Abstract
The global market of monoclonal antibodies (mAbs) is growing very fast. The aim of this study is to analyze the reimbursed market of adalimumab and pemborlizumab in Bulgaria. A retrospective cost analysis of the reimbursed mAbs adalimumab and pemborlizumab for ambulatory and hospital settings, respectively, paid by the National Health Insurance Fund (NHIF) was performed based on official NHIF data. The share of their costs from the total mAbs costs was calculated. The results showed that the most cost-consuming mAbs were adalimumab in ambulatory and pembrolizumab in hospital settings: 31% and 31.59% in 2020 out of the total reimbursed mAbs budget, respectively. Regardless of the extension of the reimbursed therapeutic indications, the costs for adalimumab decreased from 25,575,836.32 Euro in 2018 to 20,493,187.02 Euro in 2019 as a result of inclusion of 3 biosimilars and international price referencing. Despite the reduction of the registered price with 20%, the pembrolizumab costs have almost tripled probably because of reimbursement of five additional therapeutic indications. Overall, the reimbursed costs for pemborlizumab have been increased every year as a result of reimbursement of new therapeutic indications and increase in the number of patients treated with mAbs. The adalimumab trend is for a stable decline in the reimbursed costs because of entrance of biosimilars.
Introduction
Аntibodies (Abs) are protein components of the immune system related to cellular and humoral reactions to different substances [Citation1]. Monoclonal antibodies (mAbs) are made by one type of immune cells, which makes them identical. Since 1975, when Kohler and Milstein created the first mAb by fusion of murine myeloma cells with murine–antibody-secreting lymphocytes, many more mAb have been developed and used for different purposes such as for diagnostic procedures and immunotherapy [Citation2].
The number of approved mAbs as medicines reached almost 100 [Citation1]. Their revolutionary potential in the treatment of various nonmanageable diseases with no existing or specific therapies such as cancers, autoimmune diseases, cardiovascular, respiratory, rare diseases, neurological diseases, infections including COVID-19 etc., has been revealed not only during clinical studies but in the real-world settings [Citation3–7]. mAbs ensure better therapeutic outcomes, delay of progression and prolongation of overall survival, respectively, and improved quality of life of those affected [Citation8–13].
Historically, human and humanized monoclonal antibodies (mAbs) were approved as the most suitable therapeutic antibodies [Citation14]. By detecting novel biomarkers, identification of the intimate molecular mechanisms of different diseases, modifications in the structure and application of significant technological advances, scientists discover new mAbs with higher specificity which is associated with better efficacy [Citation15]. Crucial issues is reaching high therapeutic effect parallel with overcoming the side effects and the significant ecnomic burden of mAbs [Citation15]. The percentage of successfully marketed mAbs motivates hundreds of companies to engage themselves in mAbs development. Over 500 mAb names have been provided by World Health Organization till 2017. Clinical trials of more than 570 mAbs are currently ongoing and about 90% of them are in the early phase of safety and efficacy assessment in different patient populations. About 70% of the mAbs entering phase 1 clinical trials are targeted to cancer. The ratio of mAbs entering or located in Phase 2 and Phase 3 with onco-indication and those with another indication is almost equal [Citation16]. In the last 10 years, the number of mAbs in the last phases of clinical trials has tripled to 79 compared to 2010, when it was 26.
Fastly, mAbs became the most preferrable class of new medicines by the pharmaceutical companies as their market share has been growing every year: US$115.2 billion in 2018 and the expectations are to reach almost 300 billion dollars in 2025 [Citation15]. The impact of mAbs on the budget is high with increasing trend leading to challenges for the reimbursement institutions [Citation17]. The entrance of biosimilars is defined as an instrument “in reducing costs for medication and increasing patient access to treatment” [Citation18]. They are shown to decrease the prices and to influence utilization of biologic medicines [Citation19].
The aim of the study is to analyze the reimbursed market of the most cost consuming mAbs for the ambulatory and hospital practice in Bulgaria: adalimumab and pemborlizumab.
Methods
This is a cross-sectional top down cost study performed through the analysis of three data sets. A qualitative analysis was applied to identify the number and INNs of all marketing authorized mAbs in the European Union and reimbursed in Bulgaria.
