Abstract
This study aims to investigate the effects of nano zinc oxide (ZnO) loaded on eggshell on Actinobacillus actinomycetemcomitans (AA) and Actinomyces viscosus in order to find new oral antibacterial agents. The AA standard strain ACTT 700685 and A. viscosus standard strain ACTT 27044 were used as experimental strains. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of nano-ZnO loaded on eggshell against AA and A. viscosus were determined. By prolonging the action time, the MIC of nano-ZnO loaded on eggshell against AA was decreased from 160 to 20 μg/mL, and the MBC was decreased from 2560 to 80 μg/mL. The MIC of nano-ZnO loaded on eggshell against A. viscosus was 640 μg/mL and the MBC was 2560 μg/mL, which were not associated with the action time. Nano-ZnO loaded on eggshell has certain inhibitory effects on the growth of AA and A. viscosus.
Introduction
Periodontitis is a highly prevalent and multifactorial inflammatory disease caused by dental plaque colonisation along the gingival sulcus. It is characterised by the progressive destruction of the periodontal supporting tissue. If not treated timeously, periodontitis will lead to tooth loosening or even falling [Citation1]. This disease affects 50% of adults over the age of 30, and the incidence rate increases with age [Citation2]. At present, it has been recognised that there are two main forms of destructive periodontal disease: chronic and aggressive periodontitis.
Actinobacillus actinomycetemcomitans (AA) is one of the pathogens responsible for aggressive periodontitis and is found in 90% of cases of localised aggressive periodontitis and 30–50% of severe adult periodontitis [Citation3]. AA is a non-motile, gram-negative (G−), carbon dioxide tolerant, fermentative bacterium. It is found in dental plaque, the periodontal pocket and the buccal mucosa of 36% of healthy people [Citation4]. In the mid-1990s, a specific JP2 clone belonging to the serotype B of AA was isolated. The JP2 clone has a high leukocyte toxicity; it can kill human immune cells and is closely related to the aggressive periodontitis [Citation5]. After the basic treatment of periodontitis, the bacteria in the tooth neck change from periodontal pathogens to root caries pathogens [Citation6]. Actinomyces viscosus is associated with dental caries (especially root caries) and periodontal disease; it can store polysaccharides and can prolong the acid production time in the case of exogenous sugar deficiencies. Under anaerobic conditions, A. viscosus produces exopolysaccharides by increasing the metabolism of sugars [Citation7]. A. viscosus is one of the most common gram-positive (G+) bacteria found in oral cavities, and the primary condition of its pathogenicity is its adhesion to and settlement in the host. A. viscosus has a strong adhesion to the tooth surface, which determines its important role in dental caries. A. viscosus also has a strong affinity for collagen and its metabolic activity in root surface caries is significantly higher than in normal root surfaces.
It is necessary to find effective substitutes for antibiotics to solve the increasing problem of antibiotic resistance. Zinc oxide (ZnO) can effectively inhibit the growth and proliferation of bacteria, and because of its low price, low toxicity and good biocompatibility, it has attracted attention [Citation8, Citation9]. ZnO is recognised as a safe substance by the U.S. Food and Drug Administration (21CFR182.8991). It has been approved for use in cosmetics in the European Union, with a maximum concentration of 25% (regulation [EC] No 1223/2009); and is widely used in local products, including sunscreen, baby powder, diaper cream and mineral cosmetics [Citation10]. ZnO has a strong inhibitory effect on Staphylococcus aureus (S. aureus), Staphylococcus epidermidis, Bacillus subtilis and Klebsiella pneumoniae [Citation11].
