Abstract
Rice blast is a major threat to the rice crop in rice-growing areas around the globe. However, synthetic fungicides are injurious for the ecosystem and the existing bio-control agents facing the resistance from the pathogens. In this study, an isolated novel Streptomyces strain (TA-47), from the rice field was identified by culturing on different media, physiological tests, biochemical tests, chemo-taxonomy and molecular biological approaches. In-vitro and in-vivo bio-control activity of the isolated strain was evaluated. Growth on the media, spore production, utilization of sole carbon and nitrogen sources, presence of diaminopimelic acid (DAP) in the cell wall of the strain and fatty acid production were observed.16S rRNA (gene sequence) and a neighbour-joining hood tree indicated that the strain TA-47 is Streptomyces koyanogensis. In-vitro inhibition zone of 29 mm against the M. grisea showed the antagonistic potential of the novel strain TA-47. On the other hand, in the in-vivo studies, strain TA-47, showed the strong bio-control effect, as disease severity decreased to 19.66 ± 1.6%, while in the non-treated sample the disease severity was recorded as 44.66 ± 5.249%. Moreover, after the treatment of the strain, effects on the growth, such as increased height and root length by 72.22 and 16.54 cm, respectively, were also recorded. In conclusion, rhizosphere Streptomyces TA-47, was a novel Streptomyces koyanogensis strain. The study found that the strain had strong bio-control and growth-promoting abilities.
Introduction
Magnaporthe grisea causes rice blast, which is the most devastating disease in virtually all rice-growing countries [Citation1]. Rice is economically significant since it feeds 60% of the world’s population. Blast infections may be effectively controlled by cultivating resistant types with chemical control [Citation2]. Blast disease has been treated by using a variety of fungicides. Synthetic fungicides, on the other hand, pollute the environment, produce residual issues, lead to pesticide resistance, reduce soil quality and harm natural ecosystems [Citation3]. The development of fungicide-resistant organisms, necessitates immediate agricultural disease control measures [Citation4].
Natural materials that are environmentally benign and have minimal toxicity to living creatures are gaining popularity as a significant resource for the creation of fungicides. Streptomyces are naturally occurring soil bacteria that have been widely employed as biological control agents [Citation5]. Streptomyces species are capable of producing a wide range of bioactive chemicals with antibacterial, antifungal, antiviral, anticancer and antioxidant activities [Citation5]. In recent years, rice blast disease has been controlled by the two products of Streptomyces (Kasugamycin and blasticidin- S) [Citation3,Citation5,Citation6]. The occurrence of rice blast has been controlled by pyrroles (pyrroles [1,2-a] pyrazine-1,4-dione, hexahydro) Oligomycin A and rapamycin [Citation7]. Chemical fungicides could be replaced by bio-control antagonists in the form of Streptomyces spp. Since fungal pathogens can develop resistance against the existing bio-control agents, we need to explore novel antagonistic agents to combat the fungus phytopathogen. In this study, a Streptomyces strain was isolated from soil and identified and evaluated against rice blast pathogen (M. grisea).
Materials and methods
Microorganism
Streptomyces strain TA-47 isolated from the rice soil from a rice growing area in Gujrat, Pakistan (32°37′N, 74°7′44′′E). The strain was cultivated on nutrient media and maintained at −4 °C. Fungus pathogen M. grisea was obtained from the Department of Agriculture (Plant Protection) Warning and Quality Control of Pesticides, Gujrat, Pakistan. Fungus pathogen was cultivated on potato dextrose agar (PDA) and maintained at −4 °C for further work.
Taxonomy of strain TA-47
Potent strain was grown on different medium. Seven-, fourteen- and twenty-one-day-old cultures of the isolated TA-47 were grown in several agar media, including the four ISP media (ISP 2–5) recommended by Shirling and Gottlieb [Citation8], the Bennett agar medium, the Nutrient agar medium and the S-agar medium (SEM). Tests were conducted on the formation of melanin in peptone-yeast extract-iron (ISP6) and trypsin (ISP7) medium agars. SEM was used to analyse the morphological features of spores and aerial mycelia of strain TA-47 cultivated on the medium at 28 °C for 120 h. The method of Holding and Collee [Citation9] was used to assess physiological and biochemical properties.
