Abstract
In this pilot study, we set a goal of developing collagen-induced arthritis (CIA) in normal mice of both sexes, and compared the susceptibility, prevalence and the degree of inflammation. To assess the grade of inflammation, CIA was evaluated by a visual scale, plethysmometer score and locomotor activity. Seventeen male mice (85%) and only two female mice (10%) developed symptoms of arthritis. None of the female mice that developed arthritis were rated ‘4’ on the visual scale, while 5 male mice (29.42%) were rated with ‘4’. Only animals with a degree of inflammation of ‘4’ and ‘3’ had a significant initial decrease in spontaneous locomotor activity compared to the unaffected animals. The results from this study showed that the susceptibility to development of arthritis was significantly higher in male mice compared to females.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by inflammation of the joints and tendons, impaired mobility and irreversible damage to the cartilage and bones, often leading to systemic manifestation, with significant social and cost-effective consequences [Citation1–3]. The worldwide prevalence of the disease is around 1% [Citation4]. Different factors have implication in the development of RA: genetic (in about 60% of cases), gender, age, hormonal, socio-economic and co-morbidity [Citation5]. In humans, RA is usually more common among females, and the female to male ratio is 3:1 [Citation6].
To understand the pathogenic processes of RA in humans, in vivo models are the most useful. Animal models of RA can be classified into induced and spontaneous [Citation7,Citation8]:
Induced models include collagen-induced arthritis (CIA), adjuvant-induced arthritis, Streptococcal cell wall-induced arthritis, cartilage oligomeric matrix protein (COMP)-induced arthritis, pristane-induced arthritis, antigen-induced arthritis, proteoglycan-induced arthritis (PGIA) and glucose-6-phosphate isomerase (G6PI)-induced arthritis, etc.
Spontaneous models develop RA spontaneously, like K/BxN mice, SKG mice, Human tumour necrosis factor gene (HTNFG) mice, IL-1ra-/- transgenic mice, etc. Spontaneous models are chronic and progressive and can more accurately mimic the processes of improvement and deterioration observed in humans compared to induced models [Citation7].
The two model classes show different aspects of RA. The most widely used models for identifying RA pathology and evaluating different treatment strategies are CIA and PGIA [Citation8]. In the mid-1970s, Trentham et al. [Citation9] successfully developed antibodies against collagen type II (CII) in rats. They found that rats immunized with CII emulsified in the incomplete Freund’s adjuvant developed arthritis, which in many ways resembles human RA [Citation9]. CII is a major component (> 90%) of collagen in the cartilage of a mature adult rat. Analyses indicate that the pathogenesis of CIA is complex and multigenic [Citation10–14]. The first signs of paw inflammation may occur on the 9th day after immunization, but the typical course of the disease in the most used mouse line (DBA/1 LacJ) is characterized by onset between 14 and 21 days. After the first signs, the disease develops rapidly within a few days. The number of paws affected varies widely, depending on the mouse line used, and not all affected paws have the same severity of the inflammation. However, Brand et al. [Citation15], recommended avoiding the use of older male DBA/1 mice for CIA experiments, as there is evidence that ∼80% of ageing male DBA/1 mice develop arthritis spontaneously at the age of 4 months [Citation16]. Interestingly, no arthritis developed after castration of the male mice and the disease susceptibility was restored by testosterone treatment after that [Citation17]. On the contrary to mice, many authors claim that female rats exhibit greater susceptibility to CIA [Citation18–21].
The presence or absence of arthritis in rodents is relatively easy to diagnose. The pattern of arthritis development in a limb or among limbs can vary depending on the species and strain of the animals immunized. It can affect all four paws. The most widely used system for assessment of the swollen paw is a simple visual scoring of 0 to 4. Unfortunately, this approach does not allow to evaluate the severity of the inflammation. In addition, two other methods, plethysmometer and footpad thickness measurements, can be used to feature the severity of arthritis [Citation22].
This pilot study aims to develop a method for inducing CIA in the normal mice line ‘H’, as well as to determine its productivity and reproducibility, as an arthritis model for further research in our lab. This study also aims to define the effect of gender on CIA in this mouse strain.
