and/or α’) and two regulatory subunits (β). Its broad-spectrum action involves CK2 in almost all aspects of cell physiology, and its key role in the regulation of gene expression and signal transduction is unquestionable [Citation11].
Although the significance of MAPK for the lens physiology has been studied, the participation of CK2 in MAPK pathway in the lens was not. This study aimed to evaluate the role of CK2 in the EMT of LECs.
Subjects and methods
Ethics statement
All patients gave their informed consent. The protocol was approved by the Ethics Committee of The Medical University of Sofia (Bulgaria). The present study adhered to the tenets of the Declaration of Helsinki.
Patients
Explants of anterior lens capsules and adherent LECs with an approximate size of 5.50 mm were obtained during standard phacoemulsification procedure by continuous curvilinear capsulorhexis in 10 patients (10 eyes), aged from 55 to 75 years of either sex, having age-related cataract (5 eyes) or T2D-induced cataract (5 eyes).
Obtaining primary LECs from an anterior capsule
The explants were placed with the epithelial cells at the bottom of six-well plates (Greiner Bio-One GmbH, Frickenhausen, Germany) in a complete nutrient medium-Dulbecco’s Modified Eagle Medium (DMEM, AppliChem GmbH, Darmstadt, Germany), containing 20% foetal bovine serum (FBS, Sigma-Aldrich, USA) and 2 mmol/L glutamine, and incubated at 37 °C and 5% CO2. The complete culture medium was changed every third day of the in vitro cultivation of the explants. Upon reaching about 60% of cell confluency of the primary LECs cultures, the cells were prepared for subsequent studies.
Passage of LECs cultures
The primary cultures were seeded with 0.05% trypsin/ethylenediaminetetraacetic acid (Gibco, Germany), the cell count was determined by hemocytometry and 5 × 103 cells/well were seeded in 96-well plates (Greiner Bio-One GmbH) and cultured in a complete nutrient medium containing 10% FBS at 37 °C and 5% CO2. Upon reaching 50% cell confluency, the complete culture medium was replaced with one containing 0.5% FBS to create autocrine culture conditions, and 24 h later the cells were subjected to further experiments.
Phase-contrast light microscopic observation of LECs
Capsular explants were monitored every 24 h with a 10× lens, Brightfield illumination from Acquisition Mode of the Automated cellular and subcellular imaging system IN Cell Analyzer 6000 (GE Healthcare Life Sciences, GE Healthcare UK Ltd Amersham Place, UK, www.gelifesciences.com). Capsular explants and the primary LECs that migrated from them were imaged at various time intervals.
Stimulation with TGFβ2
Exponentially growing LECs (∼65% cell confluency) were subjected to a 24-h incubation with human recombinant TGFβ2 growth factor (Sigma-Aldrich) added to the medium at 37 °C and 5% CO2. After the first 12 h of the incubation period, the cells were transfected with scrambled siRNA or CK2α siRNA for the next 12 h of TGFβ2 stimulation. After 24 h of stimulation, the cells were indirectly multifluorescently labelled and analysed.
siRNA-mediated attenuation of the endogenous catalytic subunit (α) of the protein kinase CK2 in TGFβ2-stimulated LECs
At 12 h from the start of TGFβ2 stimulation, cells were transfected with 12 pmol scrambled siRNA (control cells) or CK2α siRNA (Santa Cruz Biotechnology, USA). A complex transfection kit (siRNA Reagent System, Santa Cruz, Biotechnology) was used according to a protocol provided by the manufacturer. After 12 h of transfection, in the presence of TGFβ2, the cells were indirectly multifluorescently labelled and analysed. Each experiment was performed in triplicate.
Immunofluorescence labelling of TGFβ2-stimulated, scrambled siRNA/CK2α siRNA-transfected LECs
Cells were washed three times with PBS, fixed for 30 min at room temperature with 2% paraformaldehyde, permeabilized for 10 min at room temperature with 0.5% TrytonX-100, washed three times with PBS, and incubated with blocking buffer (PBS, 3% BSA, 0.02% Tween20) for 1 h at room temperature. After washing three times with PBS, the cells were incubated for 2 h at room temperature with anti-human phospho-ERK1/2 ab or with anti-human CK2α ab, washed three times with PBS and incubated for 1 h at room temperature, in the dark, with IgG-CFL 555 antibody (sc-362,267). After washing three times with PBS, the cells were incubated for 2 h at room temperature with anti-human vimentin antibody or with anti-human α-SMA antibody, washed three times with PBS, and incubated for 1 h at room temperature, in the dark, with IgG-FITC antibody (sc-2990). All antibodies used were manufactured by Santa Cruz Biotechnology, USA. After washing three times with PBS, the cell nuclei were labelled with DAPI (Termo Fisher Scientific, USA) for 15 min at room temperature, in the dark. Immunofluorescently labelled cells were analysed on an automated cellular and subcellular imaging system IN Cell Analyzer 6000 (GE Healthcare Life Sciences).
