 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Capparis trinervia (family Capparaceae) is a common wild plant distributed in various localities in the mountainous region of Vietnam. While essential oils have been found in the leaves and stems of C. trinervia, there are no reports to date describing the chemical composition and biological activities to guide further exploration and application of the oils. This study determined the essential oil composition of C. trinervia; 8 and 23 distinct essential oil compounds were identified from C. trinervia leaves and stems, respectively. Stigmasterol (C29H48O) was identified as the primary compound of essential oil from leaves, accounting for 75.775% of the oils, while 3,7,11,15-tetramethyl-2-hexadecen-1-ol (C20H40O) was identified as the main essential oil component in C. trinervia stems, accounting for 18.337%. This study also explored the cytotoxic effects of essential oils extracted from C. trinervia against five cancer cells lines (A549, MCF7, HeLa, HepG2 and KB). A strong cytotoxic effect on all cancer cell lines was demonstrated by 100 µg mL−1 essential oils derived from C. trinervia leaves and stems, which suggests the species is a promising candidate for use in life and public healthcare.
Introduction
Capparis is the largest genus of the Capparaceae family; it includes approximately 250 species distributed in tropical and subtropical regions [Citation1]. Many species of this genus are consumed as food and utilized in traditional medicine [Citation2,Citation3]. Traditional medicine values Capparis for its use as a diuretic, as well as for treatment of gastrointestinal issues, inflammation, bronchitis, nasal mucositis, menstruation and asthma [Citation1,Citation4]. Extracts of some Capparis species have been noted to contain biologically active secondary compounds.
Numerous studies on the biological activity and pharmacological and phytochemical properties of C. spinosa have been conducted [Citation5,Citation6]. The content and biological activity of substances is dependent upon the sampling site and extraction method [Citation7,Citation8]. Extracts from C. spinosa were shown to have protective effects against cytotoxicity and tumour growth, as well as anticancer, antibacterial and antifungal properties [Citation9–12].
The biological activities of several other species of Capparis have also been assessed. Leaf extracts of Capparis brevispina have antioxidant, cytotoxic and anticancer effects [Citation13–15]. Methanol extracts from the fruit of Capparis ovata has demonstrated anticancer activity in mouse models [Citation16]. Stem and leaf extracts of C. flavicans have also exhibited anticancer activities [Citation17]. Numerous published reports on the composition and bioactivity of essential oils (EOs) from various plants, such as Thymus daenensis, Chromolaena odorata, Artemisia fragrans and Psidium guajava, have provided information on the composition and antioxidant, antimicrobial and cytotoxic activities of EOs [Citation18–21].
To date, there have been several studies conducted to elucidate the composition and EO bioactivities of C. spinosa, C. spinosa var. aegyptiaca, and C. ovata var. canescens. However, there are no published studies on the EOs found in C. trinervia, a species that is widely distributed in Vietnam and in secondary forests at altitudes up to 300 m [Citation4]. This study aimed to serve as an initial report on C. trinervia EOs with respect to compositional analysis and exploration of their cytotoxic effects on cancer cell lines.
Materials and methods
Materials
Samples of C. trinervia were collected in Phuc Yen Town, Vinh Phuc Province, Vietnam (21°23′02″N; 105°42′43″E; altitude 58 m), in the spring of March 2020. Classification was based on the comparative morphology method established by Assoc. Prof. Dr. Sy Danh Thuong, Department of Botany, Faculty of Biology, TNU-University of Education, Vietnam (). The voucher specimens (Code number: Sy Danh Thuong 30) were stored at the Museum of Biology, TNU, University of Education, Vietnam. The leaves and stems with an identical code number were collected separately, cut into small pieces of approximately 1 cm, and washed to extract EOs.
Figure 1. Morphology of C. trinervia. (A) C. trinervia wildly growing in the secondary forest along with some other plants; (B) leaf-bearing branchlet (upper side); (C) leaf-bearing branchlet (underside) and fruit.