After three days of incubation at 28 °C in shake-flasks with ISP 2 broth, sufficient biomass for chemotaxonomic investigations was obtained. In the entire cell hydrolysates, thin layer chromatography (TLC) was used to determine the isomeric form of diaminopimelic acid (DAP), glycine and sugars [Citation10]. As a part of a two-dimensional TLC analysis, phospholipids were detected utilizing spray reagents and comigration with standards [Citation11,Citation12]. A conventional Microbial Identification System (MIDI) was used to extract fatty acids, methylate them, and then, analyse them using gas chromatography (GC) [Citation13].
PCR amplification of the 16S rRNA gene of strain TA-47 was performed using the universal primers: 27(5′AGTTTGTCMTGGCTCAG-3′) and 1492 R (5′-GGTTACCTTACGACTT-3′) (verity TM 96-well PCR, Applied Biosystems, Singapore). The PCR products were sent to Sangon Biotech (Shanghai, China) Co., Ltd for sequence determination. Phylogenetic analysis was conducted by using Mega version 6 [Citation14], Gene sequences were submitted to the NCBI and waiting for accession number.
Fermentation process to produce antifungal compound
A two-stage fermentation process was carried out: seed growth and the generation of a potent antifungal compound Gausses medium was used to cultivate strain TA-47 for 5 d at 28 °C after spore formation in the liquid fermentation medium [Citation15]. After 48 h of incubation at 28 °C and 160 rpm, two spore cakes (5 mm) from Strain 128 were used to seed 40 mL of medium into a 250 mL flask. Seed culture (5%, v/v) was inoculated aseptically into a 250 mL flask containing 40 mL of fermentation media. The medium comprised: 47 g soluble starch, 3 g yeast extract, 22 g peanut meal, 2.7 g (NH4) 2 SO4, 2.7 g NaCl and 2.7 g CaCO3 dissolved in 1 L distilled water and pH were adjusted to 6.8–7.2 and incubated at 28 °C in a rotary shaker (HZQ-F16 Harbin Dong Lian Electronic Technology Production. Co., Ltd., Harbin, China) at the speed of 160 rpm for 96 h. This fermented culture was then centrifuged, and the supernatant was kept at 4 °C for future research. The diameter of the inhibitory zones was measured to evaluate antifungal activity.
In-vitro antifungal assay against M. grisea
For initial screening, a spore cake was used against the pathogen. The spores of the pathogen were mixed in PDA and 5 mm spore cake of antagonistic strain TA-47 was challenged with it. Oxford cup method was used to study the activity of fermentation broth (200 µL) for each trail.
Bio-control activity of strain TA-47 in a greenhouse
In greenhouse settings, the antagonistic Streptomyces spp. was tested for its ability to suppress rice blast in vivo. The rice variety 1121 White Basmati Rice (Oryza sativa L.) was chosen as the host plant because it is particularly vulnerable to rice blast disease. All the experiment performed in the greenhouse under same and control conditions. All the plants were grown on sterilized paddy soil. Four treatments with ten plants each were carried out. (1) Ck (Check) control, without any treatment. (2) Inoculated with strain TA-47 and M. grisea. The treatment was given as 2000 µL of the fermentation broth per10 plants and 2000 µL of spore suspension of M. grisea per 10 plants. (3) Inoculated with strain TA-47 given as 2000 µL of the fermentation broth per 10 plants). (4) Inoculated with 2000 µL of spore suspension of M. grisea per 10 plants. Treatments were applied to the plants by spraying method.
Statistical analysis
Minitab software version 17 was used to analyse the data. Values are the means with standard deviation (±SD). Mean values were compared by least significant difference (LSD) to determine the significant differences at a level of p ≤ .05.