Image-based high content screening analysis
Microscopic images were obtained with a 20× lens and 405-, 561-, 488-nm lasers excited by DAPI-labelled nuclei, IgG-CFL 555-labelled FOXM1 and, respectively, IgG-FITC-labelled BrdU, which was incorporated into the DNA nuclei.
The area of the whole well, for each of the experimental conditions, was microscopically analysed by image-based high content screening: an algorithm was applied to calculate the 555-, 488-, and 405-fluorescent radiation of the cells occupying the area.
Statistical analysis
Data obtained from Image-Based High Content Screening analyses was analysed by Student’s test and one-way analysis of variance conducted on SigmaPlot Version 12.0 (Systat Software, San Jose, CA, USA). Differences were considered statistically significant at the p < 0.05 level.
Results
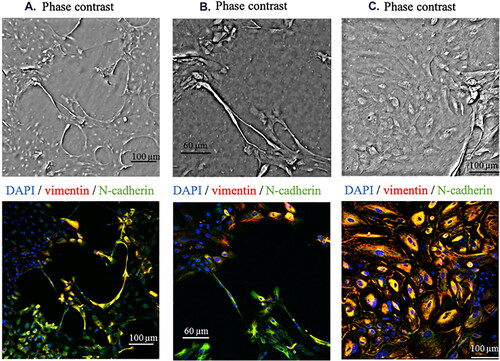
The morphology of LECs was examined by phase-contrast microscopy. In LECs isolated from patients with T2D-induced cataract, an in vitro initial change into fibroblast-like epithelial phenotype was observed. There was a change in morphology from cubic to fibroblast-like in 60% of the initial LECs cultures of patients with T2D. Such a change was not observed among LECs of non-diabetic patients. The morphological changes began about 14 days after the cultivation of the patient explants, and the duration of the process was 7–8 days. At the end of the period, fibroblast-like cells represented 20% of the cell composition of the original cultures. These cells were proliferatively inactive, and empty spaces formed between them and proliferatively active LECs (). Heterogeneous (composed of LECs and fibroblast-like cells) primary cultures were not used in subsequent experiments but were subjected to immunofluorescence analysis. Cell nuclei were identified based on DAPI. The presence of Vimentin and N-cadherin around the nuclei was analysed (). Higher expression of Vimentin and N-cadherin was found among LECs from non-diabetics, when compared with LECs from diabetic patients.
Figure 1. Spontaneous differentiation in vitro of LECs from anterior lens capsule isolated from T2D cataract (A) and (B). LECs from anterior lens capsule isolated from age-related cataract (C).
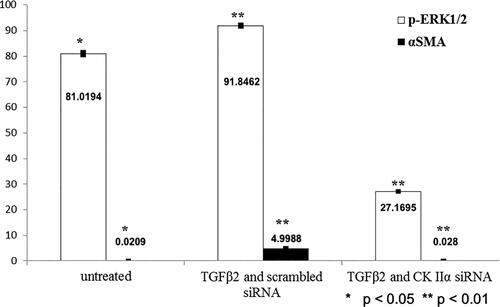
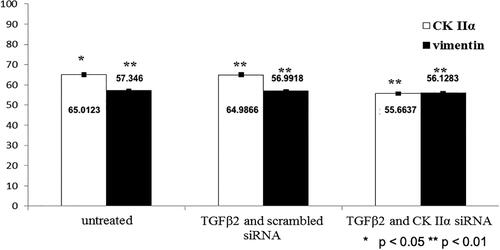
Our study revealed that 24-h treatment of LECs with TGFβ2 was associated with statistically significantly stimulated ERK1/2 activity (). ERK1/2 activity increased by 10% in the presence of TGFβ2. TGFβ2-induced increased phosphorylation of ERK1/2 did not correlate with the expression of the cytoskeletal protein Vimentin (), whose expression remained the same but correlated with the appearance of expressed α-SMA (). α-SMA expression increased significantly from 0.02% to 4.99% in the presence of TGFβ2. Twelve-hour siRNA-mediated attenuation of the endogenous catalytic subunit (α) of the protein kinase CK2 statistically significantly inhibited TGFβ2-induced increased ERK1/2 activity and decreased TGFβ2-induced α-SMA expression to 0.028% ().