The cancer cell lines used in the study included A549 (human lung carcinoma), MCF7 (human breast carcinoma), HeLa (human cervix carcinoma), HepG2 (human hepatocarcinoma) and KB (human carcinomas in the mouth). These cancer cell lines were used in the cytotoxic assays at the Bioassay Laboratory, VAST-Institute of Biotechnology, Vietnam. Five human cancer cell lines were grown in Dulbecco’s Modified Eagle Medium (DMEM; Hyclone, Logan, Utah, USA) with 10% foetal bovine serum, 2 mmol L−1 L-glutamine, 10 mmol L−1 HEPES and 1 mmol L−1 sodium pyruvate at 37 °C in a 5% CO2 humidified atmosphere.
Essential oil extraction method
EOs from C. trinervia leaves and stems were extracted by a steam-aspirated distillation method for 4 h under normal pressure at approximately 70 °C–80 °C [Citation22]. The extracts were dissolved in n-hexane and n-butanol to obtain the dissolved EOs. The EOs were stored in glass bottles, which were then wrapped in sealed plastic bags, covered and stored at 4 °C. Soluble EO was utilized for determining the composition and testing for cytotoxic activity against selected human cancer cell lines.
Essential oil chemical composition analysis method
A n-hexane-soluble EO sample was used to analyze the chemical composition of the EO. One milliliter of the EO of the plant was obtained following evaporation of the n-hexane solvent, and 0.1 mL of extracted EO was dissolved in 5 mL n-hexane to determine the chemical composition using gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS).
GC was performed using an Agilent Technologies HP 6890 N Plus attached to a FID detector (Agilent Technologies, USA). An HP-5MS chromatographic column with a 30 m length, inner diameter (ID) of 0.25 mm and thin-film layer of 0.25 m was used. The injection chamber temperature was maintained at 250 °C. The detector temperature was set to 260 °C. The initial 60 °C thermostatic chamber temperature was held for 2 min prior to being increased at a rate of 4 °C/min until 220 °C was reached and held for 10 min. Helium was used as the carrier gas.
GC/MS was performed using gas chromatography equipment, and GC/MS conjugate spectroscopy was performed on an Agilent Technologies HP 6890 N paired with a mass selective detector (Agilent HP 5973 MSD). The HP-5MS column measured 0.25 m × 30 m × 0.25 mm, and the HP1 measured 0.25 m × 30 m × 0.32 mm. The temperature was held at 60 °C for 2 min before being increased by 4 °C/min to 220 °C; the temperature was subsequently increased at a rate of 20 °C/min to 260 °C. Helium was the carrier gas.
Constructor validation was performed by comparing MS spectral data with published standard spectra available in the Willey/Chemstation HP library [Citation7].
Cytotoxic assays on human cancer cell lines
To assess the cytotoxic activity of EOs from the leaves and stems of C. trinervia, four concentrations of EO extracts (100, 20, 4 and 0.8 µg mL−1) were tested on five cancer cell lines (A549, MCF7, HeLa, HepG2 and KB).
The in vitro cytotoxicity testing method utilized was confirmed by the National Cancer Institute (NCI) as a standard cytotoxicity test to screen and detect substances with inhibitory ability on the in vitro growth or destruction of cancer cells. This test was based on cellular protein content and performed in accordance with the methods described by Skehan et al. (1990) [Citation23,Citation24]. Protein content was determined based on optical density (OD), as measured by staining content with sulforhodamine B (SRB). The measured OD value was directly proportional to the amount of SRB bound to the protein molecule; therefore, a greater quantity of cells and protein content yielded a higher OD value.
The experiment was carried out under the following conditions: (1) trypsinization was used to loosen and count cells to adjust for density suitable for the experiment. (2) Different concentrations of reagents were added to a 96-well plate. A well containing 190 µL of cancer cells was used as a control on day 0. After 1 h, the cells in the control well were fixed with 20% trichloroacetic acid (TCA). (3) The sample was placed in an incubator for 72 h. TCA was applied for 1 h to fix cells and SRB was then used to stain cells for 30 min at 37 °C prior to washing the sample in triplicate with acetic acid and allowing time to dry. (4) SRB was dissolved with an unbuffered Tris base (10 mmol L−1) and then gently shaken for 10 min. (5) The OD results were read at 540 nm using an ELISA plate reader.
The percentage of cell growth inhibition in the presence of the reagent was determined using the following formula:
(%)
(%)
Each test was repeated 3 times. Ellipticine at 10, 2, 0.4 and 0.08 µg mL−1 concentrations was used as a positive control. Dimethyl sulfoxide (DMSO) 10% was used as a negative control.