Materials and methods
Study approval
All animal procedures were conducted in strict accordance with the institutional guidelines and were approved by the Animal Care and Use Committee of the Medical University of Sofia and Bulgaria Food Safety Agency (BFSA). Mice were euthanized via isoflurane overdose [Citation23].
Experimental animals
Forty normal white mice, line ‘H’ (20 male and 20 female), 8 weeks of age, were purchased from the Experimental and Breeding Base for Laboratory Animals, Slivnitsa, Bulgaria. They were allowed to acclimate in the Vivarium of the Faculty of Pharmacy, Medical University of Sofia for seven days before the beginning of the experiment. Mice (n = 5/cage) were placed in polycarbonate cages (20 × 10 × 15 cm) in a controlled environment: food and water ad libidum, temperature 22 ± 3 °C, humidity 60% ± 4%, and 12 h light/dark controlled cycle.
In vivo model of collagen-induced arthritis
For the preparation of the emulsion, equal amounts of collagen type 2 (CII) and Freund’s complete adjuvant (CFA) were mixed. Mechanical tissue homogenizer (IKA-Werke Model # T8.10) was used for preparing the emulsion. At medium speed emulsification, CII was added dropwise to CFA. Once all of the CII was added, the speed of the homogenizer was increased to the maximum for 2 min. All reagents for preparing the emulsion were kept cool throughout the whole procedure. The prepared emulsion was stored in ice to prevent the denaturation of CII. A visual inspection of the emulsion was performed during the transfer of the emulsion from the homogenizer. If the degree of emulsification was not sufficient, re-homogenization was made to improve the state of the emulsion. Each mouse was injected subcutaneously with 50 μl of the emulsion, 1.5 cm distal to the base of the tail without affecting a blood vessel. The injection site was carefully selected because when booster immunization is performed, at a later stage, it should be above the site of the first injection. For a higher degree of CIA manifestation, booster was performed after 21 days. For the booster injection, an emulsion of CII and incomplete Freund’s adjuvant (IFA) in equal amounts was prepared following the same procedure as the initial emulsion. Injection around initially inflamed areas was strictly avoided.
Assessment of arthritis
Visual scale, plesthysmometer score, and determination of the spontaneous locomotor activity were used for assessing the grade of inflammation.
Visual scale
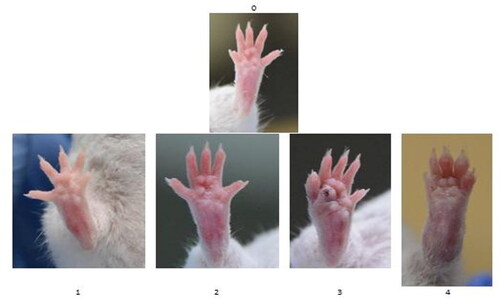
The clinical scoring of arthritis was commenced on day 14 after the initial immunization. The mice were examined daily for visual appearance of inflammation. A subjective evaluation of each limb on a scale of 0 − 4 was performed, where ‘4’ was the most severe form of inflammation, and ‘0’ was a healthy-looking paw (). Assessment of the arthritis score was performed by two independent observers.
Plethysmometry
The study was performed 14–21 days after the booster with a plethysmometer, Model 7140, Ugo Basile, Italy. The plethysmometer consists of two communicating cylindric vessels; one of them is equipped with a pair of electrodes. During the measurement, the vessels are filled with liquid (electrolyte - wetting compound in concentrate 2–3 ml). Immersing the paw to the start line of the hair changes the fluid level and this leads to a change in the conductance between the two electrodes. Registered changes in displacement are reported in millilitres on an electronic display.
The methods of visual assessment and plethysmometry for the determination of arthritis severity are described by Rosloniec et al. (2010).
Spontaneous locomotor activity
The determination of spontaneous locomotor activity was performed 14–21 days after the booster in a Ugo Basile Biological Research Activity Cage Milan, Italy. It is a 40 × 32 × 28 cm acrylic box; its floor consists of metal rods, with even pairs of rods grounded and odd rods active. Based on the connection that mice make or break between rods, random pulse configurations are generated. The electronic counter summarizes the impulses and displays the results. Animals were individually placed in the apparatus for 120 min. The results were registered every 10 min. Before the start of the test, the experimental animals spent 15 min in the apparatus to acclimate. The tests were performed under standard conditions, at the same time of day - from 9 to 13 h. During the studies, the lateral sounds and stimuli were eliminated.