Discussion
According to the present study, LECs isolated from patients with T2D-induced cataract are more likely to undergo an in vitro initial change from cubical to fibroblast-like epithelial phenotype. Vimentin and N-cadherin are proteins expressed in the epithelial cell phenotype [Citation12, Citation13]. Although Vimentin is considered a biomarker for EMT due to its expression as an intermediate filamentous protein and a major cytoskeletal component of mesenchymal cells, it is also involved in epithelial cell migration [Citation14]. As Vimentin is an integral part of cell structural integrity, lamellipodia formation and adhesion signalling, decreased expression of Vimentin is sufficient to alter cell morphology in vitro as well as to inhibit cell motility [Citation14]. N-cadherin is a glycoprotein responsible for intercellular adhesion, involved in the structure of both the fibrous cells of the lens and the structure of LECs [Citation15]. Its expression leads to enhanced cellular motility and also stimulates cell growth by blocking apoptotic signals [Citation16]. Decreased N-cadherin synthesis leads to impaired intercellular adhesion and EMT initiation [Citation17]. The established lower expression of Vimentin and N-cadherin in LECs by patients with T2D leads to a change in their cell morphology, reduced motility and, accordingly, to reduced migratory activity, as previously found by our team [Citation18]. The observed in vitro initial change of the epithelial phenotype of LECs to fibroblast-like in the group of LECs from diabetic patients can be considered as an initial stage of epithelial-mesenchymal transition. PCO formation among diabetics compared to nondiabetics is a highly controversial subject. Many ophthalmologists have the clinical impression that diabetic patients develop more severe PCO than non-diabetic ones [Citation19–22]. On the other hand, there are reports of similar or even lower incidence of PCO formation among diabetics in comparison to non-diabetics [Citation23–26]. In our previous study, we found reduced proliferative activity of LECs in diabetic patients [Citation18]. The reduced proliferation of LECs and the lower expression of Vimentin and N-cadherin may offer a possible explanation for the observed fibrous PCO among diabetics at an earlier stage.
CK2 is widely expressed, regulates more than 300 substrates and the signalling pathways in the control of cell cycle progression, the maintenance of cell survival, morphology, proliferation, the cellular responses to stress or DNA damage, as well as regulation of apoptosis [Citation27]. There is no data that gene expression silencing was performed on LECs until now. It is the most sophisticated method for gene suppression and determination of its significance on the cells physiology. CK2 activity suppression in cultured human astrocytes and vascular endothelial cells causes morphological alteration and reorganization of actomyosin cytoskeleton, which affects their adhesive properties and migratory activity [Citation28]. CK2 inhibition can also block the neoangiogenesis in the retina [Citation29]. We found that inhibiting CK2 activity in LECs by inactivation of its catalytic subunit (α) has similar effect on α-SMA, the marker for myofibroblastic cells, which manifest in reduced α-SMA expression. However, such a correlation was not found regarding Vimentin.
Conclusions
In summary, the study demonstrates that growth factor TGFβ2, which is thought to be the major signal inducing the epithelial-mesenchymal transition in the pathogenesis of capsular opacification, is transduced by ERK1/2-dependent signalling, leading to gene expression of non-epithelial cytoskeletal protein α-SMA in in vitro cultured LECs isolated from patients explants. And since there is currently no data in the literature on the involvement of protein kinase CK2 in signalling supporting the cellular physiology of LECs, it reveals for the first time that the function of ERK1/2 is regulated by the constitutively active heterotetrameric (2 catalytic α and 2 noncatalytic β subunits, products of different genes) protein kinase CK2 in this cellular phenotype. The defined novelties enrich the knowledge on LECs physiology in normal and in pathological conditions. An in-depth analysis of the biochemical processes underlying the development of PCO could lead in the future to the creation of new methods for prevention of its formation by the usage of CK2 inhibitors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gupta VB, Rajagopala M, Ravishankar B. Etiopathogenesis of cataract: an appraisal. Indian J Ophthalmol. 2014;62(2):103–110.
- Javadi MA, Zarei-Ghanavati S. Cataracts in diabetic patients: a review article. J Ophthalm Vis Res. 2008;3(1):52–65.
- Nibourg LM, Gelens E, Kuijer R, et al. Prevention of posterior capsular opacification. Exp Eye Res. 2015;136:110–115.
- Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127(4):555–562.
