?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the field of the microbial ecology of biofilms and activated sludges, it is widely accepted that the microstructure of the communities depends on the environmental factors. Nevertheless, due to their complexity, the exact mechanisms are still unknown. In this study, we applied a stepwise increase of an azo-dye concentration as a selective factor for adaptation towards biodegradation. The degrading biofilm was developed in a lab-scale sand biofilter. It functioned in a semi-continuous regime for 623 h. The concentration of the azo-dye amaranth was increased from 10 to 55 mg L−1. The effectiveness was 90% and the rate of amaranth elimination was 1.136 mg h−1. The fluorescence in-situ hybridisation (FISH) revealed zones with high activity of Pseudomonas sp. Also increasing importance of the unculturable Pseudomonas sp. and the relationships in the biofilm were found. At the final stage of the experiment, a decrease of the azoreductase activity and an increase of the catechol-1,2-dioxygenase activity were established in the depth of the biofilter. The obtained results were linked with different Pseudomonas microstructures (shown by FISH). The obtained data showed that the changes in the biofilm structure occurred accordingly to the biodegradation of the toxic compound and it included the development of cooperative microbial relationships in the key genus Pseudomonas.
Introduction
In 1856 the first synthetic dye was discovered by Sir William Henry Perkin. Its color was purple and it was used on the fabrics for Queen Victoria of England and Empress Eugenie, wife of Napoleon Bonaparte [Citation1]. By the end of the nineteenth century, more than 100 000 synthetic dyes were found with an annual production of more than 0.7 million tons [Citation2]. Among the largest synthetic dyes consumers are the printing industry, the textile industry, the plastic production companies, the photography, the cosmetics, the pharmaceutical industry, etc [Citation3]. Azo-dyes are the most widely used type of dyes in the textile industry [Citation4]. Because the adsorption of all of the dye stuff to the fibers is impossible, 2-50% of the colorants remain in the wastewater [Citation5]. The azo-dyes pose serious environmental and health risks. Some of the dyes can be seen when present in concentrations lower than 1 mg L−1 [Citation5]. When colored wastewater is present in the natural water bodies it can diminish the light penetration. This affects negatively photosynthesis and as a result - the oxygen concentrations. Another big issue is the toxicity of the dyes which have recalcitrant xenobiotic molecules [Citation6,Citation7].
Different physico-chemical and chemical methods for dyes removal have been developed but they have serious drawbacks such as generation of sludge, high operating/energy costs and production of byproducts [Citation4]. One of the most promising non-biological groups of methods for dye removal is membrane-based. These technologies include the use of a porous membrane that separates the contaminants from the water [Citation8,Citation9]. They have simple operation and are very suitable for the practice. Their main drawbacks are related to the concentration of the pollutants that have to be treated additionally and the fouling of the membranes [Citation10]. The biological methods for the treatment of dye contaminated wastewater are considered suitable, non-costly and flexible means for achieving highly efficient water detoxification [Citation11]. Many organisms have the ability to degrade azo-dyes – bacteria, fungi and algae [Citation12]. Bacteria are microorganisms known for their ability to adapt to hostile environments. That is why they possess impressive diversity of enzymes that degrade most of the known xenobiotics, including the azo-dyes [Citation5,Citation13].
The biological methods for the treatment of azo-dyes contaminated wastewater are based on aerobic and anaerobic regimes [Citation14]. Both stages are usually needed because of the chemistry of the azo-dyes biodegradation. In the absence of oxygen, the azo bond in the dyes molecules is reductively cleaved. This leads to decolorisation and accumulation of toxic aromatic amines. Their full biodegradation requires the presence of oxygen [Citation3]. It is known that the complete azo-dye biodegradation is achieved more easily when microbial communities are used compared to the cases with pure cultures [Citation15].
In complex microbial communities, more diverse and sophisticated degrading mechanisms can evolve. Their nature is still poorly understood. The aim of this study is to contribute to the elucidation of bacterial catabolic interplay during azo-dye elimination. Information for this is obtained by the biodegradation parameters, the enzymological activity of the community and conventional and FISH analysis.
