Abstract
Zinc finger proteins bind nucleic acids or act in transcriptional or translational regulation. The present study aimed to explore the effect of heterologous expression of the Medicago truncatula gene (Mt-Zn-CCHC), which encodes a Zinc finger CCHC type protein, in Arabidopsis thaliana. The Mt-Zn-CCHC gene, which affects seed size in M. truncatula, was used for construction of transgenic A. thaliana transcriptional reporter plants expressing pMt-Zn-CCHC::GUS::GFP, as well as lines with modified expression – overexpressed (OE) and knockdown (RNAi). In silico analysis of the promoter cis-elements of pAt-Zn-CCHC and pMt-Zn-CCHC suggested regulation during meristem activity, seed development, as well as cold stress. The expression of pMt-Zn-CCHC was localized in shoot apical meristem and in the base of the siliques. In the RNAi lines, successfully repressed endogenous At-Zn-CCHC expression resulted in shortened stem and reduction in silique number, silique size, seed number per silique, and decreased expression of the meristem marker AtSWP. In the gain-of-function lines, overexpression of Mt-Zn-CCHC acted as a positive regulator in silique and seed parameters, as well as increased AtSWP expression. Cold treatment of WT plants demonstrated upregulation of the endogenous At-Zn-CCHC and the RD29A cold marker gene. In the OE line, RD29A transcription was induced by cold faster but in the RNAi line, slower. The overall data support the roles of the studied Zn-CCHC gene in the development of shoot meristem, seeds and cold response, which highlights this protein as a conserved regulator in plant reproduction and stress signal transduction.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.2006786 .
Introduction
Regulation of the gene expression in living organisms is important for their growth, development and stress responses [Citation1]. Posttranscriptional regulation of gene expression – known as RNA metabolism – includes RNA processing, pre-mRNA splicing, and RNA export and decay. RNA metabolism has a key role in many processes in eukaryotic cells [Citation2]. The regulation of RNA metabolism is carried out by direct or indirect binding of RNA-binding proteins (RBPs) to target RNAs [Citation1]. RBPs include some conserved motifs, like RNA-recognition motif (RRM), zinc (Zn) finger motif, K homology (KH) domain, glycine-rich region, etc. [Citation3]. There is a wide range of RBPs in plants, indicating that their functions in plant growth, development and stress responses is also diverse [Citation4, Citation5]. For example, some RBPs that are central to plant growth and stress responses harbor an RRM at the N-terminus and a glycine-rich region at the C-terminus, i.e. glycine-rich RBP (GRP), zinc finger-containing GRP, cold shock domain protein (CSDP) and RNA helicase (RH) [Citation6–8]. RBPs could have various types of Zn finger motifs in their structure. There are nine different types of Zn finger motifs described [Citation9]; one of them is the CCHC Zn knuckle domain. The proteins possessing the Zn finger domains are widely distributed in plants and their role is related to regulation of nucleic acids function or participation in transcriptional or translational regulation [Citation10].
The gene coding for a Zn finger CCHC type protein (ABE91952.1) was discovered from a Tnt1 transposon mutant collection of Medicago truncatula [Citation11] and studied by application of reverse genetic approach in M. truncatula overexpressing (OE) lines, lines with silenced transcription of the gene (RNAi), and promotor lines pMt-Zn-CCHC with reporters [Citation12]. Expression and phenotypical observations demonstrated localization in anthers of OE lines and reduction of seed size in RNAi M. truncatula lines. In this study, M. truncatula gene harboring Zn finger motif CCHC type (MT2G005460) was investigated in a heterologous system of Arabidopsis thaliana. In silico approach was applied to asses the promoter sequences of Mt-Zn-CCHC (MT2G005460) and its ortolog in A. thaliana At-Zn-CCHC (At2G01050). Vectors carrying the open reading frame of Mt-Zn-CCHC gene were introduced in A. thaliana plants. Both type of transgenic lines overexpresing (OE) and knockdown (RNAi) were succesfully developed. Additionaly, A. thaliana transcriptional reporter lines harboring the construct of the promoter sequence of pMt-Zn-CCHC fused to reporter genes were generated. The phenotypes of lines with modified expression were evaluated during plant development and their reproduction, as well as in response to cold stress.