The half-maximal inhibitory concentration (IC50) is defined as the EO concentration that will inhibit 50% of cancer cells. The IC50 was determined using TableCurve 2Dv4 computer software. According to the American National Cancer Institute (NCI) standards, an extract is considered to have good activity with an IC50 ≤ of 20 μg mL−1 [Citation24].
Statistical analysis
The cytotoxic effects of EOs from C. trinervia leaves and stems against human cancer cell lines in vitro were analyzed using the Statistical Package for the Social Sciences (SPSS) software, and Duncan’s test was used to determine differences at p < 0.05.
Results and discussion
Chemical composition of essential oil from leaves and the above-ground portions of stems in C. trinervia
The EOs from C. trinervia leaves were extracted by steam aspiration distillation and dissolved in n-hexane. The EO from C. trinervia leaves is liquid, white and transparent. The analytical results of the GC/MS spectrum of leaf EO ( and ) showed that the chemical composition consisted of eight compounds, of which the most abundant compound was stigmasterol (C29H48O) with 75.775%, while nonane, 2,2,4,4,6,8,8 heptamethyl (C16H34), was the lowest yielding compound (0.079%). Much like the EO found in C. trinervia leaves, the EO from the plant’s stems is liquid, white and transparent. A GC/MS spectrum analysis of EOs from the above-ground stems of C. trinervia in and identified 23 compounds; 3,7,11,15-tetramethyl-2-hexadecen-1-ol accounted for the highest yield (18.337%), while humulenol-II was the lowest yield (0.628%). Overall, the primary constituent of EOs from C. trinervia leaves and stems were stigmasterol and 3,7,11,15-tetramethyl-2-hexadecen-1-ol, respectively.
Figure 2. GC/MS spectrum of leaf and stem essential oil from Capparis trinervia Hook. F. & Thomson. (A) GC/MS spectrum of leaf essential oil; (B) GC/MS spectrum of stem essential oil.
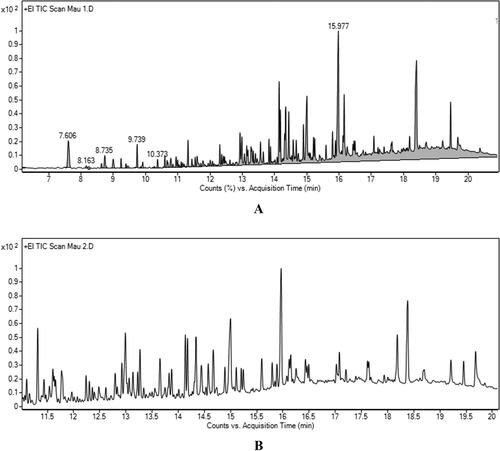
Table 1. Chemical composition of essential oils from C. trinervia leaves.
Table 2. Chemical composition of essential oils from C. trinervia stems.
Existing published studies identifying the chemical compositions and cytotoxic activities on cancer cell lines of plants native to Vietnam’s mountainous regions have been concentrated on a handful of species [Citation15,Citation26,Citation27]. In C. spinosa, methyl isothiocyanate (92.06%) has been identified as the major component of EOs extracted from leaves and flower buds. These EOs have the ability to inhibit lipid oxidation in both animal and human digestion [Citation28]. EOs from C. spinosa var. aegyptiaca, a plant widely grown in Egypt, are also primarily composed of methyl isothiocyanate (24.66%) [Citation29]. El-Ghorab et al. (2007) identified 86 and 100 compounds in EOs extracted from the buds and leaves of C. ovata var. canescens, respectively. Benzyl alcohol was identified in EOs extracted from buds (20.4%) and methyl isothiocyanate was identified in leaves (20.0%) [Citation30].
Components of EOs have been receiving great attention in research for their potential utilization; nevertheless, there have been no attempts to date to study the EOs of C. trinervia. This is the first study, to the best of our knowledge, to analyze the EO extracted from C. trinervia. GC/MS was selected for C. trinervia EO analysis due to its demonstrated efficacy as an analytical testing method.