Information about the authorized mAbs in the EU (by October 2020) was obtained from the European Medicines Agency website [Citation20]. The availability of mAbs in Bulgaria was analyzed by crossing the medicines identified in the European list with those in the Bulgarian Positive Drug List (PDL) Annex 1 and in Annex 2, group B (medicinal products guaranteed by the National Health Insurance Fund (NHIF) budget, intended for treatment of malignant diseases paid in hospital care beyond the cost of medical services).
A retrospective 5-year cost analysis of mAbs paid by the National Health Insurance Fund (NHIF) in Bulgaria for ambulatory (group A, Annex 1 of the Positive Drug List (PDL)) settings (at community pharmacy level) and a 3-year analysis for hospital (group B, Annex 2 of PDL) settings were performed. Data for the reimbursed costs and the number of health insured patients treated with mAbs were received officially from the NHIF website; more detailed information was obtained through a dedicated application to the National Health Insurance Fund for access to public information in 2020 (entry No. 24-03-381/12.11.2020).
The most cost-consuming mAbs for both settings were identified and the share of their costs in the total mAbs costs was calculated. A detailed costs reimbursed packages analysis for the most cost-consuming mAbs by NHIF code and ICD code was conducted.
The costs were extrapolated for the next 5-year period using the TREND function in Microsoft Excel.
The expenditures for every year were presented in euro at the fixed exchange rate of 1 BGN = 0.5113 Euro (European Central Bank/Euro foreign exchange reference rates).
Results
Availability and affordabily of monoclonal antibodies in Bulgaria
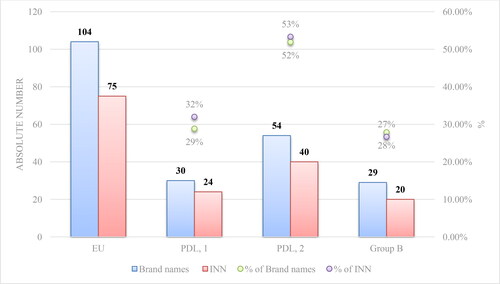
There are 74 monoclonal antibodies authorized for sale in the European Union as of October 2020, the number of brand names in total being 104. Antineoplastic and immunomodulating agents (section L of the Anatomical Therapeutic Chemical Classification System) composed 76% of all brand names and 69% of all mAbs international nonproprietary names ().
Table 1. Monoclonal antibodies authorized in the EU (by October 2020) and included in the PDL in Bulgaria.
We found that 30 out of 104 mAb-containing medicinal products are reimbursed by the National Health Insurance Fund of Bulgaria for ambulatory settings, 54 out of 104 are included in the PDL, Annex 2 for hospital use and 28 out of 104 are oncology products part of group B (Group B contains medicines for malignancies, life-threatening haemorrhages and emergency surgical and invasive interventions for congenital coagulopathies, paid by the NHIF beyond the cost of hospital medical services).
The percentages of reimbrused mAbs in Bulgaria from the total authorized mAbs in the EU are presented in .
Figure 1. Comparison of the authorized mAbs in the EU and the reimbursed ones in Bulgaria by brand name and INN.
Most mAbs are reimbursed for the purposes of hospital use probably because of the ‘restricted’ medical prescription of some biologic medicines, reserved for use only in certain specialized areas. Twenty-six biosimilar mAbs are authorized by the European Commission, corresponding to 5 INNs (adalimumab, bevacizumab, infliximab, rituximab and trastuzumab). Health-insured Bulgarian patients have access to biosimilars containing adalimumab, bevacizumab, infliximab, rituximab and trastuzumab ().
Utilization analysis
mAbs included in PDL, Annex 1: Adalimumab costs
The total number of mAbs in Annex 1 of PDL is 24. Of these, 6 trade names are biosimilars with INN adalimumab or infliximab. Compared to 2016, the total costs for mAbs in 2020 increased by nearly 56% (45 203 510.19 Euro in 2016 and 70 694 933.36 Euro in 2020). An increasing trend is observed for almost all mAbs with some exceptions. Given the presence of biosimilars of infliximab, its costs decreased from 3 703 887.33 Euro in 2018 to 1 952 417.63 Euro in 2020 ().