Materials and methods
Work hypothesis
In our previous studies a characteristic pattern of development of the biodegradation activity of the microbial communities with gradual unlocking of different biodegradation mechanisms (key enzyme activities) was found. Similar data were also obtained by other research groups [Citation16,Citation17]. For the present study as a model process, we used the biodegradation of the azo-dye amaranth (C.I. Acid Red 27). It is known that the biodetoxification of the azo-dye amaranth is in two phases. The first phase is the reduction of the azo-bond by the enzyme azoreductase (AzoR). The second phase includes the cleavage of the benzene ring of the aromatic amines generated during the first phase. This step is performed mainly by ortho- or meta-mechanisms, respectively with the participation of the enzymes catechol-1,2-dioxygenase and catechol-2,3-dioxygenase.
We hypothesised that the processes of development of the biodegradation potential are tightly related not only to the key enzymes activities but also to the formed microorganism’s relationships which are formed in the community. We selected the genetic technique FISH (fluorescence in-situ hybridization) for investigating the micro-distribution of a key genus of microbial degraders (g. Pseudomonas). The main advantage of FISH is that it is possible to obtain information simultaneously for the micro-distribution and the amount of the unculturable microorganisms of the target group.
Experimental design
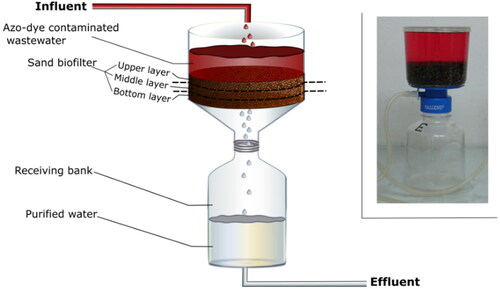
In the experiments, a lab-scale down-flow sand biofilter was used (). The working volume of the model biofilter was 192 cm3. The depth of the biofilter layer was 3 cm. In our experiments, the biofilter layer was divided into three parts: afirst upper part, where the concentration of the substrates was the highest, a second middle part, where their concentration was lowered by the microorganisms and a third bottom part, where the biodegradation mainly affected the intermediates passed from the upper two layers. The period of functioning was 26 days. To ensure a constant flow of the wastewater, the biofilter was connected to a peristaltic pump. The height of the sand layer was 30 mm, the flow of the wastewater varied between 520 − 790 mL day−1, COD was 550-600 mgO2 L−1 and the concentration of the total organic carbon (ТОС) was 230 − 245 mg L−1.
We applied a gradual increase of the inflow concentration of amaranth from 10 mg L−1 up to 55 mg L−1. The dosing was controlled by a peristaltic pump connected to a relay which additionally decreased the flow of the wastewater entering the biofilter. The amaranth concentration was determined for each portion of model wastewater passing through the pump.
The detoxification process was separated into three time stages: an early stage (0 h − 191 h), a late stage (191 h − 455 h) and a final stage of the process (455 h − 623 h). During the three stages, the amaranth concentration was increased while considering the activity of the biofilm. This was monitored using the following indicators: the rate and the effectiveness of decolorisation, the quantity of the biomass dropping from the biofilm and the activities of the key enzymes. The inflow amaranth concentration was 10 − 30 mg L−1 during the early stage, 30 − 45 mg L−1 during the late stage and 45 − 55 mg L−1 at the end of the experiment. Also, in depth of the biofilter, we studied the amount of the culturable and the unculturable bacteria from genus the Pseudomonas, which are known as azo-dye biodegrading bacteria. We divided the sand carrier in the biofilter into 3 layers of 10 mm each (an upper, a middle and a bottom layer). The described parameters have been analysed for every layer separately.
Formation of biofilm
For biofilm formation, we used an inoculum of activated sludge (AS) from the wastewater treatment plant (WWTP) of Sofia. Before the immobilisation, AS was sonicated with a US disintegrator (UD-20 automatic) for 3 × 10 s with a frequency of 22 kHz. The flocs of AS were destroyed and a homogenous microbial suspension was obtained.
The immobilisation was carried out based on the spontaneous adsorption of the cells in the suspension to the sand particles. 40 mL of the inoculum were spread on the quartz sand layer. A peristaltic pump ensured an even penetration of the microorganisms in the sand layer. The adsorption of the microorganisms to the sand layer was allowed for two hours. In the next 24 h, synthetic wastewater (described below) without amaranth was supplied in the bioreactor to wash away the unattached microorganisms and to aid the development of the biofilm.