Results
Taxonomy of the isolated strain TA-47
Soil isolated strain has been identified by different methods. Strain TA47 grew well on different ISP media, and especially on ISP2, it grew well with whitish-grey aerial and substrate mycelium, while on ISP3 the growth was good with whitish-grey aerial and blackish-grey substrate. However, the growth of the strain on ISP4 was normal without aerial and substrate mycelium appearance, and the growth of the strain was poor on ISP5 with grey appearance of aerial and substrate mycelium. Similarly, the strain grew well on nutrient agar media with whitish-grey substrate and aerial mycelium. A good growth appeared on Bennet agar media too, with whitish-grey substrate and grey aerial mycelium, while moderate growth on Sabouraud agar medium without clear observation of aerial and substrate mycelium as seen in . Sole nitrogen sources, such as L-asparagine and L-proline, were utilized by strain TA-47, while L-cysteine and L-tyrosine were not utilized. As sole carbon sources, D-sorbitol was not utilized by strain TA-47, while D-fructose, maltose and galactose were utilized by TA-47. In sole energy sources, sodium citrate was consumed, while sodium acetate was not consumed by strain TA-47. Melanin and diffusible were produced on ISP6 medium while both were not produced on ISP7 (). GC-MS profile of fatty acid included several types of saturated and saturated iso- and anteiso-branched chains and straight-chain fatty acids. The C 17.0 2-OH Cis 30% was the major cis form long-chain fatty acid ().
Table 1. Growth characteristics of Streptomyces strain TA-47, on different nutrient medium.
Table 2. Physiological characteristics of Streptomyces strain TA-47.
Table 3. Cellular fatty acid contents %, chemical characteristics of Streptomyces strain TA-47.
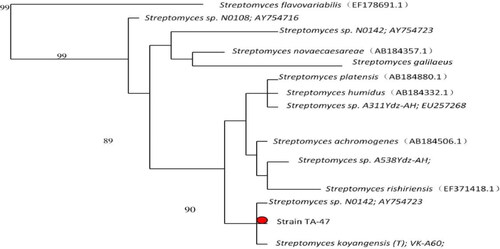
TLC results showed the presence of ribose, galactose, mannose and DAP in the cell wall of the strain TA-47. Strain TA-47 produced different types of enzymes such as catalase, urease and esterase. Metabolites results showed the production of metabolites as the nitrogen was reduced, consumed by the strain TA-47. The Voges-Proskauer test indicated that there was no production of metabolites. Coagulation and methyl red showed negative results, while peptonization showed positive results (). Scan electron microscopic analysis indicated the presence of spores and morphologically, a cylindrical shape of TA-47 (). These results showed that strain TA-47 was a Streptomyces spp. In molecular biological identification, 16S rRNA sequences used to construct the phylogenetic tree. The nucelotide sequences were analysed by BLAST by using Mega software version 6. The nearest close match was 90% with the Streptomyces koyangensis genes (T): VK-A60. The evolutionary tree indicated that strain TA-47 belongs to the S. koyangensis ().
Figure 1. SEM observation of spore chain and morphological mycelium of Streptomyces strain TA-47 on ISP2 medium.
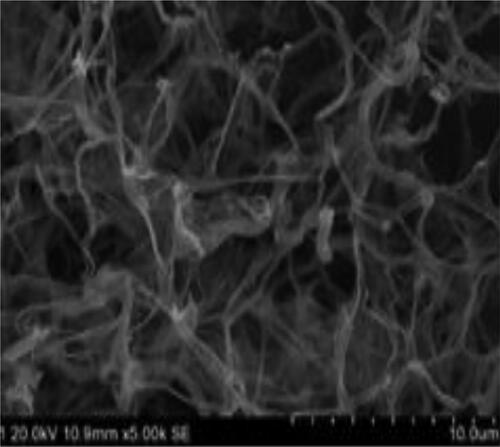
Table 4. Biochemical characteristics of Streptomyces strain TA-47.
Table 5. Bio-control and growth under effects of Streptomyces Strain TA-47 on rice in greenhouse conditions.
In-vitro antifungal activity of Streptomyces TA-47 against M. grisea
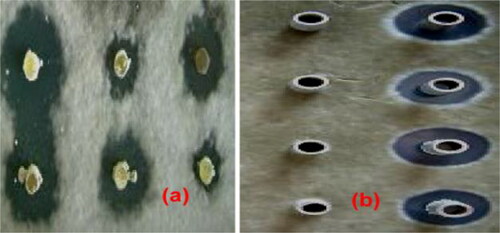
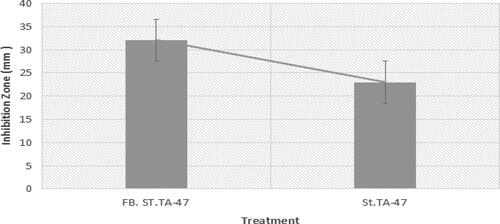
In oxford cup method, fermented broth showed a strong antifungal activity against M. grisea, with an inhibition zone of 32.72 ± 1.054. In the same way, in the direct method of microbial inhibition, the zone was 22.28 ± 5.714 mm (). In-vitro antifungal potential showed a strong antagonistic potential against M. grisea. The results are presented in .