Table 2. Reimbursed costs for mAbs included in PDL, Annex 1 for the period 2016–2020.
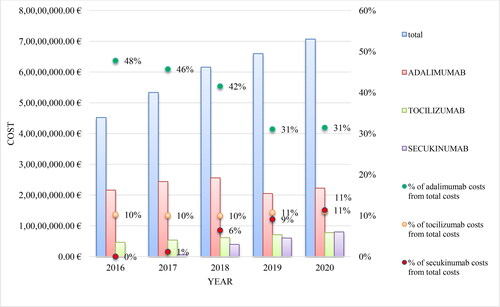
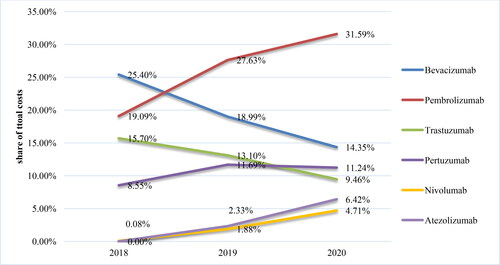
For the period 2016–2020, adalimumab, tocilizumab and secukinumab were the most cost-consuming mAbs. The costs for adalimumab amounted to 21 596 486.83 Euro in 2016 and 25 575 836.32 Euro in 2018. А decrease of nearly 5 million Euro was reported in 2019 (20 493 187.02 Euro), which is most likely a result of the entrance of adalimumab biosimilars in late 2018 and early 2019 and the reference pricing. The costs for adalimumab have increased to 22 231 046.97 Euro in 2020 on the background of the increased number of patients, inclusion of new indications for adalimumab and reduction in the relative share of adalimumab costs from the total mAbs costs (from 48% in 2016 to 31% in 2020). NHIF paid more for tocilizumab each following year: from 4 632 684.91 Euro in 2016 to 7 772 726.31 Euro in 2020, but the share of the total expenditures remains relatively constant around 10–11%. For secukinumab there was an increase in 2020 in comparision with 2017 by almost 13 times: 627 051.86 Euro in 2017 and 8 004 032.82 Euro in 2020 (from 1% of the total costs in 2017 to 11% in 2020) ().
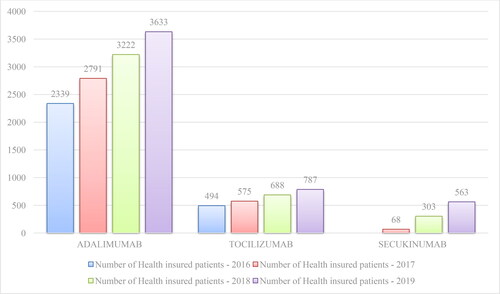
The total costs paid by the NHIF for mAbs for the period 2016 − 2020 have increased each year. This can be explained by the inclusion of new mAbs in the PDL, extension on eligible patient populations related to reimbursement of new therapeutic indications, and the overall increase in the number of patients treated with mAbs (). Tocilizumab cost followed an increasing trend, perhaps due to the lack of biosimilars and expansion of eligible patients’ population. The cost for secukinumab increased sharply in accordance with the growing number of patients for the period 2019 − 2020.
When a new product is included in the PDL, a strident increase in the costs of the National Health Insurance Fund is observed in the second year, followed by a smoother one. For the products launching in the BG market after 2017 in the second year, NHIF paid an average of 7.6 times more than in the first year (min. 2.4 times more for alemtuzumab, and max. 15.9 times more for mepolizumab) ().