Eggshells are very common in household garbage because most people include eggs in their daily diet. Many eggshells are discarded as rubbish and lose their useful value, but eggshell materials have been used in various fields. Li et al. prepared 3D eggshell-supported titanium dioxide as a photocatalyst for dye degradation [Citation12]. In addition, eggshells can be combined with other materials to recover calcium oxide, remove pollutants and promote antibacterial activity [Citation13]. In this study, ZnO was used to coat the surface of eggshells to study the antibacterial activity of nano-ZnO loaded on eggshells. It was proven that nano-ZnO has a stronger antibacterial effect than micron ZnO due to its greater surface area [Citation14]. Nano-ZnO inhibits the growth and proliferation of Escherichia coli and S. aureus [Citation15]. Furthermore, nano-ZnO can inhibit Candida albicans, the herpes simplex virus, Streptococcus mutans, and other oral pathogenic bacteria [Citation16]. However, it has not been reported whether nano-ZnO can inhibit AA and A. viscosus. This study primarily investigates the inhibitive effect of nano-ZnO loaded on eggshell on AA and A. viscosus.
Materials and methods
Materials
The eggshell was provided by Quanzhou Normal University. Zinc nitrate was provided by Sinopharm Chemical Reagent Co., Ltd., China. The AA strain ACTT 700685 and A. viscosus strain ACTT 27044 were provided by the Guangdong microbial species preservation Center, China. The brain heart infusion (BHI) broth was provided by Guangdong Huankai Microbial Sci. & Tech Co., Ltd., China. The blood agar plate was provided by Guangzhou Detgerm Microbiological Science Ltd., China.
Synthesis of nano-ZnO loaded on eggshell
First, the eggshell was rinsed with tap water until all the booty and eggshell intima were cleared out. The washed eggshell was rinsed twice more with deionised water and dried at room temperature for 24 h. The dried eggshell was ground into a powder using a grinder, passed through a 200-mesh sieve and stored in a desiccator for further study.
Two grams of the eggshell powder was soaked in 100 mL of zinc nitrate solution, stirred evenly using a magnetic stirring device for 24 h, and the filtrate was obtained by filtration. The filtrate was then calcined in a muffle furnace at 500 °C for 3 h and naturally cooled to room temperature. The end result of this process was eggshell loaded with nano-ZnO.
Characterisation of nano-ZnO loaded on eggshell
The morphological structure of the samples was examined by field emission scanning electron microscopy (FESEM, Zeiss, Germany). The lattice parameters and crystal phases were analysed by X-ray diffraction (XRD) on an X-ray diffractometer (RIGAKU Ultima IV, Japan). The particle size and agglomeration state of egg shell loaded nano zinc oxide in deionised water and the surface charge of egg shell loaded nano-ZnO were determined by nano particle size potentiometer (Zetasizer nano ZS 90 Malvern instruments, Worcestershire, UK). The sample was detected by 3-flex three-stop full-function multi-purpose adsorption instrument, and the specific surface area of the sample was obtained.
Preparation of experimental strains and bacterial suspension
The standard strains of AA and A. viscosus were inoculated in a BHI liquid medium by the plate marking method after 48 h of resuscitation and placed in an incubator (5% CO2) at 37 °C to undergo 48 h of anaerobic culture. After smear examination and biochemical identification as a pure culture, the bacteria were subcultured on the blood agar plate. A single colony was obtained from the solid medium using an inoculating loop, and the bacteria were prepared into a 0.5 McFarland suspension with sterile normal saline for standby.
The effects of nano-ZnO loaded on eggshell on AA and A. viscosus
Inhibition zone test
The eggshell loaded nano zinc oxide is used to do a series of antibacterial activities against Actinobacillus actinomycetes and Actinomycetes viscosus. Fresh single colonies of A. actinomycetes and A. viscosus were selected and configured to 0.5 Michaelis turbidity. A sterile cotton swab was dipped into the bacterial solution, rotated and squeezed from the excess bacterial solution on the inner wall of the tube. Inoculum was applied evenly on the agar surface for inoculation for 3 times while rotating the plate 60 degrees each time and finally applied along the inner edge of the plate for one week. The same blood plate was perforated and divided into an experimental hole and a control hole. The experimental hole: 15 mg eggshell loaded with nano zinc oxide was added to 40 mL sterile water to form a suspension by ultrasonic vibration. The control hole: 15 mg pure eggshell was added to 40 mL sterile water to form suspension by ultrasonic vibration. Incubation was held at 37 °C, 5% CO2, and the results were observed after 48 h. The diameter of the bacteriostatic circles was measured with Vernier caliper and compared to each other.