Materials and methods
Gene cloning and plant transformation
The promoter sequence of the Mt-Zn-CCHC gene (MT2G005460) was previously cloned into the vector pExK7SWFm14GW carrying β-glucuronidase (GUS) and green fluorescent protein (GFP) reporter genes [Citation12]. Gene cloning procedures were described in detail by [Citation12]. Briefly, the open reading frame of the Mt-Zn-CCHC gene was amplified and cloned in plant transformation vectors under the control of CaMV35S promoter [Citation13]. RNA interference protocol [Citation14] was applied for development of lines with silenced gene function. The successful gene silencing was in silico predicted, and a 153-bp fragment located between 729 bp and 882 bp from the ORF of Mt-Zn-CCHC was used (). In the present work, the M. truncatula constructs were introduced into Agrobacterium tumefaciens strain C58C1, which was used for A. thaliana transformation following the floral dip procedure [Citation15]. Seeds from OE and RNAi lines were selected on Km media. T3 progeny positive plants were grown in greenhouse for seed production and phenotyping.
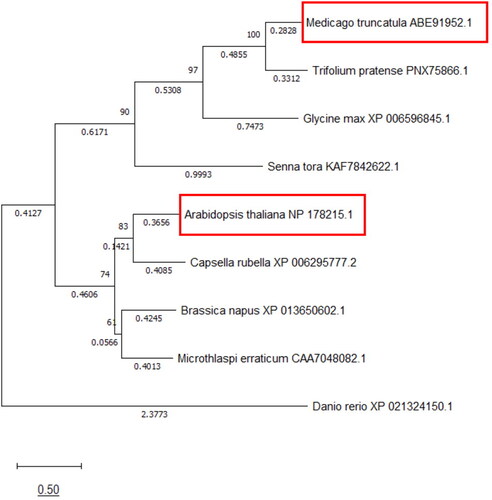
Figure 1. Phylogenetic tree of proteins containing a Zn-CCHC domain. The At-Zn-CCHC and Mt-Zn-CCHC protein sequences were used for BLASTP search for proteins with a Zn-CCHC domain. The phylogenetic tree was constructed using the MEGAX software by the Maximum Likelihood method and JTT matrix-based model. The tree with the highest log likelihood (-10431.62) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained by applying the Neighbor-Joining method to a matrix of pairwise distances estimated using the JTT model. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (next to the branches).
Plant material
A. thaliana (L.) Heynh. ecotype Columbia-0 (Col-0) was used in this work. Positive plants were selected on half-strength Murashige and Skoog (MS) medium containing 50 mg L−1 kanamycin selective agent and were used for analyses. The sterilized seeds were incubated in the dark at 4 °C for 48 h and transferred to light. Plants were grown under controlled growth conditions (at 21 °C, continuous light, 100 µmol m−2 s−1 photosynthetically active radiation, 55% humidity) on vertical plates containing ½ MS medium supplemented with 0.8% plant tissue culture agar (LAB M Ltd, Heywood, UK) and 1% sucrose.
Bioinformatic analyses
The alignment between At-Zn-CCHC (NP178215.1) and Mt-Zn-CCHC (ABE91952.1) proteins was performed by T-Coffee with M-Coffee [Citation16] and visualized by Boxshade 3.21 - Pretty Printing and Shading of Multipe-Alignment files (created by Hofmann K and Baron MD, Swiss Institute of Bioinformatics http://www.ch.embnet.org/software/BOX_form.html) (). For phylogenetic tree construction, the At-Zn-CCHC and Mt-Zn-CCHC protein sequences were used for BLASTP search for proteins with a Zn-CCHC domain. The alignment of 9 amino acid sequences and subsequent construction of phylogenetic tree were performed using the MEGAX software by the Maximum Likelihood method and JTT matrix-based model [Citation17–19].