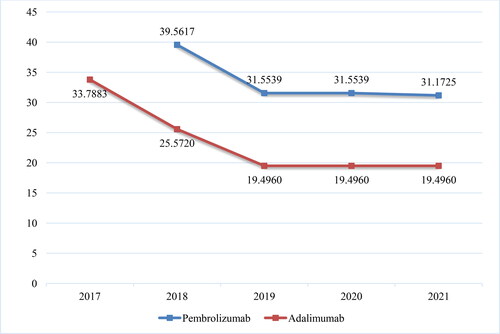
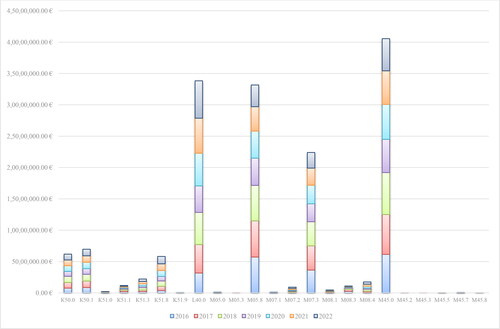
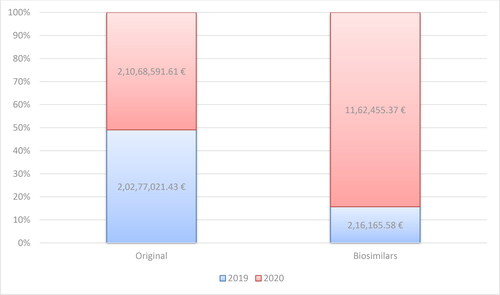
Bisomilars for adalimumab have been available in the PDL since the end of 2018. Up to May 2021, NHIF has been covering the cost of adalimumab for 21 different therapeutic indications: Other disorders of the eye and adnexa (H20.1, H20.8, H30.0, H30.1, H30.2, H30.8), Diseases of the digestive system (К50.0, К50.1, К51.0, К51.1, К51.3, К51.5, К51.8, К51.9), Diseases of the skin and subcutaneous tissue (L40.0, L73.2) and Diseases of the musculoskeletal system and connective tissue (M05.0, M05.1, M05.3, M05.8, M07.1). For all biosimilars, there is an increase in the reimbursed costs for 2020 compared to 2019 related to an almost double decrease in the reference price defined per DDD (2.9 mg) of Adalimumab (33.788 Euro in 2017 to 19.496 Euro in 2020) (). This is because of the introduction of new local legislation in April 2019, concerning the price of biosimilars on the one hand, and on the other – international price referencing every 6 months for origrinal product before biosimilars. When included in the PDL the registered price of biosimilars cannot be higher than 80% of the price of the original product. This requirement is in addition to the previous one that the price should be lower among the referent countries. The costs for the reference product have increased, but not as significantly as those for biosimilars (). The original product’s market share has decreased in 2020 in constrast to biosimilars: 98.95% vs. 1.05% in 2019 and 94.77% vs. 5.23% in 2020.
Figure 5. Reimbursed costs for the original product and biosimilars with INN Adalimumab for the period of 2019–2020.
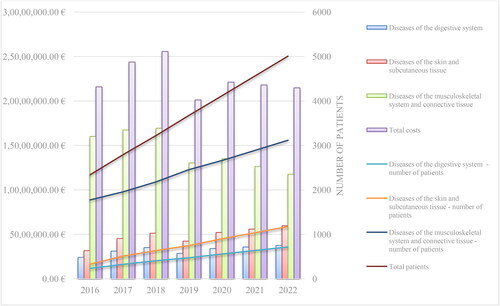
The costs for the period 2016-2020 and the extrapolated costs and number of patients for the period of 2021–2022 are shown in . The expectations are for a decrease in the total costs for adalimumab in the period of 2020–2022 and an increase in the total number of patients. The revealed trend in 2020 can be explained by the fact that the NHIF is starting to pay for several additional therapeutic indications: Chronic iridocyclitis (H20.1), Other iridocyclitis (H20.8), Focal chorioretinal inflammation (H30.0), Disseminated chorioretinal inflammation (H30.1), Posterior cyclitis (H30.2), Other chorioretinal inflammations (H30.8) and Hidradenitis suppurativa (L73.2). It is important to mention that these new indications are reimbursed for the original product only and there is no internal referencing of a defined price per DDD with biosimilars for a very short period (January–May 2020).