Synthetic wastewater: In the experiments synthetic wastewater was used. The content included mineral solution (NaH2PO4 − 3.5 g L−1, K2HPO4 − 5.0 g L−1, (NH4)2SO4 − 2.5 g L−1, MgSO4.7H2O − 0.3 g L−1, FeSO4 − 0.05 mg L−1, CuSO4 − 0.01 mg L−1, ZnSO4 − 0,005 mg L−1, CoCl2 − 0.005 mg L−1, MgCl2 − 0.005 mg L−1, CaCl2 − 0.005 mg L−1, Na2MoO4 − 0.005 mg L−1), 3% nutritive solution (NaCl 5 g L−1; peptone − 10 g L−1; yeast extract 5 g L−1) and a certain quantity of amaranth (from 10 to 55 mg L−1).
Quartz sand: The sand was provided by the Drinking water treatment plant ‘Bistritza’ (Sofia, Bulgaria). The size of the sand particles was between 0.8 and 1.6 mm.
Amaranth (C.I.Acid Red 27): The model xenobiotic was supplied from Fluka Chemical Corp. The concentrations of amaranth were determined spectrophotometrically (Utrospec3000, Pharmacia Biotech), λ = 520 nm.
Quantity of carbon-containing pollutants (ТOC, COD)
The chemical oxygen demand (COD) was measured according to the standard procedure [Citation18]. The total organic carbon (TOD) was measured with TOC-VCPN Analyzer (Shimadzu Corp.).
The effectiveness of elimination of amaranth was calculated with the following formula (1):
(1)
(1)
where Cin is the concentration of amaranth in the influent in mg L−1 and Ceis the concentration of amaranth in the effluent in mg L−1.
The elimination rate of amaranth was calculated with the following formula (2):
(2)
(2)
where Cres is the concentration difference of amaranth in the influent and the effluent in mg mL−1, Q is the flow rate in mL h−1.
Enzyme activities
For determining the key enzyme activities cell-free extract was used (obtained by the method of Ref. [Citation19] in modification by Ref. [Citation20]).
For the study of the AzoR activity/EC 1.7.1.6/we used the method by Ref. [Citation21]. Catechol-1,2-dioxygenase activity (C12DO)/EC 1.13.11.1/was defined by the method of Ref. [Citation22]. The accumulation of the product of the reaction cis, cis-muconic acid was monitored at 260 nm. The succinate dehydrogenase activity (SDH)/EC 1.3.5.1/was determined by the method described by Ref. [Citation23] by measuring the accumulation of fumarate at 455 nm. All the three enzyme activities were indicators for the rate of the key stages from the biodegradation of the amaranth: AzoR activity was an indicator for the reduction rate of the azo-bond, C12DO was an indicator for the rate of benzene rings cleavage and detoxification and SDH activity provided information for the metabolic activity of the microorganisms as well as for performing full biodegradation of the azo-dye.
The total protein content was determined by the micro-biuret method using bovine serum albumin as a standard.
Microbiological assays
The abundance of the key microbial groups in the biofilm was studied by using the plate count techniques [Citation24]. The azo-degraders (AzoD) were cultivated on Nutrient agar (HiMedia Laboratories) with 50 mg L−1 amaranth. The colonies with decolorisation of the medium were count. The aerobic heterotrophs were cultivated on Nutrient agar (HiMedia Laboratories), and the bacteria from genus Pseudomonas were cultivated on Glutamate Starch Pseudomonas Agar (HiMedia Laboratories). The ratio of the two key groups - Pseudomonas sp. and AzoD were calculated on the base of aerobic heterotrophs (cultivated on Nutrient agar (HiMedia Laboratories)) with the following equations [Citation3,Citation4]:
(3)
(3)
where NPs is the number of Pseudomonas sp. in CFU g−1 and NAH is the number of aerobic heterotrophs in CFU g−1.
(4)
(4)
where NAzoD is the number of azo-degrading bacteria in CFU g−1and NAH is the number of aerobic heterotrophs in CFU g−1.
