Abstract
Terpenoids are aromatic metabolites in plants. They can improve the adaptability of plants to the environment. Some specialized terpenoids have immense value to people because of the applications to medicine, industry and agriculture. However, it is impossible to produce terpenoids in large quantities because of their complex chemical structures and low content in plants. The regulatory mechanism of terpenoids biosynthesis has gradually become a research hot spot. Here, we provide an overview on the synthesis and metabolic pathways, regulatory pathways, and key enzymes in the biosynthetic pathways of terpenoids. This paper will provide references for further exploring the molecular regulation and improving the content of terpenoids in plants.
Introduction
Terpenoids or terpenes, with various structure and functions, exist in different living organisms including plants, animals, fungi, as well as bacteria [Citation1–3]. The chemical formula of terpenoids is (C5H8)n, where n represents the number of isoprenes contained in the compound. According to the number of n, terpenoids are divided into hemiterpene (C5, n = 1), monoterpene (C10, n = 2), sesquiterpene (C15, n = 3), diterpene (C20, n = 4), sesterterpene (C25, n = 5), triterpene (C30, n = 6), tetraterpene (C40, n = 8), and polyterpene [Citation4]. The contents of monoterpenes and sesquiterpenes are higher in various volatile oils. Diterpenes such as resins, chlorophyll and phytoalcohol are crystalline solids characterized by high molecular weight as well as poor volatility [Citation5–7]. Triterpenes are mostly found in saponins, resins and phytosterols; tetraterpenes are mostly found in plant carotenes; and polyterpenes are mostly found in plastoquinones [Citation8,Citation9].
In plants, terpenoids are classified into primary metabolites and secondary metabolites. The primary metabolites include phytohormones, steroids, carotenoids and polyterpene alcohols, while the secondary metabolites include monoterpene menthol, sesquiterpene artemisinin, diterpene paclitaxel, triterpene ginsenoside and tetraterpene carotenoid [Citation10]. The main functions of terpenoids include enhancing plant disease resistance and helping plants to resist natural enemies in a direct and indirect manner [Citation11]. Terpenoids also can be used in drugs, industrial production and agricultural production [Citation12].
The robust development of research methods promotes the research of the biological functions, synthetic pathway and regulatory mechanism of terpenoids. In recent years, a large number of useful terpenoids are obtained through genetic engineering and other approaches. Some enzymes and regulators involved in terpenoid biosynthesis have been identified and analyzed [Citation13]. This paper summarizes the latest research on the functions of plant terpenoids, the anabolic pathways and regulatory factors affecting the biosynthesis of plant terpenoids. We also summarize the regulatory factors participating in terpenoid biosynthesis. These information resources provide important references for the further exploration of plant terpenoids.
Biological functions of terpenoids
Participation in the growth, development and defense of plants
Terpenoids participate in many physiological metabolic activities in higher plants. Primary metabolites, for example, abscisic acid and brassinolide play an important role in the regulation of plant growth; while chlorophylls and carotenoids take part in photosynthesis [Citation14–17]. Gibberellin participates in plant development by the crosstalk with other plant hormones [Citation18,Citation19]. As a special signal of communication between plants and pollinators, volatile terpenoids can attract specific pollinators to participate in plant pollination. For example, the pollinators of Mimulus lewisii and Mimulus cardinalis are different because of the different content of myrcene, ocimene and limonene [Citation20]. Plants pollinated by moths released more phenylpropanoids, terpenes and nitrogenous compounds; while, the flower volatiles of plants pollinated by birds were terpenoids and aliphatic derivatives [Citation21,Citation22].
A large number of studies have shown that terpenoids can be used as signal molecules to mediate plant defense in response to herbivorous insect and pathogenic bacteria’s invasion by direct or indirect means of defense [Citation23–25]. Direct defense means that the plants directly prevent insects or herbivores from feeding on them by releasing poisonous terpenoids [Citation26]. Indirect defense means that plants synthesize and release terpenoids after being invaded by pests. After being bitten by insects, some plants could release some amount of volatile terpenoids to attract natural enemies of pests [Citation27]. It was previously believed that the mechanical damage caused by insect bites led to direct plant defense, but recent in-depth studies have found that the oral secretions of the insects or jasmonic acid can induce the production of a large number of terpenoids from plants, thereby inducing the indirect defense [Citation25,Citation26].
Allelopathy of terpenoids
Allelopathic substances of terpenoids can be released into the surrounding environment and cause autotoxicity or rejection, which make plants to maintain population density, compete for space, and gain growth advantages [Citation28,Citation29]. The allelopathic mechanism of plant terpenoids is mainly manifested in inhibiting plant growth, affecting the function of cell membrane and hindering the physiological metabolism. Several volatile monoterpenoids, such as alpha-pinene and camphor produced by Salvia leucophylla, inhibit the germination, DNA synthesis, meristem cell elongation of Brassica campestris seeds [Citation30]. Allelochemicals produced by Juglans nigra could inhibit the growth of apple trees and many common grasses, but not pear, peach, plum and June grass [Citation31]. The allelochemicals sometimes inhibit the growth and development of their own seedlings. For example, sesquiterpene secreted by Juncus effusus inhibited seed germination and seedling growth [Citation32].