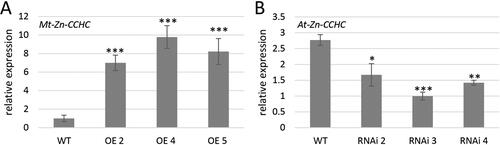
Figure 2. A. thaliana OE and RNAi transgenic lines. Expression levels of Mt-Zn-CCHC on OE lines (A) and At-Zn-CCHC in RNAi lines (B) in T3 generation.
Note: The expression levels were calculated and normalized according to the housekeeping gene AtACTIN. Data are mean values ± SEM. Asterisks indicate statistically significant differences compared to the control: p < 0.05*; p < 0.01**; p < 0.001***.
The analysis for cis-elements in At-Zn-CCHC (At2g01050) and Mt-Zn-CCHC promoters was performed by PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace) [Citation20].
Detection of GUS and GFP reporter genes
Tissue specific localization of β-glucuronidase (GUS) activity was detected by the protocol of Jefferson et al. [Citation21]. The procedure is described in detail in [Citation12]. Binocular microscope (MZ16, Leika) equipped with a camera (Nikon) was used for image tracking. Fluorescence stereomicroscope SZX7 (460–490 nm excitations and 510–550 nm emission) with a DP73 digital camera (Olympus) for the images of plants expressing green fluorescent protein (GFP) were used.
Expression analysis
Total RNA was extracted from 18-day-old seedlings. The protocol of RNeasy Plant Mini Kit (EurEx) was followed. Extracted RNA was quantified with a Nanodrop2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Copy DNA synthesis was performed with 1 µg RNA according to the protocol of the iScript cDNA Synthesis Kit (BIO-RAD). The relative transcript level was monitored using 7300 Real-Time PCR System (Applied Biosystems). PCR reaction was performed with 5 µL of 5x diluted first-strand cDNA in a 20-µL reaction including 0.5 µmol/µL gene-specific primers and iTaqUniversal SYBR green Super mix including ROX and fluorescein (BIO-RAD). The housekeeping gene AtACTIN was used as a reference gene for data normalization. All primers used in this study are listed in . The relative expression levels were calculated by qBase 1.3.5 software.
Table 1. Regulatory cis-elements in the promoter regions of pMt-Zn-CCHC and pAt-Zn-CCHC.
Phenotypic characterization
OE and RNAi transgenic lines of A. thaliana selected after qRT-PCR data were grown in a greenhouse and monitored phenotypically. The growth dynamics expressed by the length of the main stem was measured at two time points: 20 and 35-day-old plants. The characteristics related to the flower and seeds development, such as flowering time, number and size of siliques, as well as the seed number per siliques were assessed, and compared with the control plants of A. thaliana.
Cold treatment
Two-week-old plants from the T3 generation of A. thaliana OE and RNAi lines were grown in square Petri dishes 120/120 mm on MS medium at 22 °C under constant illumination. The plants were subjected to cold treatment at 4 °C for 0 h, 24 h and 48 h in a cold room. Total RNA from all tested plants was extracted at each time point and used for the following investigations. The relative expression under cold treatment was compared with the expression level of the gene AtRD29A, which was used as a positive control.
Statistical analysis
All experiments were performed in triplicate. Results are expressed as means values with standard error of the means (±SEM). Differences were assessed by Student’s t-test. Differences were considered statistically significant at the p < 0.05 level.
Results and discussion
Phylogenetic analysis of Mt-Zn-CCHC
In a previous report, the Mt-Zn-CCHC was shown to have a Zn-binding domain and to control seed size in M. truncatula [Citation12]. To explore whether there are closely related proteins to Mt-Zn-CCHC in A. thaliana, BLASTP search was performed. Proteins with an identified Zn-CCHC domain were found and used for construction of a phylogenetic tree (). In one cluster, the Mt-Zn-CCHC protein was grouped together with Trifolium pratense and Glycine max, as well as another member of the Fabaceae family, Senna tora. The A. thaliana ortholog, designated as At-Zn-CCHC, was grouped in a second cluster together with the Zn-CCHC representatives of the Brasicaceae family members Capsellea rubella and Brassica napus. Alignment between the amino acid sequences of Mt-Zn-CCHC and At-Zn-CCHC showed 34% homology and confirmed the presence of common protein domains: a DUF domain of unknown function and a Zn-binding domain ().