Determination of the minimum inhibitory concentration (MIC)
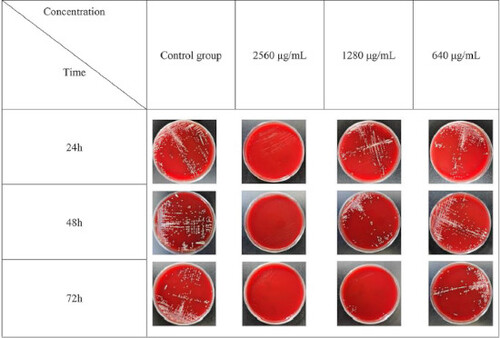
The bacterial suspension (0.5 McFarland) was diluted with BHI broth at a ratio of 1:1000 in the logarithmic growth period. In the experimental group, 100 μL of the diluted bacterial solution was added to each well and different concentrations of nano-ZnO loaded on eggshell were prepared by the double-dilution method, making the final drug concentrations 2560, 1280, 640, 320, 160, 80, 40, 20, 10, 5, 2.5, 1.25 and 0.625 μg/mL. The experimental group were given different concentrations of nano-ZnO loaded on eggshell + the bacterial solution, the positive control group were given the BHI medium containing the bacterial solution, and the negative control group were given the BHI medium. These were sealed and placed in an incubator for 24, 48 and 72 h. When the solution in the blank control group was clear and transparent, the growth of bacteria in each test tube was observed with naked eye. The MIC of nano-ZnO loaded on eggshell against the bacteria was the lowest concentration of clearly transparent bacterial fluid.
Determination of MBC against AA
The MIC of AA was observed at 24, 48 and 72 h, and 1 µL of the culture was collected from the bacterial solution with MIC, the bacterial solution with two concentration gradients higher than the MIC, the positive control group, and the negative control group, and these were inoculated in a blood agar plate. After 24 h in the incubator, the presence of colonies was observed. The lowest drug concentration showing the absence of colonies was taken as the MBC of nano-ZnO loaded on eggshell against the pathogenic bacteria.
Determination of MBC against A. viscosus
After 24, 48, and 72 h of culture, 1 μL of culture from the experimental group, positive control group, and negative control group with different concentrations of nano-ZnO loaded on eggshell were inoculated in the blood agar plate. After 24 h of culture in the incubator, the presence of colonies was observed. The lowest drug concentration for the absence of colonies was taken as the MBC of nano-ZnO loaded on eggshell against the pathogenic bacteria.
Results
Scanning electron microscopy (SEM) analysis
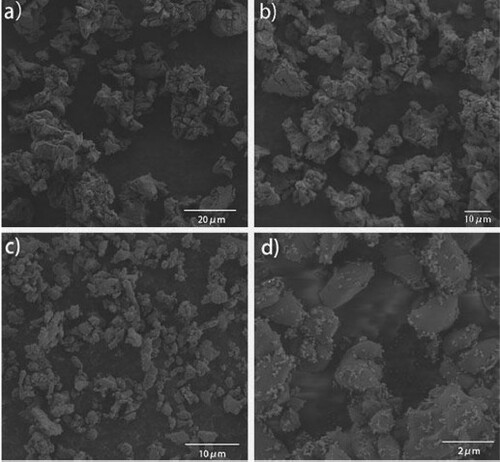
The morphology of nano-ZnO loaded on egg shell was observed by SEM. Under the scanning electron microscope at 20 and 10 μm, it was observed that the surface of the ground eggshell was smooth, without pores () and that the particle size of the nano-ZnO loaded on the surface of eggshell was much smaller than 100 nm (see ).