Medicinal value of terpenoids
Previous studies have reported that perillyl alcohol and paclitaxel have anti-tumor activity, while geraniol and linalool can inhibit the growth of gram-positive bacteria, gram-negative bacteria and some fungi [Citation33,Citation34]. In addition, menthol and artemisinin can inhibit inflammatory cytokines and have antibacterial and antimalarial effects. Currently, 98 species of hopane sesquiterpenes have been verified as having anti-inflammatory and antiviral effects [Citation35,Citation36].
Utilization of terpenoids in food and industry
Most of terpenoids are aromatic volatile substances, so terpenoids are widely used as the raw material for perfumes, flavoring agents and cosmetics. Carotenoids can be used as natural food additives and cosmetics colorants, while aromatic terpenoids such as perillyl alcoholnol, menthol and nootkatone, are the main components of plant essential oils and fragrances [Citation37]. In addition, as new sources of bioenergy, some terpenoids can be used as a substitute for gasoline, diesel and other fuels [Citation38].
Function of several important terpenoids
Carotenoids
Carotenoids, belonging to tetraterpenoids, are important natural pigments and the main source of vitamin A. The antioxidant activity of carotenoids is of great significance to human health. As free-radical scavengers, carotenoids can protect from a variety of diseases, such as cardiovascular diseases, cancer and can prevent a variety of degenerative diseases [Citation39]. At present, the chemical structures of about 700 natural carotenoids have been identified and the pathway of carotenoid biosynthesis and its influencing factors in vegetable crops have been systematically studied.
Cucurbitacin
Cucurbitacins, a kind of highly oxidized tetracyclic triterpenoids, cause the bitterness of some Cucurbitaceae plants including cucumber, pumpkin and bitter gourd. Some in vitro studies showed that cucurbitacins could have anticancer activity; they could inhibit cancer cell proliferation and induce cell cycle arrest or apoptosis [Citation40,Citation41]. Cucurbitacins also play important roles in plant resistance to adverse environment such as insect pests and mechanical damage [Citation42].
Limonoids
Mainly existing in Rutaceae plants, limonoids are the cause of lemon bitterness. More than 300 limonins, including 39 limonin ligands (aglycones), 24 neutral compounds, and 15 acidic compounds, have been identified. Limonin has important application value in agricultural production. Limonoids can be used as insecticides and growth regulators for crops. What is more, limonoids can also improve the efficacy of other biological insecticides and conventional insecticides. Limonin also has important medical value. A large number of studies have proved that limonin in citrus peel and seed has anticancer roles [Citation43,Citation44]. Limonin has higher antioxidant activity and plays important roles in inhibiting fungus such as Escherichia coli, Staphylococcus aureus and Bacillus thuringiensis [Citation45,Citation46].
Artemisinin
As a sesquiterpenoid drug extracted from Artemisia annua, artemisinin and its derivatives not only have very strong antimalarial activity but also protect people from other parasitic diseases. A large number of in vitro and in vivo experiments have shown that artemisinin could inhibit or kill multiple cancer cells, such as leukemia, breast cancer and cervical cancer.
Biosynthetic pathways of terpenoids
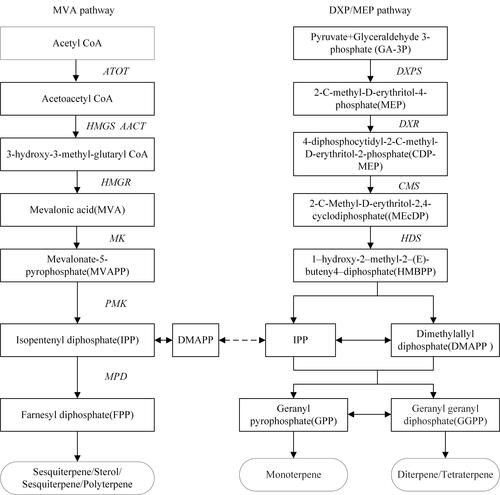
In vivo, terpenoids can be synthesized by two different pathways, the mevalonate (MVA) pathway and the 2-C-Methyl-D-erythritol-4-phosphate (MEP) pathway. The MEP pathway is also known as the 1-deoxy-D-xylulose-5-phosphate (DXP) pathway [Citation47,Citation48]. This section gives a brief introduction to the specific processes of the two synthesis pathways ().