Figure 3. In-vitro inhibition zone of strain TA-47 against M. grisea. Note: FB. ST. TA-47 represented fermentation broth from strain TA-47 (in antifungal assay, fermented broth was used as inhibitory antagonist); St. TA-47 represented strain TA-47 (in antifungal assay, direct strain TA-47 was challenged against the M. grisea). All the values are mean ± standard error of three replicates.
Bio-control assessment in greenhouse conditions
Greenhouse experiments showed the strong antifungal effects of strain TA-47 against M. grisea. Rice plant treated with Streptomyces strain TA-47, had positive effects. There was no blast lesion produced on the plant and no dry leaves were observed. Besides the disease control, strain TA-47 had even positive effects on the growth of the rice. Results showed that the height of rice plants was 72.22 ± 0.9195 cm, the root length 16.54 ± 1.311 cm, the weight of dry roots 3.396 ± 0.142 g and the number of tillers produced was 7.0 ± 0.408. Compared to the control (without inoculation of strain TA-47 and M. grisea), the height of rice plants was 54.41 ± 4.379 cm, the root length 9.52 ± 0.3630 cm, the weight of dry roots 2.03 ± 0.042 g and the number of tillers 3.66 ± 0.471.
In mixed treatment (inoculated with strain TA-47 and M. grisea), strain TA-47 had strong antifungal effects. A small lesion of blast disease was produced in the treatment with M. grisea, however strain TA-47 controlled the expansion of the blast infection. As the results showed, lesion was 0.76 ± 0.08, the number of dry leaves was 1.33 ± 0.23 and disease severity was 19.66 ± 1.6%. Rice plants also had positive effect from strain TA-47 in the company of M. grisea: the height of rice plants was 65.68 ± 0.240 cm, the root length 12.72 ± 0.496 cm, the weight of dry roots 2.31 ± 0.095 g and the number of tillers 5.33 ± 0.235. While the results of inoculation of M. grisea only had negative effects, such as presence of infection and increased disease severity. Growth factor also showed negative effects. As the disease lesion was 1.18 ± 0.089, the number of dry leaves was 2.33 ± 0.23, and disease severity was 44.66 ± 5.249%, as the height of rice plants was 46.786 ± 4.379 cm, the root length 5.609 ± 0.3634 cm, the weight of dry roots 0.96 ± 0.024 g and the number of tillers 2.33 ± 0.471.
represents the biological control impact of strain TA-47 in greenhouse on the rice plant. In , plants were treated with strain TA-47 and M. grisea. The blue arrows point to the infection. The results indicated that there was an infection produced by M. grisea, inhibited by strain TA-47. There was no infection produced on rice plants treated with fermentation broth of strain TA-47 only (). In plants treated with M. grisea only () there were lesions and even drying leaves due to infection. In Ck control plants (), there was no treatment given and no infection occurred under greenhouse condition.
Figure 5. An illustration of biological control in greenhouse on the rice plant. (A) Treated with strain TA-47 and M. grisea; the results indicated there was infection produced by M. grisea, but Strain TA-47 inhibited it. (B) Treated with strain TA-47 only; in the figure shown, there was no lesion or infection occurred. (C) Treated with M. grisea only; there were lesions and drying leaves as symptoms of infection. (D) Ck (check) control value; there was no treatment given. No symptoms of infection occurred. Note: Blue arrows point to the symptoms of infection.

presents the effects of strain TA-47 on rice growth under greenhouse conditions. There was poor growth of the plant. The plant size, the root length and the number of tillers were less. Lesions and symptoms of infection appeared. Strain TA-47 showed positive impact on the rice plant, though the infection occurred as by the mix treatment. The height of plant, the root length and the number of tillers increased. No disease occurred and growth was normal under greenhouse conditions. Strain TA-47 had a positive impact on the growth of the rice plants. The height of the plants, the root length and the number of tillers revealed positive impact of strain TA-47.