FISH (fluorescence in-situ hybridisation)
Samples were taken from the three layers of the biofilm (upper, middle and bottom). The samples were fixed in 4% paraformaldehyde. The dehydration and permeabilisation were carried out according to Ref. [Citation25]. A fluorescent signal was obtained by a 5′- labeled oligonucleotide probe (5′- GCT GGC CTA GCC TTC −3′) with fluorescent dye Cy3. As a negative control, the non-sense probe NON338 (5′- ACT CCT ACG GGA GGC AGC −3′) was used [Citation26]. The hybridisation was performed with 20% formamide. After the hybridisation, the samples were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (AppliChem GmbH). The images were taken with a fluorescent microscope Leica Microsystems DFC310FX, under 400 times magnification. The digital processing of the images was done with the software daime [Citation27]. The ratio of the hybridised Pseudomonas sp. to the total quantity of the microorganisms was calculated on the base of the images with DAPI.
Statistical analysis
All data are mean values from three independent repetitions. The results were assessed according to Student & Fisher [Citation28].
Results
The present study demonstrated spatial rearrangement in the biofilm community degrading azo-dye in heavily contaminated wastewater. The gradual increase of the amaranth concentration was used as a selective factor for the development of amaranth degrading biofilm. The increasing toxicity provoked the adaptive changes in the biofilm. The microorganisms from the key genus Pseudomonas participated in a cooperative relationship and formed the necessary for this kind of relations specific spatial distribution. Studied with FISH this effect looked like clustering of cells with strong fluorescence which indicated a high metabolic activity. They formed ‘hot spots’, where the detoxification catabolic processes were very active.
The early stage of the wastewater treatment
At the early stage of the functioning of the lab-scale biofilter (0 h − 191 h), the inflow concentration of amaranth increased from 10 mg L−1 to 30 mg L−1. The effectiveness of elimination of the model xenobiotic was 88.35%. COD of the effluent was 372.74 mgO2 L−1, and TOC − 116.02 mg L−1.At this early stage, the biofilm community showed initial adaptation towards amaranth biodegradation. This was proved by the increase of the effectiveness of elimination of amaranth from 44.44% up to 97.56%. The rate of biodegradation increased more than 5 times and the removed quantity of the azo-dye increased from 4.68 mg L−1 to 33.48 mg L−1. Simultaneously during the studied period, there was an increase of the xenobiotic load in the wastewater with 20 mg L−1.
The results obtained from the cultivation studies showed that the quantity of the azo-degrading bacteria and the bacteria from genus Pseudomonas was highest in the middle layer of the biofilter (1.59 × 107 CFU mL−1). In the middle part of the biofilter, Pseudomonas sp. were 257.28% more than those in the upper part and 66.04% more than the ones in the bottom biofilter part. At this stage of the process, Pseudomonas sp. were almost 92% of the aerobic heterotrophs (Тable 1). This was probably related to the higher toxicity in the upper layer and the relatively low adaptation level of the community. At the same time, it possessed a high potential for adaptation (demonstrated by the high part of the Pseudomonas sp). The three investigated enzyme activities were found at their highest values in the upper layer of the biofilter (AzoR − 57.65% more than the other layers; SDH − 127.27%; C12DO − 158.60%) since the concentrations of the substrates there were the highest (Тable 1).
The Middle stage of wastewater treatment
In the middle treatment stage (191 h − 455 h) under the increased concentrations of amaranth (from 30 mg L−1to 45 mg L−1) the effectiveness of elimination of the azo-dye increased up to 90.60%. The rate of elimination of amaranth was 2.4 times higher than the one in the early stage. The COD of the effluent was 392.78 mgO2 L−1 and TOC − 115.85 mg L−1.The plate count techniques showed the highest quantity of microorganisms from the key groups – AH, AzoD, genus Pseudomonas in the upper layer of the biofilter (Тable 1). The aerobic heterotrophs were more with 98.71% compared to the middle layer and 366.26% than the bottom layer. The difference in the abundance of the key groups was more clearly distinguishable for the microorganisms from the genus Pseudomonas. In the upper layer, they were 3.28 times more compared to the middle and 10.29 times more compared to the bottom layer. The highest activity of the key enzymes was registered in the middle layer of the biofilter. Their values hadn’t changed much compared to the previous time period (AzoR − 7.40 μmol min−1 mg protein−1; SDH − 0.73 μmol min−1 mg protein−1; C12DO − 2.94 μmol min−1 mg protein−1) (Тable 1). A role for this probably played the inhibition in the upper layer due to the increased concentrations of the toxic substance and insufficiently developed cooperative relationships among the microorganisms.