The MVA pathway mainly exists in the cytoplasm of eukaryotes. Firstly, two molecules of acetyl CoA are catalyzed to form 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA). The obtained HMG-CoA is catalyzed by 3-hydroxy-3-mothylglutaryl coenzyme A reductase (HMGR) to form MVA, followed by the production of isopentenyl diphosphate (IPP). A part of IPP is catalyzed by isopentenyl diphosphate isomerase to form dimethylallyl diphosphate (DMAPP) [Citation49]. Finally, the remaining IPP and DMAPP are catalyzed by polyisoprene pyrophosphate synthase to form precursors of sesquiterpenes, triterpenes and steroids. The MEP pathway mainly exists in the plastid of prokaryotes and plants. IPP and DMAPP are synthesized through the condensation of glyceraldehyde 3-phosphate (GA-3P) and pyruvate catalyzed by several enzymes. This pathway forms monoterpenes, diterpenes and tetraterpenes in plants [Citation50].
The MVA and MEP pathways are divided into three stages: intermediate products formation (IPP and isomer DMAPP), direct precursor substances formation and the terpenoids generation and skeleton modification stage. Previous studies have reported that although MVA and MEP pathways in eukaryotes are isolated in the subcellular space, they are not independent of each other [Citation51]. In general, the terpenoid biosynthetic process is closely associated with the photosynthetic process, and intermediates of the Calvin cycle are directly used as precursors of the MEP pathway [Citation52]. However, the two terpenoid biosynthetic processes are decoupled when photosynthesis is inhibited under severe drought stresses. Under the circumstances, carbon sources other than carbon dioxide are used in the biosynthesis of terpenoids, such as carbohydrates [Citation53].
Key enzymes and functions in the biosynthetic pathways of terpenoids
The key enzymes in the terpenoid biosynthetic pathways encode HMGR, 1-deoxy-D-xylulose-5-phosphate synthase (DXS), 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR), isoprene pyrophosphate synthase and terpene synthase (TPS).
HMGR
The HMGR catalytic domain contains four conserved domains, namely two HMG-CoA binding regions and two NADP(H) binding regions, which are associated with substrate recognition and binding. The gene encoding HMGR was firstly cloned in Arabidopsis thaliana, and then was identified in Panax notoginseng, Malus domestica, Andrographis paniculata, Ginkgo biloba and other plants [Citation54–56]. Up-regulation of this gene will increase the content of isoprene substances in plants. OkHMGR in camphor tulsi (Ocimum kilimandscharicum) could provide an endogenous isoprene unit into the terpenoids synthesis [Citation57]. In addition, latex biosynthesis increases in HbHMGR1-overexpressed Hevea brasiliensis plants [Citation58].
DXS
As the first key rate-limiting enzyme in the MEP pathway, DXS exists in the thylakoid of plants. MEP pathway begins with the condensation reaction of pyruvate and glyceraldehyde 3-phosphate catalyzed by DXS, and the reaction can produce DXP and release CO2 [Citation59,Citation60]. DXS genes are subdivided into three types I, II and III [Citation61,Citation62]. Type I DXS exists in photosynthetic tissues and may be involved in the biosynthesis of isoprenes such as carotenoids and phytols. Type II is involved in activating defense response genes including terpene synthase, while type III usually exists in the plant genome and the function of its encoding enzymes is unclear. Overexpression of DXS increased the accumulation of terpenoids, indoles and alkaloids in the hairy roots of periwinkle [Citation61]. Reduced expression of DXS decreased the level of isoprene, thereby causing the occurrence of potato late blight [Citation62].
DXR
DXR is a key enzyme in the second step of the MEP pathway. DXR can catalyze the conversion of DXP into intermediate metabolites of IPP [Citation63]. DXR has a bidirectional N-terminal transport domain, which contains chloroplast transport peptide (CTP) and thylakoid transport peptide (ITP) [Citation17]. In addition, large amounts of diterpenes and perilla alcohol were accumulated in the flowers of transgenic plants when LiDXS and LiDXR genes were overexpressed in tobacco [Citation17].
Isoprene pyrophosphate synthetase
Isoprene pyrophosphate synthase catalyzes IPP and the isomer DMAPP. The synthetic products can be divided into three categories: geranyl diphosphate synthase (GPPS), farnesyl diphosphate synthase (FPPS) and geranyl geranyl diphosphate synthase (GGPPS).
GPPS
GPPS catalyzes a molecule of IPP and DMAPP to form GPP, which provides a carbon skeleton for monoterpenes [Citation64]. GPPS proteins have conserved domains. BbGPPS protein from Blumea balsamifera has high homology with the GPPS protein obtained from other plants, and it has an isoprene domain.
FPPS
FPPS has two conserved domains FARM and SARM. FARM contains the DDXX(XX)D conserved sequence, which is associated with the length of the product chain. SARM contains the DDXXD conserved sequence, which is associated with the catalytic activity of enzymes. FPPS can increase the content of isoprene in transformed plants. The activity of FPPS in FPS1-overexpressed Artemisia annae transgenic plants was 2 ∼ 3 times higher than that in the control plants [Citation65].
GGPPS
The GGPPS gene has been cloned from various plants such as Salvia miltiorrhiza, Solanum lycopersicum, Chimonanthus praecox and Catharanthus roseus [Citation66,Citation67]. A comparison of their sequence similarity indicated that nucleic acid sequence of the gene and the amino acid sequence of GGPPS were highly consistent among the different species.