The highest average annual reimbursed costs are those for M45.0 (Ankylosing spondylitis, Multiple spinal involvement) (around 5.7 million Euro), psoriasis (L40.0) (around 4.8 million Euro), M05.8 (Other seropositive rheumatoid arthritis) (around 4.7 million euro) and M07.3 (Other psoriatic arthropathies) (around 3.2 million Euro). The annual costs are without significant fluctuations, but show a tendency for a greater impact in 2020–2022 (). For the whole period the lowest expenditures are those for M45.2-M45.8.
mAbs included in group B: Pembrolizumab costs
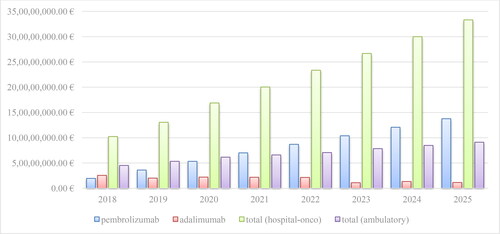
The total number of mAbs in PDL, Annex 2 is 40 and 12 of the brand names are biosimilars with INN - rituximab, trastuzumab, bevacizumab, infliximab and adalimumab. Their number in Group B is 20 and biosimilars are available for rituximab, trastuzumab and bevacizumab. For the purposes of this analysis, the data officially published in the NHIF website about the reimbursed costs for mAbs from group B (medicinal products included in the package guaranteed by the NHIF budget for the treatment of malignant diseases and medicinal products for life-threatening bleeding) were used. In general, there is an increase in the total cost for mAbs in group B due to the inclusion of new INNs and an increase in the number of patients: from 102 393 242.82 Euro in 2018 to 168 827 502.53 Euro in 2020. The highest are the costs for pembrolizumab: 19 547 559.82 Euro in 2018, 36 072 364.92 Euro in 2019 and 53 327 370.83 Euro in 2020. This increase is nearly 3 times in 2020 compared to 2018 (; ). The reference price for pembrolizumab followed a tendency for lower values: 39.5617 Euro/mg in 2018 to 31.5530 Euro/mg in 2020. The costs for pembrolizumab have increased despite the reduction in the registered price by 20% (), most likely due to the inclusion of 5 additional therapeutic indications which are paid by the NHIF.
Table 3. mAbs costs from group B*.
Trends in the market of mAbs adalimumab and pembrolizumab
The trend for the analyzed period shows growing of pembrolizumab and decline in adalimumab reimbursed market. The extrapolated data for the next 5-year period illustrate the same trend for both mAbs without considering any other influencing factors (such as international price referencing and inclusion of other biosimilars with INN adalimumab) ().
Discussion
Our study revealed that more than half of the mAbs authorized in the EU are included in the reimbrusement medicines list for hospital use, more of them being for the oncology and antireumatic practice. In contrast, the number of mAbs for ambulatory practice is far below 50% (around 32%) of all 74 mAbs accessible at the European market. We could conclude that Bulgarian patients have a good access to mAbs, as the health-insurance fund covers both ambulatory and hospital settings. A study conducted in Bosnia and Herzegovina also concluded that there appeared to be a relatively good availability of mAbs despite some limitations [Citation21].
Similar to other studies, our study confirmed that biosimilars decrease the prices of mAbs at the moment of their entrance in the reimbursement list due to legislation reguirements, as the external and internal prices referencing. Further reduction can be expected as a rezult of relevant hospital tender procedures. By reducing the treatment costs, the opportunity for better access to effective biological medicines for the citizens grows [Citation19,Citation20,Citation22–26]. Despite this correlation, the trend is for expansion of mAbs reimbursed market for both ambulatory and hospital settings in Bulgaria as a result of treatment of more patients and reimbursment approval for additional therapeutic indications.