Figure 6. An illustration of effects of strain TA-47 on rice growth under greenhouse conditions. (T1) Treated with M. grisea. Results showed that, there was poor growth of the plant, the plant size, the root length and the number of tillers were reduced. Symptoms of infection appeared. (T2) treated with M. grisea and strain TA-47., Strain TA-47 showed positive impact on the rice plant, though some symptoms of the infection occurred. The height of plant, the root length and the number of tillers were increased. (T3) Ck (check), control plants, there was no treatment. No symptoms of disease occurred and growth was normal. (T4) Plant treated with strain TA-47. Results showed a positive impact of the strain on the growth of the rice plants. The height of the plant, the root length and the number of tillers were increased. Note: Blue arrows point to the symptoms of infection.

Discussion
This work was intended to identify Streptomyces strains from the rice field in order to explore their potential in vitro and under greenhouse conditions to suppress the rice blast fungus M. grisea. The soil is a rich source of Streptomyces strains [Citation5], and these microbes are reported as potential antagonists against fungus pathogens [Citation3,Citation16]. Growth on different medium, presence of spore, colour of aerial and substrate mycelium, utilization of sole nitrogen and carbon sources, melanin production, production of enzymes and metabolites. These findings correspond to our results for Streptomyces koyangensis strain TA-47 [Citation17].
The presence of DAP in the cell wall and the presence of several types of saturated and iso- and anteiso-branched chains and straight-chain fatty indicated that this strain belongs to the Streptomyces genus, as previously described [Citation18]. Molecular analysis indicated that strain TA-47 is closely related to Streptomyces. Applications of 16S rDNA or rRNA are reliable approaches to identify the bacterial strains [Citation19].
Disease management of rice blast has become a major concern, as the rice is major staple crop throughout the world. In this study, S. koyangensis potently inhibited the M. grisea and controlled the rice blast. Besides the antagonistic potential, strain TA-47 even induced rice growth. Several Streptomyces strains were reported to inhibit the fungus pathogen in in-vitro conditions. Recently, Ahsan et al. [Citation20] found that Streptomyces sp. strongly inhibited the growth of Rhizoctonia solani AG-3, by inhibiting basidiospores, mycelia and seclerotia in in-vitro conditions. Ahsan et al. controlled the F. oxysporum in in-vitro conditions.
Strain TA-47 showed bio-control effects in the greenhouse, as the treated plants did not develop disease symptoms. Pathogen-inoculated samples of rice gained a strong resistance from strain TA-47. Streptomyces strain TA-47 had a significant contribution in disease control under greenhouse conditions. Streptomyces albus successfully inhibits Macrophomina phaseolina under greenhouse conditions [Citation21].
Bacteria additionally worked as growth promoters in the plants. In this study, strain TA-47 helped the plant growth as a bio-control agent. Rice plants tread with this strain, showed increase in height, root size and number of tillers. As previously reported [Citation22,Citation23], actinobactria have the potential to promote growth in plants.
Conclusions
Strain TA-47 is a novel strain S. koyangensis. S. koyangensis TA-47 had a strong antifungal potential against M. grisea in vitro. In-vivo studies under greenhouse conditions, revealed that S. koyangensis had potential bio-control abilities, to inhibit M. grisea and control the exploitation of the blast disease. Additionally, S. koyangensis TA-47 acted as a growth promoter. In conclusion, S. koyangensis TA-47 could be a potential biological control agent for the rice blast disease management.
Disclosure statement
The authors declare no conflict of interest.
Conceptualization: L.B.X, W.Y and T.A.; methodology: T.A, L.B.X and W.Y; software: T.A., L.H., Y.S. and T.Z.; investigation: T.A.; data curation: T.A., L.B.X., W.Y and M.A.; writing – original draft preparation: T.A. M.A. and L.B.X.; writing – review and editing: T.A. and L.B.X.; visualization: T.A.; supervision: L.B.X. and W.Y.; funding: W.Y. All authors have read and agreed to the published version of the manuscript.