Regulatory cis-elements in the promoters of Mt-Zn-CCHC and At-Zn-CCHC
The promoter assay of Mt-Zn-CCHC and At-Zn-CCHC could identify putative signaling pathways that are common for both plant species including seed development as reported by [Citation12]. In confirmation of the functional study in M. truncatula, Mt-Zn-CCHC and At-Zn-CCHC were found to be enriched in regulatory cis-elements related to seed development (; and ). Strikingly, both promoters included motifs linked to cold stress. Some cis-motifs were annotated to be related to seed development and cold stress response together (). The approximate density of the cis-elements per promoter length was compared (). The results demonstrated that both genes have equal capacity to regulate seed development, and in respect to cold, the At-Zn-CCHC promoter seems to be more responsive (). Interestingly, there were meristem-related motifs in the At-Zn-CCHC promoter, whereas such were not observed in the Mt-Zn-CCHC sequence. In a study describing the Zn-CCHC gene family in Triticum sp., the authors discussed the existence of lots of cis-acting elements in the promoter regions of TaCCHC-ZF genes associated with environmental stress and phytohormone responsiveness, which might take part in multiple signalling pathways [Citation22].
Figure 3. Tissue-specific pMt-Zn-CCHC expression in A. thaliana. GFP signal localization in shoot apical meristem (A); GUS signal in the base of shoot apical meristem at 13 (B) and 27 days-old seedlings (C), and in the base of the siliques (D).
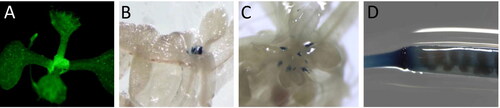
Table 2. Density of cis elements in the promoter regions of prMt-Zn-CCHC and prAt-Zn-CCHC.
Generation of transgenic A. thaliana lines
In order to investigate the Mt-Zn-CCHC function in a heterologous system, stable transgenic A. thaliana plants with modified expression of the Mt-Zn-CCHC (overexpressed-OE) and knockdown of At-Zn-CCHC gene (RNAi) were developed. Three overexpressing lines (OE 2, 4 and 5) with a higher transcript level of the exogenous Mt-Zn-CCHC and three RNAi A. thaliana lines with silenced expression of the endogenous gene At-Zn-CCHC (RNAi 2, 3 and 4) were selected after analysis of transcript levels ().
Tissue localization of pMt-Zn-CCHC in A. thaliana
The produced A. thaliana transcriptional reporter lines (pMt-Zn-CCHC::GUS::GFP) were analyzed for tissue localization of the marker genes expression. A strong GFP signal was detected in the base of the shoot apical meristem (SAM) (). GUS monitoring during SAM development showed that the signal was localized specifically in this point (). Later in the development, the GUS signal was detected in the base of siliques (). The data demonstrated the specific localization of the Mt-Zn-CCHC gene in tissues with high dividing cell activity. It has been reported that A. thaliana RNA-binding protein AtGRP2 was specifically detected in SAM of 6-day-old seedlings [Citation23]. In another study [Citation24], the tissue localization of AtCSP2 was in shoot and root apical regions, as well as in the reproductive organs. Specific accumulation in tissue with high meristematic activity was described for OsCSP1 and OsCSP2 genes in rice [Citation25]. These type of proteins are RBPs that harbour Zn-CCHC domains.