- Wormstone I, Liu C, Rakic J, et al. Human lens epithelial cell proliferation in a protein-free medium. Investig Ophthalmol Vis Sci. 1997;38(2):396–404.
- Zhang R, Wei Y, Zhao C, et al. EDIL3 depletion suppress epithelial-mesenchymal transition of lens epithelial cells via transforming growth factor β pathway. Int J Ophthalmol. 2018;1:1–7.
- Meng F, Li J, Yang X, et al. Role of Smad3 signaling in the epithelial‑mesenchymal transition of the lens epithelium following injury. Int J Mol Med. 2018;42(2):851–860.
- Ma B, Jing R, Liu J, et al. CTGF contributes to the development of posterior capsule opacification: an in vitro and in vivo study. Int J Biol Sci. 2018;14(4):437–448.
- Whitmarsh AJ. Casein kinase 2 sends extracellular signal-regulated kinase nuclear. Mol Cell Biol. 2011;31(17):3512–3514.
- Filhol O, Cochet C. Protein kinase CK2 in health and disease: cellular functions of protein kinase CK2: a dynamic affair. Cell Mol Life Sci. 2009;66(11–12):1830–1839.
- Son E, Do H, Joo H-M, et al. Induction of alkaline phosphatase activity by L-ascorbic acid in human osteoblastic cells: a potential role for CK2 and ikaros. Nutrition. 2007;23(10):745–753.
- Velez-del Valle C, Marsch-Moreno M, Castro-Muñozledo F, et al. Epithelial cell migration requires the interaction between the vimentin and keratin intermediate filaments. Sci Rep. 2016;6(1):24389.
- Logan CM, Rajakaruna S, Bowen C, et al. N-cadherin regulates signaling mechanisms required for lens fiber cell elongation and lens morphogenesis. Dev Biol. 2017;428(1):118–134.
- Richardson AM, Havel LS, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion . Clin Cancer Res. 2018;24(2):420–432.
- Maisel H, Atreya P. N-cadherin detected in the membrane fraction of lens fiber cells. Experientia. 1990;46(2):222–223.
- Wheelock MJ, Shintani Y, Maeda M, et al. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–735.
- Saénz-de-Santa-María I, Celada L, Chiara M-D. The leader position of mesenchymal cells expressing N-cadherin in the collective migration of epithelial cancer. Cells. 2020;9(3):731.
- Haykin V, Oscar A, Dimitrova V, et al. Bioimage analysis of cell physiology of primary lens epithelial cells from diabetic and non-diabetic cataract patients. Biotechnol Biotechnol Equip. 2021;35(1):170–178.
- Ebihara Y, Kato S, Oshika T, et al. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2006;32(7):1184–1187.
- Ionides A, Dowler JG, Hykin PG, et al. Posterior capsule opacification following diabetic extracapsular cataract extraction. Eye 1994;8(Pt (5):535–537.
- Hayashi K, Hayashi H, Nakao F, et al. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Am J Ophthalmol. 2002;134(1):10–16.
- Praveen M, Vasavada A, Shah G, et al. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: a case–control study. Eye. 2014;28(6):720–727.
- Elgohary MA, Dowler JG. Incidence and risk factors of Nd:YAG capsulotomy after phacoemulsification in non-diabetic and diabetic patients. Clin Exp Ophthalmol. 2006;34(6):526–534.
- Knorz MC, Soltau JB, Seiberth V, et al. Incidence of posterior capsule opacification after extracapsular cataract extraction in diabetic patients. Metab Pediatr Syst Ophthalmol (1985). 1991;14(3–4):57–58.
- Zaczek A, Zetterstrom C. Posterior capsule opacification after phacoemulsification in patients with diabetes mellitus. J Cataract Refract Surg. 1999;25(2):233–237.
- Nekolová J, Pozlerová J, Jirásková N, et al. Posterior capsule opacification in patients with type 2 diabetes mellitus. Cesk Slov Oftalmol. 2008;64(5):193–196.
- Cen LP, Liu YF, Ng TK, et al. Casein kinase-II inhibition promotes retinal ganglion cell survival and axonal regeneration. Exp Eye Res. 2018;177:153–159.
- Kramerov AA, Ahmed K, Ljubimov AV. Cell rounding in cultured human astrocytes and vascular endothelial cells upon inhibition of CK2 is mediated by actomyosin cytoskeleton alterations. J Cell Biochem. 2012;113(9):2948–2956.
- Kramerov AA, Saghizadeh M, Caballero S, et al. Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites. Mol Cell Biochem. 2008;316(1–2):177–186.