Data availability statement
The data sets analysed in this study are available from the corresponding author upon reasonable request.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Additional information
Funding
References
- Asibi AE, Chai Q, Coulter JA. Rice blast: a disease with implications for global food security. Agronomy. 2019;9(8):451.
- Shahriar SA, Imtiaz AA, Hossain MB, et al. Rice blast disease. Ann Res Rev Biol. 2020;35:50–64.
- Law JWF, Ser HL, Khan TM, et al. The potential of streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol. 2017;8:3.
- Kunova A, Palazzolo L, Forlani F, et al. Structural investigation and molecular modeling studies of Strobilurin-Based fungicides active against the rice blast pathogen Pyricularia oryzae. Int J Mol Sci. 2021;22(7):3731.
- Newitt JT, Prudence SM, Hutchings MI, et al. Biocontrol of cereal crop diseases using streptomycetes. Pathogens. 2019;8(2):78.
- Copping LG, Duke SO. Natural products that have been used commercially as crop protection agents. Pest Manag Sci. 2007;63(6):524–554.
- Yang P, Li MG, Zhao JY, et al. Oligomycins a and C, major secondary metabolites isolated from the newly isolated strain Streptomyces diastaticus. Folia Microbiol (Praha). 2010;55(1):10–16.
- Shirling ET, Gottlieb D. Methods for characterization of streptomyces species1. Int J Syst Evol Microbiol. 1966;16(3):313–340.
- Holding A, Collee J. Chapter I routine biochemical tests. Methods in microbiology. Amsterdam, Netherlands: Elsevier; 1971. p. 1–32.
- Staneck JL, Roberts GD. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol. 1974;28(2):226–231.
- Collins M, Jones D. Lipids in the classification and identification of coryneform bacteria containing peptidoglycans based on 2, 4-diaminobutyric acid. J Appl Bacteriol. 1980;48(3):459–470.
- Minnikin D, Collins M, Goodfellow M. Fatty acid and polar lipid composition in the classification of cellulomonas, oerskovia and related taxa. J Appl Bacteriol. 1979;47(1):87–95.
- Sasser M. Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. Newark (DE): MIDI Inc.; 1990.
- Ahsan T, Chen J, Wu Y, et al. Screening, identification, optimization of fermentation conditions, and extraction of secondary metabolites for the biocontrol of Rhizoctonia solani AG-3. Biotechnol Biotechnol Equipment. 2017;31(1):91–98.
- Gao X, He Q, Jiang Y, et al. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae xjy and its biocontrol efficacies against fulvia fulva and botryosphaeria dothidea. J Phytopathol. 2016;164(3):155–165.
- Dasgupta S, Meisner C, Wheeler D, et al. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int J Hyg Environ Health. 2007;210(2):121–132.
- Gao F, Wu Y, Wang M. Identification and antifungal activity of an actinomycete strain against alternaria spp. Span J Agric Res. 2014;12(4):1158–1165.
- Smaoui S, Mathieu F, Fguira B, et al. Taxonomy and antimicrobial activities of a new streptomyces sp. TN17 isolated in the soil from an oasis in Tunis. Arch Biol Sci (Beogr). 2011;63(4):1047–1056.
- Zheng J, Zhu J, Chen B, et al. Application of 16s rDNA sequencing in the analysis of pathogenic bacteria in sputum of severe bacterial pneumonia. Adv Microbiol. 2021;11(02):109–116.
- Ahsan T, Chen J, Zhao X, et al. Action mechanism of Streptomyces diastatochromogenes KX852460 against Rhizoctonia solani AG-3 involving basidiospores suppression and oxidative damage. Iran J Sci Technol Trans Sci. 2019;43(5):2141–2147.
- Gopalakrishnan S, Sharma R, Srinivas V, et al. Identification and characterization of a Streptomyces albus strain and its secondary metabolite organophosphate against charcoal rot of sorghum. Plants. 2020;9(12):1727.
- Monteiro P, Borba MP, Van Der Sand ST. Evaluation of the antifungal activity of streptomyces sp. on Bipolaris sorokiniana and the growth promotion of wheat plants. J Agric Sci. 2017;9(12):229.
- El-Tarabily KA, AlKhajeh AS, Ayyash MM, et al. Growth promotion of salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front Microbiol. 2019;10:1694.