Phenotypic characteristics of A. thaliana OE and RNAi lines
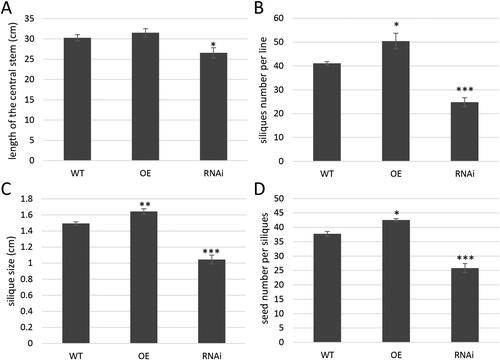
The selected T3 OE and RNAi A. thaliana lines were grown under greenhouse conditions and monitored phenotypically. The dynamics of the central stem development was measured in 20-day-old plants and 15 days later. The results demonstrated that 20-day-old plants from both OE and RNAi lines grew slowly in comparison to the wild-type (WT) control, and the length of the central stem was shortened (data not shown). At this time point it was observed that the plants from OE lines started flowering similar to the WT control plants (data not shown). In contrast, the RNAi lines were characterized with delayed flowering. On day 35 the plants from OE lines were able to compensate the delay of the central stem development in comparison to the control, but RNAi lines continued to grow slowly (). The delayed flowering described for RNAi lines was accompanied with deviation in the silique development: reduced siliques number and size (). The siliques formed on the OE lines were a bit larger than those of the control plants, while the siliques on RNAi lines were dramatically smaller (p < 0.001, ). Additionally, there was deviation from the normal development in respect to the reduced number of siliques formed by RNAi A. thaliana lines (). This observation was accompanied with a low number of seeds per siliques (). The phenotypic data demonstrated that the seeds production of A. thaliana RNAi lines was significantly disturbed due to the knockdown of the endogenous gene. A similar phenotypic defect developed in A. thaliana mutant (11-0561-1, mutation after transposon insertion – photo is available in TAIR for AT2G01050).
Figure 4. Phenotyping of OE and RNAi A. thaliana lines. Length of central stem in 35-days old plants (A); siliques number per line (B); siliques size (C) and seed number per siliques (D).
Note: Data are mean values ± SEM from analyses of two independent transgenic lines. Asterisks indicate statistically significant differences compared to the control: p < 0.05*; p < 0.01**; p < 0.001***.
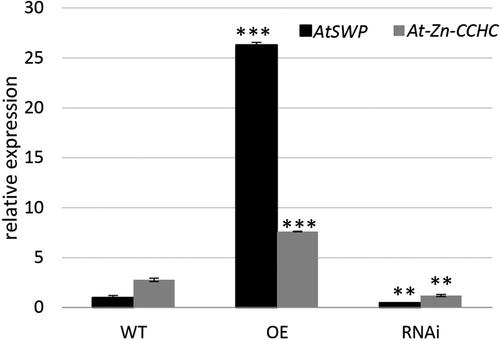
In the research of Clay and Nelson [Citation26], the STRUWWELPETER (SWP) gene known to affect cell number and shoot meristem development was studied in A. thaliana. Additionally, SWP interacts with SMP1 and SMP2 encoding CCHC zinc finger proteins with similarities to step II splicing factors. The authors demonstrated similar to the phenotypic data reported here in the mutant swp line: shorter organs, fewer siliques with reduced seed number [Citation26]. In the present study, the transcript level of AtSWP gene was monitored in two OE and two RNAi A. thaliana lines to check for a possible role of the studied Mt-Zn-CCHC gene and its ortholog At-Zn-CCHC in cell proliferation (). The obtained data demonstrated a significant expression level of the AtSWP gene in OE lines and reduction in the gene expression in RNAi lines ().
Figure 5. Meristem marker expression in transgenic lines. Relative expression level of AtSWP gene in WT, OE and RNAi lines is presented. The expression level was calculated and normalized according to the housekeeping gene AtACTIN.
Note: Data are mean values ± SEM from analysis of two independent transgenic lines. Asterisks indicate statistically significant differences compared to the control p < 0.01**; p < 0.001***.