Terpene synthases (TPS)
TPSs can catalyze GPP, FPP and GGPP to form monoterpenes, sesquiterpenes and diterpenes, respectively. TPSs can be divided into monoterpene synthase, sesquiterpene synthase and diterpene synthase according to the different synthetic products. Most TPSs have two typical conserved regions: the ‘DDXXD’ conserved region, which is rich in aspartic acid at the C-terminus, and the ‘RRx8W’ conserved region, which is rich in arginine at the N-terminus [Citation68]. TPS, isolated from many plants such as carrot (Daucus carota), mexican mint (Plectranthus amboinicus), rice (Oryza sativa) and celery (Apium graveliens), plays important roles in plant-pathogen interaction [Citation69–71]. The overexpression of OsTPS19 in rice increased the resistance to rice blast fungus Magnaporthe oryzae in the inoculated plants [Citation72].
Monoterpene synthase
Regulated by many amino acids, monoterpene synthases catalyze the synthesis of a variety of products [Citation73]. The sesquiterpene synthase of TPS-b subgroup, which is dominated by monoterpene synthase, is thought to have evolved from monoterpene synthase ancestors [Citation74].
Sesquiterpene synthase
Most proteins encoding sesquiterpene synthase contain the ‘DDXXD’ motif. If the motif mutates, it will result in the decrease in enzyme catalytic activity or abnormal products [Citation75]. Sesquiterpene synthase can produce one or more types of products. The expression of sesquiterpene synthase gene YL-1 from Salvia miltiorrhiza catalyzed the production of α-bergamotene, α-santalene, (E)-β–farnesene and β-sesquiphellandrene, while the other three genes (YL-4, YL-5 and YL-6) only got one product, valenene [Citation76].
Diterpene synthase
The catalytic mechanisms of different diterpene synthases have two patterns. One starts from the ionization of GGPP diphosphate, which is called Type A cyclization. The other pattern starts from the protonization of GGPP14, 15-double bond, which is called Type B cyclization [Citation77].
Regulatory factors affecting terpenoids biosynthesis
Transcription factors
Numerous studies have reported transcription factors such as AP2/ERF, WRKY and bHLH involved in terpenoids biosynthesis [Citation78]. The expression of CAD was activated by combination of the GA-WRKY1 transcription factor with the W-box cis-acting element in the promoter region of (+) -δ-cadinene synthase in cotton [Citation79]. Six WRKY transcription factors were found to be associated with the TPS expression in Amomum villosrm [Citation80]. In addition, the metabolism of phenylpropanol decreased in the VvMYB5b-overexpressing tomato plants, but the content of β-carotene increased [Citation81]. Phalaenopsis PbbHLH4 regulates the biosynthesis of different monoterpenes by interacting with PbbZIP4 and PbNAC1 [Citation82]. CitERF71 participated in the transcriptional regulation of E-geranol biosynthesis by directly combining with the promoter of CitTPS16 [Citation83].
Different developmental stages and circadian rhythm
The content of (-)-β-pinene synthase, encoded by artemisinin monoterpene synthase gene QH6, varies between day and night time, which means that the monoterpene biosynthesis is regulated by circadian rhythms [Citation84]. In osmanthus and Wisteria sinensis plants, the content of terpenoids increases during the day and decreases at night [Citation85]. Another study also reported that the expression of LfTPS01, LfTPS02 and LfTPS03 showed seasonal changes in Liquidambar formosana, and the contents of sesquiterpenes in the volatile oil of its leaves showed seasonal differences [Citation86]. The progress of plant growth and development has a significant impact on the synthesis of terpenoids. The content and composition of essential oil between Origanum vulgare and Thymus vulgari are significantly different at different growth stages of the same developmental stages [Citation87]. The expression patterns of TPS and the terpene oil production were also different during different development stages of lemon leaves [Citation88].
Plant hormones
Plant hormones can promote or inhibit the terpenoids biosynthesis. The expression analysis of terpenoids biosynthesis genes confirmed that methyl jasmonate (MeJA) activated the related biosynthetic pathway. MeJA can induce the expression of CsTPS genes including CsTPS23, −25, −43, −51, −52 and −76 in Camellia sinensis [Citation89]. Another study reported that MeJA had a positive regulatory effect on the synthesis of artemisinin in Norway spruce (Picea abies) [Citation90]. Moreover, the concentration of aroma substances increased significantly after spraying grapes with MeJA, especially the content of monoterpenes [Citation91].
Biotic and abiotic factors
Recent studies have shown that a variety of volatile terpenoids such as farnesene can participate in the interaction of plants and biotic stress. The increase in the content of some sesquiterpenoids can improve the disease resistance of plants, and certain monoterpenoid volatiles can be used as insect inhibitors [Citation92–94]. Insects and microorganisms can induce terpenoids synthesis. For example, after attack by Pieris rapae for 24 h, the expression of AtTPS03 and AtTPS10 was induced in Arabidopsis thaliana [Citation95].