Statistical analysis
Statistical comparison between the different experimental groups was performed by using the Mann-Whitney U test. The results are expressed as mean values with standard deviation (±SD). Differences were considered statistically significant at the p < 0.05 level. Statistical analysis was performed using GraphPad Prism 5.0 software.
Results
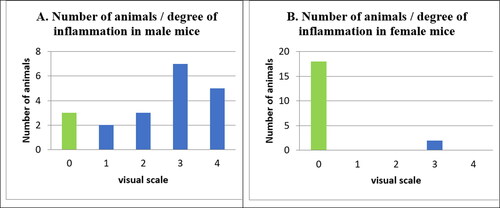
CIA was induced in 20 male and 20 female mice. No symptoms of arthritis were observed in 3 male and 18 female mice, which were termed ‘unaffected’ and were given a score of ‘0’. They appeared normal and never developed arthritis throughout the experiment. All the other mice developed arthritis, and the most affected paw, in about 70% of the animals, was the hind left one.
Seventeen male mice (85%) developed symptoms of arthritis. Five of these were scored ‘4’ (redness and severe edema affecting the whole limb or ankylosis of the limb); 7 animals had score ‘3’ (redness and medium swelling spreading to the metatarsal joints); three had score ‘2’ (redness and slight swelling spreading from ankle to tibia) and two were with score ‘1’ ().
In our experiment, in comparison to the male mice, the females developed arthritis significantly less (p < 0.05). Only two female mice out of 20 (10%) developed arthritis (). These two female mice had a score of ‘3’, whereas among the male animals 29.42% (5 mice; 8 paws) were rated ‘4’; 41.17% (7 mice; 7 paws) were rated ‘3’; 17.65% (3 mice; 3 paws) were rated ‘2’ and 11.76% (2 mice; 2 paws) were rated ‘1’.
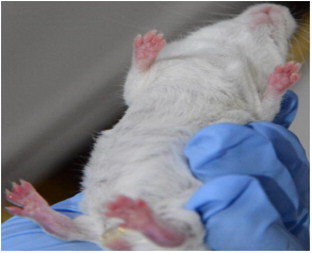
Sixteen out of 17 male mice had only one inflamed paw, and one animal, rated with a score of ‘4’ on the visual scale, developed severe inflammation of all four paws ().
In our experiment, significant difference was also observed between the affected and the unaffected mice in the measurement of inflamed paws by plethysmometer. In the unaffected mice, the mean paw size was 0.19 ± 0.03 and 0.17 ± 0.02 in male and female animals, respectively ().
Table 1. Correlation between visual scale and plethysmometer results.
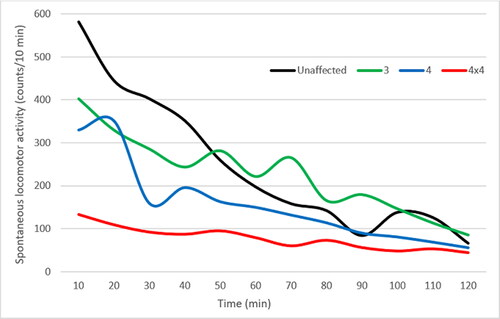
We found a strong correlation between developed CIA and locomotion. Locomotor activity was not altered in the male mice that scored ‘1’ and ‘2’ (data not shown); however, there was a significant initial decrease in spontaneous locomotor activity in the mice with a degree of inflammation of ‘3’ and ‘4’ on the visual scale compared to the unaffected animals (). The decrease in spontaneous movements was more pronounced and lasted longer in the male mice that scored ‘4’, compared to those that scored ‘3’. The animal with the four inflamed paws, which was scored ‘4’, had a visible limitation of the area of movement in the cage compared to the other mice in the group (, 4 × 4 red line). The mouse obviously had difficulty moving, visibly avoided the others in the cage, moving only along the edges of the cage, but not towards the centre. This animal was not sacrificed at the end of the experiment. It was kept alive and observed by a veterinarian for up to 6 months after the development of CIA. During that period, no deterioration or improvement of its condition was observed.