X-ray diffraction (XRD) analysis
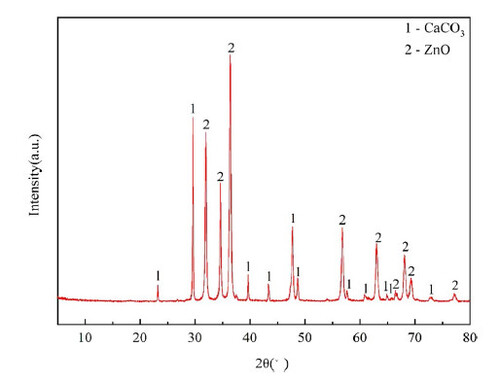
As can be seen from , when 2 θ = 31.95°, 34.61°, 36.43°, 47.75° and 56.75° etc, it coincides with the (100) (002) (101) (102) and (110) crystal planes of ZnO (standard PDF Card No. 89-0510), indicating that ZnO exists in the sample. Similarly, when 2 θ = 23.25°, 29.60°, 39.61°,43.37° and 48.69° etc, it coincides with (012) (104) (113) (202) and (116) of CaCO3 (standard PDF Card No. 99-0022), indicating that CaCO3 also exists in the sample. It can be concluded that, combined with SEM, the zinc oxide was successfully loaded on the calcined egg shell (). The specific surface area (SSA), grain diameter, polydispersity index (PDI) and Zeta potential of eggshell loaded nano-ZnO are shown in .
Table 1. Characterization of eggshell loaded with nano ZnO.
Inhibition zone experiment
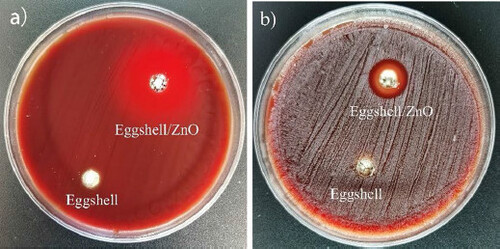
Inhibition zone experiment is a classic and effective method for studying antibacterial effects. As shown in , there is an obvious circle of inhibition around the experimental hole with actinomycetes and viscous actinomycetes in the blood plate. The diameter of the average inhibition zone around the eggshell loaded with nano-zinc oxide was measured: for AA it was 28 mm (), and for A. viscosus was 14 mm (). However, bacteria were still growing around the control wells and no sterile zone appeared.
MIC Of AA
The MIC of nano-zinc oxide loaded on egg shells for 24 h to AA was 160 μg/mL, and the MIC of nano-zinc oxide loaded on egg shells for 48 h against AA was 40 μg/mL, the MIC of nano-ZnO loaded on eggshells for 72 h against AA was 20 μg/mL.
MIC of A. viscosus
The MIC of the nano-zinc oxide loaded on eggshells at 24, 48 and 72 h for A. viscosus was 640 μg/mL.
MBC of AA
It can be seen from that the MBC of AA loaded on egg shells at 24 h, 48h and 72h were 2560 μg/mL, 160 μg/mL and 80 μg/mL, respectively.
Discussion
The conversion of biological waste into high-value-added materials currently attracts extensive research interest. Using eggshell as a coating material for nano-ZnO has two advantages. First, eggshell is a common waste product of daily life, which means it is low cost and widely available. Second, environmental pollution can be reduced by using waste products. Zinc is a trace element that the human body needs, especially at the cellular level, and it has the function of smoothing. Nano-ZnO is an inorganic nanomaterial approved by the FDA for human use [Citation17] and has been used in cosmetics and sunscreens for many years [Citation18]. ZnO is also widely used in the treatment of oral cavities, e.g. ZnO backing, temporary bonding cement, ZnO clove oil and periodontal tamponade [Citation19]. Adding nano-ZnO to a denture base can not only enhance the mechanical and optical properties of the polymer but can also inhibit G+ and G− bacterial growth [Citation20]. Nano-ZnO can inhibit Enterococcus faecalis and the herpes simplex virus [Citation21], and the inhibition ability of nano-ZnO on Candida albicans is two times that of non-nano-ZnO [Citation22].