The biosynthesis of terpenoids is also affected by non-biological factors such as light, temperature, humidity, nutrient balance, PEG (polyethylene glycol) and mechanical damage [Citation39,Citation96,Citation97]. The content of terpenoids increases after short-term exposure to high temperatures, but decreases after long-term exposure to high temperatures [Citation98]. OsTPS20 was significantly induced by oxidative stress in rice [Citation99]. A total of 80 terpene synthase genes were identified in C. sinensis, and the expression of CsTPS67, −69 and −71 were suppressed after the treatments of cold acclimation, salt and PEG [Citation89]. The expression patterns of endogenous key enzyme genes such as NtIPPI2, NtSQS and NtGGPPS2 were up-regulated, while NtIPPI1 was down-regulated after overexpressing the mustard HMGS gene in tobacco [Citation100].
Prospects
Terpenoids play important roles in the progress of plant growth, development and defense. However, terpenoids are mostly secondary metabolites, and their content in plants is relatively low and their extraction is difficult. Therefore, researchers have focused on developing new strategies for obtaining high yields of terpenoids. In recent years, obtaining terpenoids through genetic engineering and key enzyme approaches have become a hot topic in this research field. However, it is still difficult to carry out effective genetic engineering applications for terpenoids biosynthesis related genes because there are many kinds of terpenoids and their metabolism is complex. Therefore, future studies should focus on the following aspects: (1) Dig into downstream genes of MVA and MEP pathways to find related genes for increasing terpenoids content; (2) Dig into the regulatory mechanism of key enzymes and the feedback mechanism between key enzymes with the goal of reasonably increasing terpenoids content through genetic engineering.
Author contributions
YH wrote the paper; MYL, FJX and XC revised the paper, MYL contributed reagents/materials/analysis tools. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32102405, 32002027, 31801868), the Natural Science Foundation of Shandong Province (ZR2020QC156, ZR2018PC023), and Scientific Research Starting Foundation (LYDX2019BS030).
Compliance with ethical standards
This article does not contain any studies with human participants or animal performed by any of the authors.
Disclosure statement
All authors declare they have no other competing interests.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Christianson DW. Structural and chemical biology of terpenoid cyclases. Chem Rev. 2017;117(17):11570–11648.
- Gozari M, Alborz M, El-Seedi R, et al. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur J Med Chem. 2021;210:112957.
- Zhou F, Pichersky E. More is better: the diversity of terpene metabolism in plants. Curr Opin Plant Biol. 2020;55:1–10.
- Ashour M, Wink M, Gershenzon J. Biochemistry of terpenoids: monoterpenes, sesquiterpenes and diterpenes. In: Wink M, editor. Biochemistry of plant secondary metabolism. Chapter 5. Oxford: Wiley-Blackwell; 2010. p. 258–303.
- Lassen LM, Nielsen AZ, Ziersen B, et al. Redirecting photosynthetic electron flow into light-driven synthesis of alternative products including high-value bioactive natural compounds. ACS Synth Biol. 2014;3(1):1–12.
- Pateraki I, Heskes AM, Hamberger B. Cytochromes P450 for terpene functionalisation and metabolic engineering. Adv Biochem Eng Biotechnol. 2015;148:107–139.
- Vavitsas K, Fabris M, Vickers CE. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes. 2018;9(11):520.
- Haba H, Lavaud C, Magid AA, et al. Diterpenoids and triterpenoids from Euphorbia retusa. J Nat Prod. 2009;72(7):1258–1264.
- Fraser PD, Pinto ME, Holloway E, et al. Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2020;24:551–558.
- Polatolu K, Karako MC. Biologically active essential oils against stored product pests-science direct. Essen Oils Food Preserv. 2016;5:39–59.
- Xiao H, Zhang Y, Wang M. Discovery and engineering of cytochrome P450s for terpenoid biosynthesis. Trends Biotechnol. 2019;37(6):618–631.
- Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010;584(14):2965–2973.
- Kleine S, Müller C. Drought stress and leaf herbivory affect root terpenoid concentrations and growth of tanacetum vulgare. J Chem Ecol. 2014;40(10):1115–1125.
- Jonathan G, Natalia D. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–414.
- Wang GL, Xiong F, Que F, et al. Morphological characteristics, anatomical structure and gene expression: novel insights into gibberellin biosynthesis and perception during carrot growth and development. Hortic Res. 2015;2(1):e0134166.
- Leng XP, Wang PP, Wang C, et al. Genome-wide identification and characterization of genes involved in carotenoid metabolic in three stages of grapevine fruit development. Sci Rep. 2017;7(1):4216.
- Zhang T, Sun M, Guo Y, et al. Overexpression of LiDXS and LiDXR from lily (lilium siberia) enhances the terpenoid content in tobacco flowers. Front Plant Sci. 2018;9:909.
- Li J, Yang Y, Chai M, et al. Gibberellins modulate local auxin biosynthesis and polar auxin transport by negatively affecting flavonoid biosynthesis in the root tips of rice. Plant Sci. 2020;298:110545.