In this study, the EOs extracted from C. trinervia leaves consisted of eight compounds, of which the main compound was stigmasterol (75.775%), a phytosterol with potential anti-osteoarthritic properties due to its role in maintaining structural and physiological stability of cell membranes [Citation31]. Furthermore, dietary stigmasterol is known to play a role in increasing serum phytosterol content and reducing LDL (low-density lipoptotein) cholesterol levels [Citation32].
Meanwhile, EO extracted from the stems of the C. trinervia tree was comprised of 23 total compounds, with 3,7,11,15-tetramethyl-2-hexadecen-1-ol (C20H40O) identified as the main compound (18.337%).
Cytotoxic effects of essential oils from C. trinervia leaves and stems on selected human cancer cell lines
Findings on cytotoxic activities of C. trinervia EOs against selected human cancer cell lines are presented in . The results showed strong cytotoxic activities against A549, MCF7, KB, HepG2 and HeLa with IC50 values of 9.44, 9.51, 15.01, 28.09 and 30.63 µg mL−1, respectively, with inhibitory effects against human cancer cell lines inversely proportional to IC50 values. Stem-derived EOs of C. trinervia also had strong cytotoxic activities against A549, MCF7, KB, HepG2 and HeLa with IC50 values of 9.02, 8.47, 14.94, 26.10 and 28.41 µg mL−1, respectively. For both stem and leaf derived C. trinervia EOs, the inhibitory effects against the HeLa cell line were the lowest observed amongst the five selected human cancer cell lines. The strongest cytotoxic effects for both stem and leaf derived EOs were seen against the A549, MCF7 and KB, with IC50 values ranging from 8.47 to 14.94 µg mL−1.
Table 3. Cytotoxicity of essential oils from C. trinervia leaves and stems against human cancer cell lines in vitro.
The obtained results following treatment with EO from C. trinervia leaves and stems showed a concentration-dependent relationship between EO concentration and cytotoxic effects on selected cancer cell lines. Leaf and stem EOs demonstrated potent cytotoxicity against the HeLa cell line at concentrations of 100 µg mL−1 and 20 µg mL−1, respectively. Leaf and stem EOs at a 100 µg mL−1 concentration had strong toxic effects on the HepG2 cancer cell line. Cytotoxic activities of leaf and stem EOs against the KB cell line varied by concentration.
To explain the mechanisms underlying the inhibitory action of EOs, some studies have performed cell viability assays and flow cytometry analyses to elucidate the mechanism of cancer cell proliferation inhibition. The resultant literature has suggested that EO compounds inhibit gene transcription, directly inhibit proteins and enzymes [Citation33,Citation34], decrease mitochondrial membrane potential and induce apoptosis [Citation35]. The cumulative scientific experience indicates there is a clear relationship between inhibitory activities of EOs against cancer cell lines and regulation of gene expression in cells. There is a need for further studies to evaluate the role of EOs on regulating genes involved in cellular apoptosis.
The results of the IC50 analysis performed in this study showed that C. trinervia EOs had different cytotoxic effects against five selected cancer cell lines. Differences in cytotoxicity against different cancer cell lines may be attributable to the suppression of transcription of various genes, the inhibition of the activity of different proteins and enzymes, and the apoptosis-inducing effects in various cell cycle phases. In this analysis, three cancer cell lines, A549, MCF7 and KB, demonstrated greater concentration-dependent sensitivity to C. trinervia EOs when compared to HeLa and HepG2 cancer cell lines. This suggests that potential future use of EOs as an agent for cancer treatment must take into account each cancer cell type; at the same time, the match of EO concentration with each cancer cell line must be taken into account.
This study suggests that C. trinervia is a promising candidate for further research to explore its cytotoxic potential against human cancer cells and for its use in food manufacturing. As this study only tested the EO activity on cancer cell lines and not on normal cells, the cytotoxic effects of C. trinervia EOs against normal cell lines require further experimental research to establish a scientific basis for their potential pharmaceutical use.
Conclusions
In this study, eight substances in the composition of EOs from leaves and 23 EOs compounds from C. trinervia stems were identified. Stigmasterol is the main compound of EOs from leaves, accounting for the highest percentage (75,775%), and 3,7,11,15-tetramethyl-2-hexadecen-1-ol is the main compound of EOs from stems, accounting for the highest percentage (18.337%). Five human cancer cell lines (A549, MCF7, HeLa, HepG2 and A549) were treated with EOs extracted from C. trinervia leaves and stems. The EOs displayed strong cytotoxic effects on A549, MCF7 and KB cell lines, with IC50 values ranging from 8.47 to 14.94 µg mL−1. At a concentration of 100 µg mL−1, EOs from the leaves and stems had a strong toxic effect on all cancer cell lines. C. trinervia is a novel medicinal plant whose EOs demonstrate inhibitory activities against cancer cell lines. C. trinervia shows promise as a candidate for use in the realm of public healthcare.