Dental bacterial plaque is the initiating factor for periodontal disease and caries, and the prevention and treatment of periodontitis and caries aims to control dental bacterial plaque with antimicrobial agents [Citation23]. G− bacilli are the main pathogens in dental bacterial plaque, which can trigger the aggregation and activation of neutrophils. Subsequently, neutrophils phagocytise pathogens to produce a series of antimicrobial factors, e.g. reactive oxygen species (ROS) are released through the nicotinamide adenine - dinucleotide phosphate oxidation pathway [Citation24]. The detection rate of AA in patients with periodontitis was significantly higher than in patients without periodontitis [Citation25]. The results of this study revealed that with an increase in action time, the MIC of nano-ZnO against AA decreased from 160 to 20μg/mL, and the MBC decreased from 2560 to 80 μg/mL. Therefore, the results revealed that the inhibitory effective of nano-ZnO on AA is related to both action time and concentration.
After the basic treatment for periodontitis, the tooth neck is exposed. Because cementum is more highly soluble than enamel, the development of root caries is much faster. A. viscosus is the first bacterium to colonise the root surface. The fimbriae of A. viscosus can adhere firmly to the type α1 collagen of the cementum, and a wooden fence structure is formed to accelerate the formation of dental bacterial plaque and produce acid under various growth conditions, resulting in root caries [Citation7]. Whether the root surface is intact, worn, or demineralised will not affect the colonisation of A. viscosus. The cariogenic ability of A. viscosus on the root surface is greater than Streptococcus mutans [Citation26]. The present study revealed that the MIC of nano-ZnO against A. viscosus was 640 μg/mL, the MBC was 2560 μg/mL, and they did not change over time. Therefore, the results revealed that the inhibitory effect of nano-ZnO on A. viscosus is related to concentration but not to action time.
When compared with the A. viscosus, nano-ZnO is more sensitive to the AA. The most common mechanism of nano-ZnO in killing bacteria is through the production of ROS. Mirhosseini [Citation16] confirmed that the high antibacterial activity of nano-ZnO against the G+ Enterococcus faecalis and Streptococcus mutans can be attributed to the production of ROS. The production of ROS leads to the degradation of the cell membrane, and through lipid peroxidation, its cell contents are released and cell necrosis is induced [Citation27, Citation28]. After periodontal surgery, adding nano-ZnO to the occluder can not only inhibit the proliferation of AA and enhance the effect of the periodontal treatment but can also inhibit the proliferation of A. viscosus and prevent the occurrence of root caries.
Conclusions
The results of the present study demonstrate that nano-ZnO has an inhibitory effect on the G+ A. viscosus and AA, but it is more sensitive to the AA. This suggests that nano-ZnO could be further explored as a potential approach to enhance the effect of the periodontal treatment, inhibit the proliferation of A. viscosus and prevent the occurrence of root caries.
Disclosure statement
No potential competing interest was reported by the authors.
Data availability statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013;62(1):203–217.
- Ferraiolo DM. Predicting periodontitis progression? Evid Based Dent. 2016;17(1):19–20.
- Mj R, Ummer F, Dhivakar CP. Aggregatibacter actinomycetemcomitans – a tooth killer? J Clin Diagnostic Res. 2014;8(8):13–16.
- Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12(1):1–20.
- Tsai CC, Ho YP, Chou YS, et al. Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human periodontitis – a historic review with emphasis on JP2. Kaohsiung J Med Sci. 2018;34(4):186–193.
- Hoppenbrouwers PMM, Driessens FCM, Borggreven JMPM. The mineral solubility of human tooth roots. Pergamon. 1987;32(5):319–322.
- Deng L, Li W, He Y, et al. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch Oral Biol. 2019;100:106–112.
- Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45(5):1977–1983.
- Brayner R, Ferrari-Iliou R, Brivois N, et al. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6(4):866–870.
- Rosenberg M, Visnapuu M, Vija H, et al. Selective antibiofilm properties and biocompatibility of nano-ZnO/Ag coated surfaces. Sci Rep. 2020;10(1):13478.
- Riaz M, Zia R, Saleemi F, et al. In vitro antimicrobial activity of ZnO based glass–ceramics against pathogenic bacteria. J Mater Sci - Mater Med. 2015;26(12):268.
- Li Y, Zhou J, Fan Y, et al. Preparation of environment-friendly 3D eggshell membranesupported anatase TiO2 as a reusable photocatalyst for degradation of organic dyes. Chem Phys Lett. 2017; 689:142–114.
- Alsohaimi IH, Nassar AM, Elnasr TAS, et al. A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: a potent photocatalytic and antibacterial activities. J Cleaner Prod. 2020;248:119274.
- Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater. 2008;9(3):035004.
- Podporska-Carroll J, Myles A, Quilty B, et al. Antibacterial properties of F-doped ZnO visible light photocatalyst. J Hazard Mater. 2017;324(Pt A):39–47.
- Mirhosseini F, Amiri M, Daneshkazemi A, et al. Antimicrobial effect of different sizes of nano zinc oxide on oral microorganisms. Front Dent. 2019;16(2):105–112.
- Teow Y, Asharani PV, Hande MP, Valiyaveettil S. Health impact and safety of engineered nanomaterials. Chem Commun (Camb). 2011;47(25):7025–7038.
- García-Hevia L, Valiente R, Martín-Rodríguez R, et al. Nano-ZnO leads to tubulin macrotube assembly and actin bundling, triggering cytoskeletal catastrophe and cell necrosis. Nanoscale. 2016;8(21):10963–10973.
- Wang X, Fan H, Zhang F, et al. Antibacterial properties of bilayer biomimetic nano-ZnO for dental implants. ACS Biomater Sci Eng. 2020;6(4):1880–1886.
- Raj I, Mozetic M, Jayachandran VP, et al. Fracture resistant, antibiofilm adherent, self-assembled PMMA/ZnO nanoformulations for biomedical applications: physico-chemical and biological perspectives of nano reinforcement. Nanotechnology. 2018;29(30):305704–305704.
- Mishra YK, Adelung R, Röhl C, et al. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1 . Antiviral Res. 2011;92(2):305–312.
- Gondal MA, Alzahrani AJ, Randhawa MA, et al. Morphology and antifungal effect of nano-ZnO and nano-Pd-doped nano-ZnO against Aspergillus and candida. J Environ Sci Health A. 2012;47(10):1413–1418.
- Dima S, Lee YY, Watanabe I, et al. Antibacterial effect of the natural polymer ε-polylysine against oral pathogens associated with periodontitis and caries. Polymers. 2020;12(6):1218.
- Sui L, Wang J, Xiao Z, et al. ROS-scavenging nanomaterials to treat periodontitis. Front Chem. 2020;8:595530.
- Tomás I, Regueira-Iglesias A, López M, et al. Quantification by qPCR of pathobionts in chronic periodontitis: development of predictive models of disease severity at site-specific level. Front Microbiol. 2017;8:1443.
- Firestone AR, Feagin FF, Heaven TJ, et al. In vitro demineralization by strains of Actinomyces viscosus and Streptococcus sobrinus of sound and demineralized root surfaces. J Dent Res. 1993;72(8):1180–1183.
- Zabihi E, Babaei A, Shahrampour D, et al. Facile and rapid in-situ synthesis of chitosan-ZnO nano-hybrids applicable in medical purposes; a novel combination of biomineralization, ultrasound, and bio-safe morphology-conducting agent. Int J Biol Macromol. 2019;131:107–116.
- Kiwi J, Nadtochenko V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir. 2005;21(10):4631–4641.