Considering inclusion of bisoimilars for adalimumab and market protection period for pembrolizumab since 2015, the expectations are for lower total reimbursed costs for adalimumab and growing the volume of pembrolizumab market in the next years. Despite the negative trend for the adalimumab market, it is still the most cost-consuming mAb for the ambulatory practice in Bulgaria. It exceeds the cost for the next most consuming mAb, tocilizumab, almost 3 times in 2020 having more and more eligible patients. Adalimumab was the top-selling medicine of all times with sales of US$20 billion in 2018 generating 60% of AbbVie’s revenue. In contrast, the market conditions (increased competition and expiring patents) for adalimumab are leading to a negative trend and a decline in the costs, pembrolizumab seems to be the next medicine number one in sales [Citation19]. Approval of additional indications for pembrolizumab (melanoma, nonsmall cell lung cancer, small cell lung cancer, head and neck cancer, Classical Hodgkin Lymphoma, primary mediastinal large B-cell lymphoma, urothelial carcinoma, microsatellite instability-high (MSI-H) cancer, renal carcinoma etc.) affirms the hypothesis for the growth of its market [Citation27,Citation28]. A similar trend is shown in a study by Elias et al. (2016) - by 2016, pembrolizumab ranked first in the list, representing 64% of the total cost of lung cancer treatment in Lebanon [Citation29]. According to a study from 2016, the costs of oncology drugs were expected to rise above $150 billion by 2020, and pembrolizumab and nivolumab are defined as “harbingers of the market for cancer immunotherapies” [Citation30]. A Chinese report also predicts that the pembrolizumab sales revenue has increased significantly, as the Compound annual growth rate (CAGR) was 284.67% from 2018 to 2020 [Citation31]. A study revealed that ipilimumab, nivolumab and pembrolizumab have substantial costs which have tripled for only a 1-year period (120 million euros in 2015 to >340 million Euro in 2016) [Citation32]. Despite the expenses for pembrolizumab, many studies prove its cost-effectiveness and enough incremental clinical and economic benefits [Citation33–36]. The other possible reason for the market share increase migth be the fact that the products for hospital use are less affected by the internal prices referencing.
The main limitation of our study is that we did not include data for hospital use on mAbs not indicated for oncology patients due to limited access to information for a previous period. Moreover, we have not considered additional factors which could also infuence the future costs and trends for the mAbs market in Bulgaria. This is not the first study focusing on the biotechnology medicines but it seems to be one of the few developing the trends in the mAbs reimbursed market in this country. At some level, it could be used as a basis for predicting and planning the health-insurance budget and developing strategies for the sustainability of the healthcare system.
Conclusions
The reimbursed costs for pemborlizumab have increased in recent years as a result of reimbursement of new therapeutic indications and increase in the number of treated patients. This increasing is difficult to optimize with now applicable cost-containment measures like international price referencing and compensated mechanism of NHIF expenditures. The adalimumab trend is for a stable decline in the reimbursed costs probably because of biosimilars entrance and reduction in the international price referencing. The nationally-based expectations are for a stable expansion of the total mABs reimbursed market as a result of more eligible patients for treatement with mAbs and approval of additional therapeutic indications subject to reimbursement.
Funding
This research received no external funding.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Data sharing not applicable.
Disclosure statement
The authors declare no conflict of interest.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Overview of therapeutic monoclonal antibodies. Available online: https://www.uptodate.com/contents/overview-of-therapeutic-monoclonal-antibodies/print.
- Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. Am J Med Sci. 2002;324(1):14–30.
- Singh S, Kumar NK, Dwiwedi P, et al. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2018;13(2):85–99.
- Jahanshahlu L, Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother. 2020;129:110337.
- Shanmugaraj B, Siriwattananon K, Wangkanont K, et al. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19)). Asian Pac J Allergy Immunol. 2020;38(1):10–18.
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15(6):361–370.
- Nissim A, Chernajovsky Y. Historical development of monoclonal antibody therapeutics. Handb Exp Pharmacol. 2008;(181):3–18.
- Corren J, Castro M, Chanez P, et al. Dupilumab improves symptoms, quality of life, and productivity in uncontrolled persistent asthma. Ann Allergy Asthma Immunol. 2019;122(1):41–49.e2.
- Weisel K, Ludwig H, Rieth A, et al. Health-related quality of life of carfilzomib- and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma, based on german benefit assessment data. Qual Life Res. 2020;29(1):69–79.
- Mazieres J, Kowalski D, Luft A, et al. Health-related quality of life with carboplatin-paclitaxel or nab-paclitaxel with or without pembrolizumab in patients with metastatic squamous non-small-cell lung cancer. J Clin Oncol. 2020;38(3):271–280.
- Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153)). J Thorac Oncol. 2019;14(9):1628–1639.
- Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36(16):1579–1587.
- Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines – recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042.
- Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1).