Author contributions
Conceived and designed the experiments: TDS, MHC, KVP. Performed the experiments: NTTN, LTNN, QHN, TQT, KVP, TQT. Performed analyses and wrote the article: NTTN, TDS, TQT, LTNN, QHN, MHC. Did the proof-reading: TDS, LTNN, QHN, MHC
| ABBREVIATIONS: | ||
| DMEM | = | Dulbecco’s Modified Eagle Medium |
| DMSO | = | Dimethyl sulfoxide |
| GC | = | Gas chromatography |
| GC/MS | = | Gas chromatography/Mass spectrometry |
| A549 | = | Human lung carcinoma |
| MCF7 | = | Human breast carcinoma |
| HeLa | = | Human cervix carcinoma |
| HepG2 | = | Human hepatocarcinoma |
| KB | = | Human carcinomas in the mouth |
| EO | = | essential oil |
| LDL | = | Low-density lipoptotein |
| OD | = | Optical density |
| SRB | = | Sulforhodamine B. |
Data availability statement
Raw data were generated at TNU- University of Education, Vietnam. Derived data supporting the findings of this study are available from the corresponding author MHC upon reasonable request. The collection of plant materials has complied with Vietnamese and international guidelines and legislation
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sy DT. Doctoral thesis in biology: Taxonomical study of the capparaceae in vietnam. Hanoi: Institute of Ecology and Biological Resources; 2014.
- Rivera D, Inocencio C, Obon C, et al. Review of food and medicinal uses of capparis L. subgenus capparis (capparidaceae). Econ Bot. 2003;57(4):515–534.2.0.CO;2]
- Mishra SN, Tomar PC, Lakra N. Medicinal and food value of capparis - a harsh terrain plant. Indian J Tradit Know. 2007;6(1):230–238.
- Pham HH. An illustrated flora of vietnam. Vol. 1. Ho Chi Minh: Young Publishing House; 1999.
- Zhang H, Ma ZF. Phytochemical and pharmacological properties of capparis spinosa as a medicinal plant. Nutrients. 2018;10(2):116.
- Moufid A, Farid O, Eddouks M. Pharmacological properties of capparis spinosa linn. Int J Diabetol Vasc Dis Res. 2015;3(5):99–104.
- Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectrometry. 4th ed. Carol Stream (IL): Allured Publishing Co.; 2007.
- Allaith AAA. Assessment of the antioxidant properties of the caper fruit (capparis spinosa L.) from Bahrain. J Assoc Arab Univ Basic Appl Sci. 2016;19(1):1–7.
- Yu L, Yang J, Wang X, et al. Antioxidant and antitumor activities of capparis spinosa L. and the related mechanisms. Oncol Rep. 2017;37(1):357–367.
- Moghadamnia Y, Kani SMK, Ghasemi-Kasman M, et al. The anti-cancer effects of capparis spinosa hydroalcoholic extract. Avicenna J Med Biotechnol. 2019;11(1):43–47.
- Sherif MM, El-Shiekh HH, Elaasser MM, et al. In vitro evaluation of antimicrobial and cytotoxic effects of caper (capparis spinosa). J Appl Sci Res. 2013;9(4):2944–2950.
- Benzidane N, Aichour R, Guettaf S, et al. Chemical investigation, the antibacterial and antifungal activity of different parts of capparis spinosa extracts. J Drug Delivery Ther. 2020;10(5):118–125.
- Sreenivas SA, Venu Gopal Y, Ravindranath A, et al. Antitumor and antioxidant activity of capparis sepiaria against dalton’s ascites lymphoma in rodents. Acad J Cancer Res. 2012;5(2):46–52.
- Subramanian SK, Ramani P. Antioxidant and cytotoxic activities of indian caper (capparis brevispina DC (capparaceae)) leaf extracts. Eur J Integr Med. 2020;33(101038):101038.