The results presented here support previous evidence that Mt-Zn-CCHC gene can influence the plant development and seeds size of M. truncatula [Citation12]. A recent report identified genes with direct or indirect influence on plant reproduction in water yam (Dioscorea alata L.), among which was the Mt-Zn-CCHC gene [Citation27]. Additionally, high-density SNP-based association mapping of seed treat in Fenugreek identified dDocent_Contig_466_145, which indicates an association between the Znf-C2HC domain (corresponding to the domain in Mt-Zn-CCHC) and its role in gene transcription and effect on seed size [Citation28]. Fusaro et al. [Citation23] demonstrated that AtCSP2 knockdown plants had a reduced number of stamens and high rates of abnormal development of seeds/embryos. The gene AtCSP2 possesses Zn-CCHC knuckle domains. The relation between the function of CSPs (cold shock proteins) and their role in flowering time and reproductive tissue development was discussed [Citation29, Citation30].
Mt- Zn-CCHC gene and its ortolog At-Zn-CCHC play a role in the plant cold response
Based on the promoter data analysis, next, we tested the sensitivity to cold of the endogenous At-Zn-CCHC and the heterologous Mt-Zn-CCHC. First, WT A. thaliana plants were treated for 0, 24 and 48 h at 4 °C in a cold chamber. The relative expression of At-Zn-CCHC was monitored at each time point and compared to the expression of the AtRD29A gene, which is a cold-inducible marker used as a positive control [Citation31]. This gene was reported to be strongly up-regulated in transgenic A. thaliana with chilling tolerance [Citation32]. The expression analysis revealed strong induction of both genes at 24 h, corresponding to 8% upregulation for At-Zn-CCHC and 40% for AtRD29A. The induction continued and after 48 h of cold treatment up-regulation of almost 60% for At-Zn-CCHC and 85% for AtRD29A was detected ().
Figure 6. Cold response of Zn-CCHC in A. thalina. Cold stress was applied for 48 h and At-Zn-CCHC and AtRD29A expression was measured at 0, 24 and 48 h from the treatment. (A) Relative expression of At-Zn-CCHC and AtRD29A in control plants. (B) Relative expression of AtRD29A in OE and RNAi lines compared to WT. The expression levels were calculated and normalized according to the housekeeping gene AtACTIN.
Note: Data are means ± SEM from analyses of two independent transgenic lines. Asterisks indicate statistically significant differences compared to the control p < 0.001***.
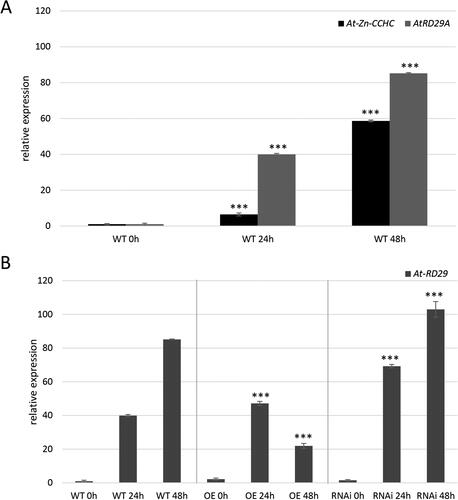
In support of the transcriptional regulation of the endogenous At-Zn-CCHC by cold, the expression profile of the gene transcript in WT and RNAi showed a similar trend with significant induction after cold treatment (). Notably, at 48 h of the treatment, the signal obtained from RNAi lines was lower than that in WT. In the OE lines, it was confirmed lack of Mt-Zn-CCHC induction under cold after 24 and 48 h of treatment – which is logical because the heterologous gene Mt-Zn-CCHC is under the 35S promoter ().