- Li Q, Li J, Zhang L, et al. Gibberellins are required for dimorphic flower development in Viola philippica. Plant Sci. 2021;303:110749.
- Byers KJ, Vela JP, Peng F, et al. Floral volatile alleles can contribute to pollinator-mediated reproductive isolation in monkeyflowers (mimulus). Plant J. 2014;80(6):1031–1042.
- Knudsen JT, Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Bot J Linn Soc. 1993;113(3):263–284.
- Dobson HE. Relationship between floral fragrance composition and type of pollinator. In: Dudareva N, Pichersky E, editor. Biology of floral scent. Boca Raton (FL): Taylor and Francis; USA, 2016. p. 147–198.
- Francesco L, Susanna P, Silvia F, et al. Volatile isoprenoids and their importance for protection against environmental constraints in the mediterranean area. Environ Exp Bot. 2014;103:99–106.
- Willmer PG, Nuttman CV, Raine NE, et al. Floral volatiles controlling ant behaviour. Funct Ecol. 2009;23(5):888–900.
- He J, Bouwmeester HJ, Dicke M, et al. Transcriptional and metabolite analysis reveal a shift in direct and indirect defences in response to spider-mite infestation in cucumber (Cucumis sativus). Plant Mol Biol. 2020;103(4-5):489–505.
- Mumm R, Posthumus MA, Dicke M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008;31(4):575–585.
- Turlings TC, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250(4985):1251–1253.
- Nicol RW, Yousef L, Traquair JA, et al. Ginsenosides stimulate the growth of soil-brone pathogens of american ginseng. Phytochemistry. 2003;64(1):257–264.
- Inderjit KG, Muker JI. Allelochemicals: Biological control of plant pathogens and disease. Vol. 157. Nertherlands: Springer Press; 2006.
- Nishida N, Tamotsu S, Nagata N, et al. Allelopathic effects of volatile monoterpenoids produced by salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of brassica campestris seedlings. J Chem Ecol. 2005;31(5):1187–1203.
- Jose S, Gillespie AR. Allelopathy in black walnut (Juglans nigra L.) alley cropping: effects of juglone on hydroponically grown corn (Zea mays L.) and soybean (Glycine max L. Merr.) growth and physiology. Plant Soil. 1998;203(2):199–205.
- Ervin GN, Wetzel RG. Allelochemical autotoxicity in the emergent wetland macrophyte juncus effusus (juncaceae). Am J Bot. 2000;87(6):853–860.
- Chen TC, Fonseca COD, Schönthal AH. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am J Cancer Res. 2015;5(5):1580–1593.
- Chen F, Li W, Jiang L, et al. Functional characterization of a geraniol synthase-encoding gene from camptotheca acuminata and its application in production of geraniol in Escherichia coli. J Ind Microbiol Biotechnol. 2016;43(9):1281–1292.
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827.
- Wu ZH, Liu D, Proksch P, et al. Punctaporonins H-M: caryophyllene-type sesquiterpenoids from the sponge-associated fungus Hansfordia sinuosae. Mar Drugs. 2014;12(7):3904–3916.
- Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10(12):594–602.
- George KW, Alonso-Gutierrez J, Keasling JD, et al. Isoprenoid drugs, biofuels, and chemicals-artemisinin, farnesene, and beyond. Adv Biochem Eng Biotechnol. 2015;148:355–398.
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6(2):466–488.
- Cheng AC, Hsu YC, Tsai CC. The effects of cucurbitacin E on GADD45β-trigger G2/M arrest and JNK-independent pathway in brain cancer cells. J Cell Mol Med. 2019;23(5):3512–3519.
- Saeed ME, Boulos JC, Elhaboub G, et al. Cytotoxicity of cucurbitacin E from citrullus colocynthis against multidrug-resistant cancer cells. Phytomedicine. 2019;62:152945.
- Balkema-Boomstra AG, Zijlstra S, Verstappen FW, et al. Role of cucurbitacin C in reaistance to spider mite (tetranychus urticae) in cucumber (cucumis sativu L.). J Chem Ecol. 2003;29(1):225–235.
- Kelly C, Jewell C, O’Brien NM. The effect of dietary sup-plementation with the citrus limonoids, lomonin and nomilinon xenobiotic-metabolizing enzymes in the liver and small intestine of the rat. Nutr Res. 2003;23(5):681–690.
- Lam K, Zhang J. Citrus limoninoid reduction of chemically induced tumorigenesis. Food Technol. 1994;7(11):104–108.
- Arnason JT, Philogène BJR, Donskov N, et al. Limonoids from the meliaceac and reduce feeding, growth and development of ostrinia nubilalis. Entomol Exp Appl. 1987;43(3):221–226.
- Sun CD, Chen KS, Chen Y, et al. Contents and antioxi-dant capaeity of limonin and nomilinin different tissues of citrus fruit of four cultivars during fruit growth and maturation. Food Chem. 2005;93(4):599–605.