- Pham VK, Sy DT, Vu VN, et al. Two new polycyclic compounds and cytotoxic activities of ethanol and ethyl acetate extract of leaves of capparis dongvanensis (Sy.) and their chemotaxonomic significance. Polycycl Aromat Compd. 2021; DOI: 10.1080/10406638.2020.1871383
- Arslan R, Bektas N, Ozturk Y. Antinociceptive activity of methanol extract of fruits of capparis ovata in mice. J Ethnopharmacol. 2010;131(1):28–32.
- Luecha P, Umehara K, Miyase T, et al. Antiestrogenic constituents of the thai medicinal plants capparis flavicans and vitex glabrata. J Nat Prod. 2009;72(11):1954–1959.
- Alizadeh A, Alizadeh O, Golnaz Amari G, et al. Essential oil composition, total phenolic content, antioxidant activity and antifungal properties of iranian thymus daenensis subsp. daenensis celak. as in influenced by ontogenetical variation. J Essent Oil Bear Plants. 2013;16(1):59–70.
- Gogoi R, Sarma N, Begum T, et al. North-East indian chromolaena odorata (L. King robinson) Aerial part essential oil chemical composition, pharmacological Activities - Neurodegenerative inhibitory and toxicity study. J Essent Oil Bear Plants. 2020;23(6):1173–1191.
- Younessi-Hamzekhanlu M, Sanjari S, Dejahang A, Karkaj ES, et al. Evaluation of essential oil from different artemisia fragrans willd. populations: chemical composition, antioxidant, and antibacterial activity. J Essent Oil Bear Plants. 2020;23(6):1218–1236.
- Borah A, Pandey SK, Haldar S, et al. Chemical composition of leaf essential oil of psidium guajava L. from North East India. J Essent Oil Bear Plants. 2019;23(5):248–253.
- Fandohan P, Gnonlonfin B, Laleye A, et al. Toxicity and gastric tolerance of essential oils from cymbopogon citratus, ocimum gratissimum and ocimum basilicum in wistar rats. Food Chem Toxicol. 2008; 46(7):2493–2497.
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112.
- Hughes JP, Rees S, Kalindjian SB, et al. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239–1249.
- Vu TTT, Vu TKL, Nguyen HQ, et al. Cytotoxic effects of steroidal glycosides isolated from the paris vietnamensis plant on cancer cell lines and against bacterial strains. Biotechnol Biotechnol Equip. 2019;33(1):1516–1524.
- Goodner KL. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax or flavor and fragrance compounds. LWT-Food Sci Technol. 2008;41(6):951–958.
- Chedraoui S, Abi-Rizk A, El-Beyrouthy M, et al. Capparis spinosa L. in a systematic review: a xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front Plant Sci. 2017;8:1–18.
- Kulisic-Bilusic T, Blažević I, Dejanović B, et al. Evaluation of the antioxidant activity of essential oils from caper (capparis spinosa) and sea fennel. (crithmum maritimum) by different methods. J. Food Biochem. 2010;34:286–302.
- Bakr RO, Bishbishy MHE. Profile of bioactive compounds of capparis spinosa var. aegyptiaca growing in Egypt. Rev Bras Farmacogn. 2016;26(4):514–520.
- El-Ghorab A, Shibamoto T, Özcan MM. Chemical composition and antioxidant activities of buds and leaves of capers (capparis ovata desf. var. canescens) Cultivated in Turkey. J Essent Oil Res. 2007;19(1):72–77.
- Ferrer A, Altabella T, Arró M, et al. Emerging roles for conjugated sterols in plants. Prog Lipid Res. 2017;67:27–37.
- Cabral CE, Klein MRST. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq Bras Cardiol. 2017;109(5):475–482.
- Weng CJ, Chou CP, Ho CT, et al. Molecular mechanism inhibiting human hepatocarcinoma cell invasion by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 2012;56(8):1304–1314.
- Singh M, Singh N. Molecular mechanism of curcumin-induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem. 2009;325(1-2):107–119.
- Zhang Y, Xin C, Qiu J, et al. Essential oil from pinus koraiensis pinecones inhibits gastric cancer cells via the HIPPO/Yap signaling pathway. Molecules. 2019; 24(21):3851.