- Liu JK. The history of monoclonal antibody development – progress, remaining challenges and future innovations. Ann Med Surg (Lond)). 2014;3(4):113–116.
- Kaplon H, Reichert JM. Antibodies to watch in 2019. MAbs. 2019;11(2):219–238.
- Biot J, Fasano C, Dos Santos C. D’orthoclone au dénosumab: l’expansion des anticorps monoclonaux à des fins thérapeutiques [from orthoclone to denosumab, the fast growing market of monoclonal antibodies]. Med Sci (Paris)). 2009;25(12):1177–1182. PMID: 20035702.
- Moorkens E, Jonker-Exler C, Huys I, et al. Overcoming barriers to the market access of biosimilars in the European union: the case of biosimilar monoclonal antibodies. Front Pharmacol. 2016;7(7):193.
- Tachkov K, Mitkova Z, Boyadzieva V, et al. Did the introduction of biosimilars influence their prices and utilization? The case of biologic disease modifying antirheumatic drugs (bDMARD) in Bulgaria. Pharmaceuticals. 2021;14(1):64.
- Tamer J, Grozev J, Kamusheva M, et al. PNS155 access of bulgarian patients to biosimilar medicines. Value Health. 2019;22(3):S787.
- Tubic B, Marković-Peković V, Jungić S, et al. Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: findings and implications. Medicine Access @ Point of Care. 2021;5:239920262110276–239920262110277.
- Stoyanova S, Yordanov E, Hristov E, et al. Availability, affordability and drug utilization of biosimilar medicinal products, containing monoclonal antibodies in Bulgaria. SAGE J Generic Med. 2021.
- Povero M, Pradelli L. Funding innovation thanks to anti-TNF-α biosimilars uptake. Econom Imp Italy. 2020; 21(1).
- Inotai A, Csanádi M, Vitezic D, et al. Policy practices to maximise social benefit from biosimilars. J Bioequiv Availab. 2017;(9):467–472.
- Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017;12(12):e0190147.
- Moorkens E, Godman B, Huys I, et al. The expiry of humira® market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy Meas Front Pharmacol. 2020;11:591134.
- Flynn J, Gerriets V. Pembrolizumab. 2021. StatPearls Publishing LLC. Bookshelf ID: NBK546616PMID: 31536223
- Kwok G, Yau TC, Chiu JW, et al. Pembrolizumab (keytruda). Hum Vaccin Immunother. 2016;12(11):2777–2789.
- Elias F, Bou-Orm IR, Adib SM, et al. Cost of oncology drugs in the Middle-Eastern country of Lebanon: an update (2014-2016). J Glob Oncol. 2018;4:1–7.
- Oncology pharma costs to exceed $150 billion by 2020. Manag Care. 2016;25(10):40.
- Investigation Report on China’s Pembrolizumab Market, 2021–2025. April 2021. Available online: https://www.researchandmarkets.com/reports/5312028/investigation-report-on-chinas-pembrolizumab?utm_source=GNOM&utm_medium=PressRelease&utm_code=d7f6c8&utm_campaign=1525398+-+China+Pembrolizumab+Market+Report+2021%3a+Market+-Size+will+Expand+in+the+Future+Due+to+the+Increased+Numbers+of+Approved+Indications&utm_exec=chdo54prd.
- Denis H, Davoine C, Bermudez E, et al. [Specific immunotherapies in the treatment of cancers]. Bull Cancer. 2019;106(1):37–47.
- Bensimon AG, Zhou ZY, Jenkins M, et al. An economic evaluation of pembrolizumab versus other adjuvant treatment strategies for resected high-risk stage III melanoma in the USA. Clin Drug Investig. 2020;40(7):629–643.
- Barrington DA, Dilley SE, Smith HJ, et al. Pembrolizumab in advanced recurrent endometrial cancer: a cost-effectiveness analysis. Gynecol Oncol. 2019;153(2):381–384.
- Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21(12):1191–1205.
- Bensimon AG, Zhong Y, Swami U, et al. Cost-effectiveness of pembrolizumab with axitinib as first-line treatment for advanced renal cell carcinoma. Curr Med Res Opin. 2020;36(9):1507–1517.