- Xu Q, He Y, Yan X, et al. Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ Exp Bot. 2018;149:81–94.
- Zhao H, Feng S, Zhou W, et al. Transcriptomic analysis of postharvest toon buds and key enzymes involved in terpenoid biosynthesis during cold storage. Sci Hortic. 2019;257:108747.
- Xiao H, Zhong JJ. Production of useful terpenoids by higher-fungus cell factory and synthetic biology approaches. Trends Biotechnol. 2016;34(3):242–255.
- Lange BM, Croteau R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: cloning and heterologous expression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase from peppermint. Arch Biochem Biophys. 1999;365(1):170–174.
- Vranová E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64(1):665–700.
- Dudareva N, Klempien AK, Muhlemann J, et al. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198(1):16–32.
- Francesco L, Jörg-Peter S. Abiotic stresses and induced BVOCs. Trends in Plant Sci. 2010;15(3):154–166.
- Caelles C, Ferrer A, Balcells L, et al. Isolation and structural characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme a reductase. Plant Mol Biol. 1989;13(6):627–638.
- Zhang M, Liu H, Wang Q, et al. The 3-hydroxy-3-methylglutaryl-coenzyme a reductase 5 gene from Malus domestica enhances oxidative stress tolerance in Arabidopsis thaliana. Plant Physiol Biochem. 2020;146:269–277.
- Shen G, Pang Y, Wu W, et al. Cloning and characterization of a root-specific expressing gene encoding 3-hydroxy-3-methylglutaryl coenzyme a reductase from ginkgo biloba. Mol Biol Rep. 2006;33(2):117–127.
- Bansal S, Narnoliya LK, Mishra B, et al. HMG-CoA reductase from camphor tulsi (ocimum kilimandscharicum) regulated MVA dependent biosynthesis of diverse terpenoids in homologous and heterologous plant systems. Sci Rep. 2018;8(1):3547.
- Jayashree R, Nazeem PA, Rekha K, et al . Over-expression of 3-hydroxy-3- methylglutaryl-coenzyme A reductase 1 (HMGR1) gene under super-promoter for enhanced latex biosynthesis in rubber tree (Hevea brasiliensis Muell. Arg.). Plant Physiol Biochem. 2018;127:414–424.
- Battistini MR, Shoji C, Handa S, et al. Mechanistic binding insights for 1-deoxy-D-Xylulose-5-Phosphate synthase, the enzyme catalyzing the first reaction of isoprenoid biosynthesis in the malaria-causing protists, Plasmodium falciparum and Plasmodium vivax. Protein Expr Purif. 2016;120:16–27.
- Rodriguez-Concepcion M, Boronat A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr Opin Plant Biol. 2015;25:17–22.
- Peebles CA, Sander GW, Hughes EH, et al . The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab Eng. 2011;13(2):234–240.
- Henriquez MA, Soliman A, Li G, et al. Molecular cloning, functional characterization and expression of potato (Solanum tuberosum) 1-deoxy-D-xylulose 5-phosphate synthase 1 (StDXS1) in response to Phytophthora infestans. Plant Sci. 2016;243:71–83.
- Yang J, Adhikari MN, Liu H, et al. Characterization and functional analysis of the genes encoding 1-deoxy-D-xylulose-5-phosphate reductoisomerase and 1-deoxy-D-xylulose-5-phosphate synthase, the two enzymes in the MEP pathway, from Amomum villosum lour. Mol Biol Rep. 2012;39(8):8287–8296.
- Burke C, Croteau R . Geranyl diphosphate synthase from Abies grandis: cDNA isolation, functional expression, and characterization. Arch Biochem Biophys. 2002;405(1):130–136.
- Han JL, Liu BY, Ye HC, et al. Effects of overexpression of the endogenous farnesyl diphosphate synthase on the artemisinin content in artemisia annua L. J Integr Plant Biol. 2006;48(4):482–487.
- Okada K, Saito T, Nakagawa T, et al. Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in arabidopsis. Plant Physiol. 2000;122(4):1045–1056.
- Ament K, Schie VC, Bouwmeester HJ, et al. Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta. 2006;224(5):1197–1208.
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70(15-16):1621–1637.
- Muchlinski A, Ibdah M, Ellison S, et al. Diversity and function of terpene synthases in the production of carrot aroma and flavor compounds. Sci Rep. 2020;10(1):9989.
- Ashaari NS, Rahim MH, Sabri S, et al. Functional characterization of a new terpene synthase from Plectranthus amboinicus. PLoS One. 2020;15(7):e0235416.
- Li MY, Feng K, Hou XL, et al. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the apiaceae family. Hortic Res. 2020;7:9.
- Chen X, Chen H, Yuan JS, et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol J. 2018;16(10):1778–1787.
- Kampranis SC, Ioannidis D, Purvis A, et al. Rational conversion of substrate and product specificity in a salvia monoterpene synthase: structural insights into the evolution of terpene synthase function. Plant Cell. 2007;19(6):1994–2005.