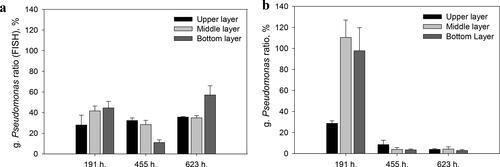
The digital analysis of the images obtained with FISH also showed a decrease of the microorganisms of genus Pseudomonas in the middle (with 13.22%) and the lower layer (with 33.52%) and their increase in the upper sand layer (with 4.36%) (, 455 h). The plate count techniques showed a considerable decrease of the part of genus Pseudomonas from the community in all the three studied layers (20.21% − 106.17%)(, 455 h). Consequently, the increased quantity of the microorganisms from this genus registered within the upper layer was due to the unculturable microorganisms from this group of bacteria.
The late stage of wastewater treatment
At the end of the experiment (455 h − 623 h), the rate of amaranth removal increased by 14.63%, but the effectiveness of its removal slightly decreased to 89.76% due to reaching the highest concentrations of xenobiotics (55 mg L−1) and its toxic effect (). Despite this, the distribution of the culturable microorganisms had not changed significantly and was still considerably lower than what’s registered during the early stage, as was mentioned.
Table 1. Technological, microbiological and enzymological parameters of the downflow sand biofilter in: early stage of the model process (0 h. - 191 h), late stage of the model process (191 h – 455 h) and end of the experiment (455 h – 623 h).
At this final stage of the experiment, when both the concentration of the model xenobiotic and its elimination rate were highest, another evidence for the significance of the unculturable microorganisms was found. The ratio of culturable Pseudomonas sp. and azo-degraders had been further lowered to only 3.72% and 0.89% respectively. The decrease was 75.27% for the microorganisms from genus Pseudomonas and with 12.60% for the azo-degrading bacteria (). At the same time an additional increase in the part of the genus Pseudomonas up to 42.57% has been registered by the FISH method. The obtained data about the genus Pseudomonas in the azo-degradation process highlights the importance of some factors (as the environment and the inter-microbial relationships) which are key for the development of the bacteria in the complex communities and cannot be studied with the standard microbiological methods.
Table 2. Key azo-degrading microorganisms’ ratio to the total quantity of the microorganisms calculated on the base of FISH and standard culture techniques.
The results from the enzyme studies showed a gradual decrease for AzoR activity from the upper (6.04 μmol min−1 mg protein−1) to the bottom layer (1.78 μmol min−1 mg protein−1) and a gradual increase for C12DO from the upper (0.88 μmol min−1 mg protein−1) to the bottom sand layer (2.72 μmol min−1 mg protein−1). In parallel with the active azo-bond reduction, the microorganisms in the biofilm developed their ability to carry out the next step from the catabolic pathway of the amaranth, more precisely – the cleavage of the benzene ring of the aromatic amines by C12DO. For the activity of C12DO, oxygen was needed and its diffusion was facilitated under the decreased density of the clusters in the bottom layer.
Discussion
In the presented study, an azo-degradation process was modelled. At the first stage of the functioning of the sand biofilter, an initial adaptive reaction was registered – a gradual increase of the efficiency and the rate of amaranth removal. The bacteria from the genus Pseudomonas were in the largest numbers in the middle part of the sand carrier. They represented almost 92% of the aerobic heterotrophs there. The highest activities of the main key enzymes from the amaranth biodegradation pathway were found in the upper part of the biofilter most probably because of the highest concentrations of the substrate (amaranth and the intermediate catechol-related metabolites) there.
Our findings are in accordance with the information of the target genus found by other studies. It is well known that the bacteria from the genus Pseudomonas are very active biodegraders of the azo-dyes [Citation29–32]. This is why their amount was found to increase when amaranth was added to the wastewater. Also the approach including the use of complex microbial communities instead of pure cultures is more close to the real wastewater treatment practice. That is why the biofilm derived from activated sludge was used in this and other similar studies [Citation33,Citation34].
In this early stage of treatment, the zones of clusters of microorganisms, showed by FISH analysis, had a small area and were found mainly in the upper and the lower layer. At this stage of the process, the biodegradation and the microbiological parameters showed that the adaptation processes were in their beginning.