Discussion
Depending on the mouse strain (C57Bl/10, DBA/1J, B10.Q, BUB, B10.RIII) and the CII type used, the susceptibility to CIA varies in a wide range. The incidence varies from 50% to 100% and the onset of arthritis is at about 3 to 4 weeks after initial immunization [Citation24].
In our experiment, in comparison to male mice, females developed arthritis significantly less (85% versus 10%, respectively; p < 0.05). None of the female mice that developed arthritis were rated ‘4’ on the visual scale, while 5 male mice (29.42%) were rated with a score of ‘4’. Only the animals with a degree of inflammation of ‘4’ and ‘3’ had a significant initial decrease in spontaneous locomotor activity compared to the unaffected animals. The results from this study showed that the susceptibility to development of arthritis was significantly higher in male mice compared to females. The evident sex difference observed in our study is in agreement with Holmdahl et al. [Citation25] and Holmdahl et al. [Citation26], who found that male mice are more susceptible to develop arthritis than females in 3 investigated strains (DBA/l, NFRN and BlOG). At the same time, while many female mice had relatively high levels of specific anti type II collagen antibodies, they did not develop arthritis [Citation26]. One explanation is that female sex hormones suppress the development of CIA and oophorectomy makes female mice as susceptible to CIA as are males. Similar results in rats were reported by Ganesan et al. [Citation27], who showed that oestrogen possessed significant anti-inflammatory effects that were diminished by progesterone. Induction of CIA is a T cell-dependent process that could be modulated by female sex hormones binding to the oestrogen receptors, which exhibit a strong protective role in arthritis [Citation28–31]. Corthay et al. [Citation32] supposed that the male aggressiveness could contribute to and enhance the susceptibility to CIA in some strains. Engelmann and Müller-Hilke [Citation33] used only male mice with histocompatibility allele Aq for their experiments because the male sex and the major histocompatibility allele Aq are deemed as crucial factors for the induction of CIA [Citation25,Citation26,Citation34]. Sexual dimorphism in disease expression of RA remained largely unelucidated. Due to the low number of female mice that developed arthritis, we could not detect any difference in the onset of the disease between sexes. The other interesting observation in our study was that 70% of affected paws in our experiment were left hind ones, while Rajaiah and Moudgil [Citation35] pinpoint that fore paws and hind paws are affected with equal frequency. In this pilot study, we cannot explain this fact.
Conclusions
Our results demonstrated that normal mice line ‘H’ are also susceptible to develop arthritis induced by immunization with type II collagen. The first clinical symptoms of inflammation became visible around 14 days after initial immunization; however, maximal joint damage occurred soon after the booster. The results of this study conclusively showed gender differences in the incidence of CIA in normal mice. Male mice were significantly more affected than females (85% to 10%, respectively). The presumption, that inflamed and swollen joints will affect the wellbeing of the animals, was confirmed in the experiment with locomotor activity. It was to be expected that an initial decrease in locomotion will strongly correlate with disease severity and arthritic score. However, the overall decrease in spontaneous locomotor activity was only observed in animals that scored in groups ‘3’ and ‘4’. The explicit male predominance in the incidence of CIA led to our decision to continue our future experiments with collagen-induced arthritis in mice only with male animals.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Lyubomir Marinov, upon reasonable request.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford). 2005;44(Suppl 2):ii3–ii7.
- Atzeni F, Doria A, Turiel M, et al. What is the role of rituximab in the treatment of rheumatoid arthritis? Autoimmun Rev. 2007;6(8):553–558.
- Atzeni F, Benucci M, Sallì S, et al. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev. 2013;12(5):575–579.
- Silva-Fernández L, Macía-Villa C, Seoane-Mato D, et al. The prevalence of rheumatoid arthritis in Spain. Sci Rep. 2020;10(1):21551.
- Alamanos Y, Drosos AA. Drosos AA epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130–136.
- Kvien TK, Uhlig T, Ødegård S, et al. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann NY Acad Sci. 2006;1069:212–222.
- Cuzzocrea S. Characterization of a novel and spontaneous mouse model of inflammatory arthritis. Arthritis Res Ther. 2011;13(5):126.
- Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40(3):193–200.
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146(3):857–868.
- DahlmanI, Lorentzen JC, de Graaf KL, et al. Quantitative trait loci disposing for both experimental arthritis and encephalomyelitis in the DA rat; impact on severity of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis and antibody isotype pattern. Eur J Immunol. 1998;28(7):2188–2196.