Further, the relative expression of AtRD29A was evaluated under cold treatment in two OE and two RNAi A. thaliana lines. The data demonstrated a significant induction of the transcript level at 24 h in OE lines in comparison to WT corresponding to 45% (). It was interesting to observe that induction was also obtained in the RNAi lines, and the transcript level in the RNAi profile was higher − 70%, in comparison with WT and OE. At 48 h cold treatment, both transgenic genotypes possessed different response, and in the OE profile the signal was reduced, whereas in RNAi lines the transcript level continued to grow (). These results suggest less sensitivity to cold in OE lines. In OE lines the accumulation of endogenous At-Zn-CCHC and overexpressed Mt-Zn-CCHC transcripts probably lead to abundance of the Zn-CCHC protein so plants become less sensitive to the cold stress. On the other hand, RNAi lines demonstrated hypersensitivity to cold probably related with deficiency of At-Zn-CCHC protein. It can be hypothesized that Мt-Zn-CCHC protein level correlates with cold tolerance, however, additional experiments are required to clarify the precise role of Mt-Zn-CCHC in the cold response.
The overall data obtained by promoter analysis and functional studies demonstrated the sensitivity of both genes Mt-Zn-CCHC and its ortholog At-Zn-CCHC to cold. Both genes have similar conservative motifs in their protein structure: DUF4283 domain and zinc knuckle - zinc binding motif () . There is scarce information about the function of these conservative domains. The DUF4283 domain is described as a binding/guiding region. The zinc binding motif was described as an important motif, largely found in a variety of regulatory proteins, which can specifically bind DNA or RNA sequences, and take part in protein interactions [Citation33].
The results presented support the observation for cold induction regulated by the promotor of the endogenous gene At-Zn-CCHC. In our study, the gene AtRD29A used as a positive control is known to encode the low-temperature responsive protein 78 (LTI78) taking part in the cold stress signaling pathway [Citation34] by overexpressing CBF/DREB1. It is clear that the behavior of Mt-Zn-CCHC and At-Zn-CCHC genes followed the dynamics of AtRD29A gene transcription level under cold treatment. These results indicate that both genes, Mt-Zn-CCHC and its ortholog At-Zn-CCHC, are sensitive to cold treatment and participated in the plant response to this abiotic stress factor.
In another study [Citation25], two cold shock proteins, OsCSP1 and OsCSP2, demonstrated the stronger influence of both genes on flower and seed development, and their transient upregulation in response to low temperature in rice cultivars. A report about AtCSP2 gene in A. thaliana confirmed that the gene is regulated by both cold and developmental signals [Citation24]. Additional experiments will broaden the knowledge about the functional role of both genes, Mt-Zn-CCHC and its ortholog At-Zn-CCHC, in plant development and plant response to cold.
Conclusions
In this study the function of Mt-Zn-CCHC gene was evaluated in the heterologous background of A. thaliana ecotype Columbia. The results demonstrated the role of this gene in plant development and reproduction, which was hardly repressed by RNAi interference of the endogenous gene At-Zn-CCHC. Additionally, the results showed that Mt-Zn-CCHC and its ortholog gene At-Zn-CCHC are sensitive to cold treatment based on the presence of cis-regulatory elements in the promotor sequences. The functional study outlines a direction to think that Mt-Zn-CCHC and At-Zn-CCHC play a role in the response to cold stress. A new family of proteins harboring a Zn binding domain was discovered by a reverse genetic approach and two members of this family had a role in seed development and cold response.
Consent to participate
All the authors have approved their participation in the final manuscript.
Consent for publication
All the authors have read and approved the final manuscript and its submission for publication.
Availability of data and material (data transparency)
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Supplemental Material
Download MS Power Point (235.4 KB)Acknowledgments
This work was supported by the technician work of our colleague Sonia Ivanova.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lee K, Kang H. Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol Cells. 2016;39(3):179–185.
- Simpson GG, Filipowicz W. Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol. 1996;32(1-2):1–41.
- Lorković ZJ, Barta A. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNAbinding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 2002;30:623–635.
- Lorković ZJ. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 2009;14(4):229–236.
- Mangeon A, Junqueira RM, Sachetto-Martins G. Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal Behav. 2010;5(2):99–104.
- Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem. Sci. 2011;36(1):19–29.