The data from the middle stage of functioning (191 h − 455 h) demonstrated that the adaptation of the biofilm progressed. The highest numbers of the key biodegraders (g. Pseudomonas and AzoD) shifted from the middle to the upper layer where they could already cope with the high amaranth concentration. The standard cultivation techniques showed that while the number of the two mentioned groups increased, their part of the community decreased ().
On the contrary, the digital image analysis which estimates Pseudomonas spp. in-situ showed that their part increased. Therefore, the significance of the unculturable bacteria of genus Pseudomonas increased. This indicated that they had a more important role at this stage when the community was well adapted to the biodegradation of the toxic compound.
A similar increase in the quantity of the bacteria engaged in the azo-detoxification process was demonstrated by Ref. [Citation34] for a consortium with attached growth. It was demonstrated also by Ref. [Citation35] for another azo-dye degrading consortium that the community is dominated by Pseudomonas sp. The approach for adaptation of the bacteria in the azo-dye treatment applied by us was used also by Ref. [Citation36].
At this stage, the FISH study of the biofilm community in the three sand layers of the biofilter showed well-formed zones with a high metabolic activity of the microorganisms (). The upper sand layer was the first that had contact with the amaranth and the microorganisms in this layer were in the environment with the highest concentrations of the toxic substance. They needed to cooperate so that they formed dense clusters with high metabolic activity, where: 1) the concentration of the oxygen was probably lower, which favours the activity of the AzoR (the highest AzoR activity was registered), 2) the spatial proximity of the cells facilitated the development of synergetic, symbiotic and co-metabolic relationships, which led to increased biodegradation, 3) due to the increased density of the clusters and the high biodegradation rate the local residual concentrations of the xenobiotic were low, which lowered also the toxicity.
Table 3. FISH of the biofilm from the three layers in the end of the process.
Other authors also highlighted that the bacteria (and especially those from genus Pseudomonas) were more efficient in the azo-dye removal when they inhabited consortia than in the case when they were used in pure cultures [Citation37,Citation38]. Ref. [Citation39] mentions specifically that the increased biodegradation capacity of the complex consortia was due to the combined action of the bacteria and the enzymes that they excrete and also to the adaptation processes carried out in such microbial communities.
At the final stage of the experiment, the biological community maintained its high biodegradation capacity (89.76% efficiency at 55 mg L−1 amaranth). The azo-degradation activity of the microbial community was distributed in the following way: upper layer – a maximal concentration of amaranth, a maximal activity of AzoR, a minimal activity of C12DO (since the concentration of the aromatic amines, obtained from the azo-dye was low); middle layer – the concentration of the azo-dye decreased in the upper layer (the substrate for AzoR was in lower concentration and respectively, AzoR activity was lower; at the same time, the aromatic amines generated in the upper layer pass with the current of the water to the middle layer and induced increased activity of C12DO in this sand layer); bottom layer – the azo-dye was almost fully depleted, which had reflected in minimal AzoR activity, but the concentration of the aromatic intermediate products was the highest in this layer (due to their accumulation from the upper and the middle layers), which was the reason for the registering maximal activity of C12DO.
We used this model azo-dye treatment process to provoke adaptation changes in a biofilm community. This enabled us to estimate some aspects of its development related to efficient treatment of azo-dye contaminated waters. Most of the studies in the scientific databases are focused only on the bacteria performing the azo-degradation [Citation32,Citation40–42] or on the technology of the azo-removal [Citation33,Citation43–45]. However, often the ecological aspects of the bacterial communities (spatial distribution within the biofilms and sludges, cooperation, and adaptation) are essential for the development of successful technology. The efficient management of the mentioned ecological aspects, on which the presented study was focused, continues to be an interdisciplinary novelty with a significant importance in real practice.
Conclusions
In this study, microstructural changes were found among the bacteria from genus Pseudomonas in an azo-dye degrading biofilm. Microhabitats with high activity had been formed. They were related to the increasing biodegradation capacity of the sand biofilter driven by the increasing amaranth concentration. The activities of the key enzymes were linked with the structure of the microbial community and the elimination of the xenobiotic.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Holme I. Sir William Henry Perkin: a review of his life, work and legacy. Color Technol. 2006;122(5):235–251.
- Paz A, Carballo J, Pérez MJ, et al. Biological treatment of model dyes and textile wastewaters. Chemosphere. 2017;181:168–177.