- Green S, Baker EN, Laing W. A non-synonymous nucleotide substitution can account for one evolutionary route to sesquiterpene synthase activity in the TPS-b subgroup. FEBS Lett. 2011;585(12):1841–1846.
- Shimada T, Endo T, Ana R, et al. Isolation and characterization of germacrene a synthases gene in citrus unshiu marc. Sci Hortic. 2012;145:102–108.
- Cui MY. Expression, purification and characterization of four sesquiterpene synthases from salvia miltiorrhiza [PhD dissertation]. Harbin: Northeast Forestry University; 2016.
- Tan H, Xiao L, Gao S, et al. TRICHOME and ARTEMISININ REGULATOR 1 is required for trichome development and artemisinin biosynthesis in artemisia annua. Mol Plant. 2015;8(9):1396–1411.
- Broun P. Transcription factors as tools for metabolic engineering in plants. Curr Opin Plant Biol. 2004;7(2):202–209.
- Xu YH, Wang JW, Wang S, et al . Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-delta-cadinene synthase-A. Plant Physiol. 2004;135(1):507–515.
- He XY, Wang H, Yang JF, et al. RNA sequencing on Amomum villosum lour. induced by MeJA identifies the genes of WRKY and terpene synthases involved in terpene biosynthesis. Genome. 2018;61(2):91–102.
- Mahjoub A, Hernould M, Joubes J, et al. Overexpression of a grapevine R2R3-MYB factor in tomato affects vegetative development, flower morphology and flavonoid and terpenoid metabolism. Plant Physiol Biochem. 2009;47(7):551–561.
- Chuang YC, Hung YC, Tsai WC, et al. PbbHLH4 regulates floral monoterpene biosynthesis in phalaenopsis orchids. J Exp Bot. 2018;69(18):4363–4377.
- Li X, Xu Y, Shen S, et al. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J Exp Bot. 2017;68(17):4929–4938.
- Lu S, Xu R, Jia J, et al. Cloning and functional characterization of a beta-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol. 2002;130(1):477–486.
- Zheng R, Liu C, Wang Y, et al. Expression of MEP pathway genes and non-volatile sequestration are associated with circadian rhythm of dominant terpenoids emission in Osmanthus fragrans lour. Front Plant Sci. 2017;8:1869.
- Chuang L, Wen CH, Lee YR, et al. Identification, functional characterization, and seasonal expression patterns of five sesquiterpene synthases in Liquidambar formosana. J Nat Prod. 2018;81(5):1162–1172.
- Christensen LP, Kai G. Effect of development stage at harvest on the composition and yield of essential oils from thyme and oregano. Develop Food Sci. 2005;43(25):261–264.
- Pandeló D, Melo TD, Singulani JL, et al. Oil production at different stages of leaf development in Lippia Alba. Rev Bras Farmacogn. 2012;22(3):497–501.
- Zhou HC, Shamala LF, Yi XK, et al. Analysis of terpene synthase family genes in camellia sinensis with an emphasis on abiotic stress conditions. Sci Rep. 2020;10(1):933.
- Fäldt J, Martin D, Miller B, et al. Traumatic resin defense in Norway spruce (picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol. 2003;51(1):119–133.
- D’Onofrio C, Matarese F, Cuzzola A. Effect of methyl jasmonate on the aroma of sangiovese grapes and wines. Food Chem. 2018;242:352–361.
- Shrivastava G, Ownley BH, Augé RM, et al. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis. 2015;65(2):65–74.
- Yoon SJ, Sukweenadhi J, Khorolragchaa A, et al. Overexpression of panax ginseng sesquiterpene synthase gene confers tolerance against Pseudomonas syringae pv. tomato in Arabidopsis thaliana. Physiol Mol Biol Plants. 2016;22(4):485–495.
- Riedlmeier M, Ghirardo A, Wenig M, et al. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell. 2017;29(6):1440–1459.
- Van Poecke RM, Posthumus MA, Dicke M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol. 2001;27(10):1911–1928.
- Hartikainen K, Riikonen J, Nerg A-M, et al. Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula roth). Environ Exp Bot. 2012;84:33–43.
- Vanhatalo A, Ghirardo A, Juurola E, et al. Long-term dynamics of monoterpene synthase activities, monoterpene storage pools and emissions in boreal scots pine. Biogeosciences. 2018;15(16):5047–5060.
- Duan Q, Kleiber A, Jansen K, et al. Effects of elevated growth temperature and enhanced atmospheric vapour pressure deficit on needle and root terpenoid contents of two douglas fir provenances. Environ Exp Bot. 2019;166:103819.
- Lee GW, Lee S, Chung MS, et al. Rice terpene synthase 20 (OsTPS20) plays an important role in producing terpene volatiles in response to abiotic stresses. Protoplasma. 2015;252(4):997–1007.
- Liao P, Wang H, Wang M, et al. Transgenic tobacco overexpressing brassica juncea HMG-CoA synthase 1 shows increased plant growth, pod size and seed yield. PLoS One. 2014;9(5):e98264.