- Jung HJ, Park SJ, Kang H. Regulation of RNA metabolism in plant development and stress responses. J Plant Biol. 2013;56(3):123–129.
- Mihailovich M, Militti C, Gabaldón T, et al. Eukaryotic cold shock domain proteins: highly versatile regulators of gene expression. Bioessays. 2010;32(2):109–118.
- Han G, Lu C, Guo J, et al. C2H2 zinc finger proteins: master regulators of abiotic stress responses in plants. Front Plant Sci. 2020;11:115.
- Klug A. Towards therapeutic applications of engineered zinc finger proteins. FEBS Lett. 2005;579(4):892–894.
- Iantcheva A, Vassileva V, Ugrinova M, et al. Development of functional genomic platform for model legume Medicago truncatula in Bulgaria. Biotechnol Biotechnol Equip. 2009;23(4):1440–1443.
- Radkova M, Revalska M, Kertikova D, et al. Zinc finger CCHC-type protein related with seed size in model legume species Medicago truncatula. Biotechnol Biotechnol Equip. 2019;33(1):278–285.
- Karimi M, Bleys A, Vanderhaeghen R, et al. Building blocks for plant gene assembly. Plant Physiol. 2007;145(4):1183–1191.
- Limpens E, Ramos J, Franken C, et al. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004;55(399):983–992.
- Clough SJ, Bent AF. Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743.
- Notredame C, Higgins DG, Heringa J. A novel method for multiple sequence alignments. J Mol Biol. 2000;302(1):205–217.
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8(3):275–282.
- Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791.
- Higo K, Ugawa Y, Iwamoto M, et al. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27(1):297–300.
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6(13):3901–3907.
- Sun A, Li Y, Zou X, et al. Comprehensive genome-wide identizcation, characterization, and expression analysis of CCHC zinc finger gene family in wheat (Triticum aestivum L.). BMC Genomics. 2021.
- Fusaro AF, Bocca SN, Ramos RLB, et al. AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta. 2007;225(6):1339–1351.
- Sasaki K, Kim MH, Imai R. Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem Biophys Res Commun. 2007;364(3):633–638.
- Chaikam V, Karlson D. Functional characterization of two cold shock domain proteins from Oryza sativa. Plant Cell Environ. 2008;31(7):995–1006.
- Clay NK, Nelson T. The recessive epigenetic swellmap mutation affects the expression of two step II splicing factors required for the transcription of the cell proliferation gene STRUWWELPETER and for the timing of cell cycle arrest in the arabidopsis leaf. Plant Cell. 2005;17(7):1994–2008.
- Mondo JM, Agre PA, Asiedu R, et al. Genome-wide association studies for sex determination and cross-compatibility in water yam (Dioscorea alata L.). Plants. 2021;10:1412.
- Abd El-Wahab MMH, Aljabri M, Sarhan MS, et al. High-density SNP-based association mapping of seed traits in fenugreek reveals homology with clover. Genes. 2020;11(8):893.
- Nakaminami K, Hill K, Perry SE, et al. Arabidopsis cold shock domain proteins: relationships to floral and silique development. J Exp Bot. 2009;60(3):1047–1062.
- Yang Y, Karlson DT. Overexpression of AtCSP4 affects late stages of embryo development in arabidopsis. J Exp Bot. 2011;62(6):2079–2091.
- Mishra MK, Chaturvedi P, Singh R, et al. Overexpression of WsSGTL1 gene of withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic arabidopsis plants. PLoS One. 2013;8(4):e63064.
- Lin KH, Sei SC, Su YH, et al. Overexpression of the arabidopsis and winter squash superoxide dismutase genes enhances chilling tolerance via ABA-sensitive transcriptional regulation in transgenic arabidopsis. Plant Signal Behav. 2019; 14(12):e1685728.
- Liu M, Wang X, Sun W, et al. Genome-wide investigation of the ZF-HD gene family in tartary buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2019;19(1):248.
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exper Bot. 2007;58(2):221–227.