- Sandhya S. Biodegradation of azo dyes under anaerobic condition: role of azoreductase BT. In: Erkurt HA, editor. Biodegradation of azo dyes. Berlin: Springer Berlin Heidelberg; 2010. p. 39–57. https://doi.org/10.1007/698_2009_43
- Vikrant K, Giri BS, Raza N, et al. Recent advancements in bioremediation of dye: current status and challenges. Bioresour Technol. 2018;253:355–367.
- Singh P, Iyengar L, Pandey A. Bacterial decolorization and degradation of azo dye. In: Singh SN, editor. Microbial degradation of xenobiotics. Berlin: Springer Berlin Heidelberg; 2012. p. 101–133. https://doi.org/10.1007/978-3-642-23789-8_4
- Rawat D, Mishra V, Sharma RS. Detoxification of azo dyes in the context of environmental processes. Chemosphere. 2016;155:591–605.
- Ribeiro AR, Umbuzeiro GA. Effects of a textile azo dye on mortality, regeneration, and reproductive performance of the planarian, girardia tigrina. Environ Sci Eur. 2014;26(1):22.
- Cao Y, Chen X, Feng S, et al. Nanofiltration for decolorization: membrane fabrication, applications and challenges. Ind Eng Chem Res. 2020;59(45):19858–19875.
- Deng D, Lamssali M, Aryal N, et al. Textiles wastewater treatment technology: a review. Water Environ Res. 2020;92(10):1805–1810.
- Shindhal T, Rakholiya P, Varjani S, et al. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered. 2021;12(1):70–87.
- Pavithra KG, Kumar PS, Jaikumar V, et al. Removal of colorants from wastewater: a review on sources and treatment strategies. J Ind Eng Chem. 2019;75:1–19.
- Khan R, Bhawana P, Fulekar MH. Microbial decolorization and degradation of synthetic dyes: a review. Rev Environ Sci Biotechnol. 2013;12(1):75–97.
- Sarkar S, Banerjee A, Halder U, et al. Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng. 2017;2(4):121–131.
- Popli S, Patel UD. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol. 2015;12(1):405–420.
- Khehra MS, Saini HS, Sharma DK, et al. Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res. 2005;39(20):5135–5141.
- Ben Mansour H, Mosrati R, Corroler D, et al. In vitro mutagenicity of acid violet 7 and its degradation products by Pseudomonas putida mt-2: Correlation with chemical structures. Environ Toxicol Pharmacol. 2009;27(2):231–236.
- Cao B, Loh KC. Catabolic pathways and cellular responses of Pseudomonas putida P8 during growth on benzoate with a proteomics approach. Biotechnol Bioeng. 2008;101(6):1297–1312.
- APHA. 2005. Standard methods for the examination of water and wastewater. 21th ed. Washington DC: American Public Health Association/American Water Works Association/Water Environment Federation.
- Feist CF, Hegeman GD. Phenol and benzoate metabolism by Pseudomonas putida: Regulation of tangential pathways. J Bacteriol. 1969;100(2):869–877.
- Topalova Y, Dimkov R, Manolov R. Influence of aryl—containing xenobiotics concentration on the oxygenase enzyme activities. Biotechnol Biotechnol Equip. 1994;8(1):62–67.
- Zimmermann T, Kulla HG, Leisinger T. Properties of purified orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem. 1982;129(1):197–203.
- Willetts AJ, Cain RB. Microbial metabolism of alkylbenzene sulphonates. Bacterial metabolism of undecylbenzene-p-sulphonate and dodecylbenzene-p-sulphonate. Biochem J. 1972;129(2):389–402.
- Veeger C, Darvactanian DV, Zeylemaker WP. Succinate dehydrogenase. In: Colowick SP, Kaplan NO, editors. Methods in enzymology. Vol. XIII. New York: Academic Press; 1969. p. 81–90. https://doi.org/10.1016/0076-6879(69)13020-7
- Collins CH, Lyne PM, Grange JM, et al. 2004. Collins and lyne’s microbiological methods. 8th ed. London: Arnold.
- Nielsen PH, Daims H, Lemmer H, et al. 2009. FISH handbook for biological wastewater treatment: Identification and quantification of microorganisms in activated sludge and biofilms by FISH. London: IWA Publishing.