- Gulko PS, Kawahito Y, Remmers EF, et al. Griffiths MM identification of a new non-major histocompatibility complex genetic locus on chromosome 2 that controls disease severity in collagen-induced arthritis in rats. Arthritis Rheum. 1998;41(12):2122–2131.
- Ibrahim SM, Koczan D, Thiesen HJ. Gene-expression profile of collagen-induced arthritis. J Autoimmun. 2002;18(2):159–167.
- Adarichev VA, Nesterovitch AB, Bárdos T, et al. Sex effect on clinical and immunologic quantitative trait loci in a murine model of rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1708–1720.
- Glant TT, Adarichev VA, Nesterovitch AB, et al. Disease-associated qualitative and quantitative trait loci in proteoglycan-induced arthritis and collagen-induced arthritis. Am J Med Sci. 2004;327(4):188–195.
- Brand DD, Latham KA, Rosloniec EF. Rosloniec EF collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–1275.
- Nordling C, Karlsson-Parra A, Jansson L, et al. Characterization of a spontaneously occurring arthritis in male DBA/1 mice. Arthritis Rheum. 1992;35(6):717–722.
- Holmdahl R, Jansson L, Andersson M, et al. Genetic, hormonal and behavioural influence on spontaneously developing arthritis in normal mice. Clin Exp Immunol. 1992;88(3):467–472.
- Cremer MA, Ye XJ, Terato K, et al. Type XI collagen-induced arthritis in the Lewis rat. Characterization of cellular and humoral immune responses to native types XI, V, and II collagen and constituent alpha-chains. J Immunol. 1994;153(2):824–832.
- Holmdahl R. Female preponderance for development of arthritis in rats is influenced by both sex chromosomes and sex steroids. Scand J Immunol. 1995;42(1):104–109.
- Griffiths MM, Cannon GW, Corsi T, et al. Collagen-induced arthritis in rats. Methods Mol Med. 2007;136:201–214.
- Dimitrijević M, Arsenović-Ranin N, Kosec D, et al. Sex differences in tfh cell help to B cells contribute to sexual dimorphism in severity of rat collagen-induced arthritis. Sci Rep. 2020;10(1):1214.
- Rosloniec EF, Cremer M, Kang AH, et al. Collagen-induced arthritis. Curr Protoc Immunol. 2010;Chapter 15:Unit 15.5.
- Seymour TL, Nagamine CM. Evaluation of isoflurane overdose for euthanasia of neonatal mice. J Am Assoc Lab Anim Sci. 2016;55(3):321–323.
- Holmdahl R. Experimental models for rheumatoid arthritis. In Kelley and Firestein’s textbook of rheumatology. 10th ed. Philadelphia, PA: Elsevier; 2017.
- Holmdahl R, Jansson L, Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986;29(12):1501–1509.
- Holmdahl R, Jansson L, Larsson E, et al. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29(1):106–113.
- Ganesan K, Balachandran C, Manohar BM, et al. Comparative studies on the interplay of testosterone, estrogen and progesterone in collagen induced arthritis in rats. Bone. 2008;43(4):758–765.
- Holmdahl R, Jansson L, Meyerson B, et al. Oestrogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987;70(2):372–378.
- Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47(7):290–301.
- Jansson L, Holmdahl R. Enhancement of collagen-induced arthritis in female mice by estrogen receptor blockage. Arthritis Rheum. 2001;44(9):2168–2175.
- Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163(5):1827–1837.
- Corthay A, Hansson AS, Holmdahl R. T lymphocytes are not required for the spontaneous development of entheseal ossification leading to marginal ankylosis in the DBA/1 mouse. Arthritis Rheum. 2000;43(4):844–851.
- Engelmann R, Müller-Hilke B. Experimental silicosis does not aggravate collagen-induced arthritis in mice. J Negat Results Biomed. 2017;16(1):5.
- Brunsberg U, Gustafsson K, Jansson L, et al. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994;24(7):1698–1702.
- Rajaiah R, Moudgil KD. Chapter 8N: Animal models. In Marc C Hochberg, editor-in-chief. Rheumatoid arthritis. Mosby: Elsevier Inc; 2009. p. 218–224.