- Loy A, Maixner F, Wagner M, et al. probeBase-an online resource for rRNA-targeted oligonucleotide probes: new features 2007 . Nucleic Acids Res. 2007;35(Database issue):D800–D804.
- Daims H, Lücker S, Wagner M. Daime, a novel image analysis program for microbial ecology and biofilm research. Environ Microbiol. 2006;8(2):200–213.
- Fisher RA. 1990. Statistical methods, experimental design, and scientific inference: a re-issue of statistical methods for research workers, the design. Oxford: Oxford University Press.
- Bera SP, Tank SK. Bioremedial approach of Pseudomonas stutzeri SPM-1 for textile azo dye degradation. Arch Microbiol. 2021;203(5):2669–2680.
- Chebet J, Masarbo RS, Karegoudar TB, et al. Studies on decolourisation of azo dye orange G by bacterium isolated from dye contaminated sites. Int J Environ Anal Chem. 2021. DOI: 10.1080/03067319.2021.1956481
- Joshi AU, Hinsu AT, Kotadiya RJ, et al. Decolorization and biodegradation of textile di-azo dye Acid Blue 113 by Pseudomonas stutzeri AK6. 3 Biotech. 2020;10(5):214
- Zhang Q, Xie X, Xu D, et al. Accelerated azo dye biodegradation and detoxification by Pseudomonas aeruginosa DDMZ1-2 via fructose co-metabolism. Environ Technol Innov. 2021;24:101878.
- Ong C, Lee K, Chang Y. Biodegradation of Mono azo dye-Reactive orange 16 by acclimatizing biomass systems under an integrated anoxic-aerobic REACT sequencing batch moving bed biofilm reactor. J Water Process Eng. 2020;36:101268.
- Zhu Y, Wang W, Ni J, et al. Cultivation of granules containing anaerobic decolorization and aerobic degradation cultures for the complete mineralization of azo dyes in wastewater. Chemosphere. 2020;246:125753.
- Tacas ACJ, Tsai PW, Tayo LL, et al. Degradation and biotoxicity of azo dyes using indigenous bacteria-acclimated microbial fuel cells (MFCs). Process Biochem. 2021;102:59–71.
- Srinivasan S, Sadasivam SK. Biodegradation of textile azo dyes by textile effluent non-adapted and adapted Aeromonas hydrophila. Environ Res. 2021;194:110643
- Didier de Vasconcelos GM, Mulinari J, de Arruda Guelli Ulson de Souza SM, et al. Biodegradation of azo dye-containing wastewater by activated sludge: a critical review. World J Microbiol Biotechnol. 2021;37:101.
- Haque M, Haque A, Mosharaf K, et al. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J Biol Sci. 2021;28(1):793–804.
- Kapoor RT, Danish M, Singh RS, et al. Exploiting microbial biomass in treating azo dyes contaminated wastewater: mechanism of degradation and factors affecting microbial efficiency. J Water Process Eng. 2021;43:102255.
- Chaturvedi A, Rai BN, Singh RS, et al. A computational approach to incorporate metabolite inhibition in the growth kinetics of indigenous bacterial strain Bacillus subtilis MN372379 in the treatment of wastewater containing Congo red dye. Appl Biochem Biotechnol. 2021;193(7):2128–2144.
- Öztürk A, Bayol E, Abdullah MI. Characterization of the biosorption of fast black azo dye K salt by the bacterium Rhodopseudomonas palustris 51ATA strain. Electron J Biotechnol. 2020;46:22–29.
- Poorasadollah D, Lotfabad TB, Heydarinasab A, et al. Biological activated carbon process for biotransformation of azo dye carmoisine by Klebsiella spp. Environ Technol. 2021;5:1–17.
- Bekhit F, Farag S, Attia AM. Decolorization and degradation of the azo dye by bacterial cells coated with magnetic iron oxide nanoparticles. Environ Nanotechnol Monit Manage. 2020;14:100376.
- Oliveira JMS, de Lima e Silva MR, Issa CG, et al. Intermittent aeration strategy for azo dye biodegradation: a suitable alternative to conventional biological treatments? J Hazard Mater. 2020;385:121558.
- Selvaraj V, Swarna Karthika T, Mansiya C, et al. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J Mol Struct. 2021;1224:129195.