Abstract
Multiple myeloma (MM) is the most common bone marrow malignancy which is defined with bone marrow infiltration of more than 10% of terminally differentiated plasma cells sive myeloma cells. The aim of this study was to find correlations between the expression of some immunohistochemical markers (Cyclin D1, p53, p21), plasmablastic differentiation and prognosis of MM. Immunohistochemistry was used to detect expression of cell cycle proteins. Bone marrow trephine biopsies of 122 MM patients were analyzed to determine the plasmablastic morphology of myeloma cells and myeloma cells expression of immunohistochemical markers (p21, p53, Cyclin D1). The following clinical data of MM patients were obtained: β2-microglobulin, albumin, haemoglobin, platelet count, glomerular filtration rate (GFR), creatinine, calcium level, M-gradient and presence of osteolytic lesions. These data were compared with the bone marrow histological findings. Plasmablastic differentiation correlated with decreased level of haemoglobin, albumin and GFR. Cyclin D1 expression correlated with significantly higher serum calcium level, more common osteolytic lesions in bones. Patients with p21 expression in myeloma cells had lower albumin levels, higher M-gradient levels and more advanced stage by Salmon–Durie. These results suggest that there are correlations between plasmablastic differentiation, expression of p53, CyclinD1 and p21 and poor prognosis in cases of MM.
Introduction
Multiple myeloma (MM) is the most common bone marrow malignancy of terminally differentiated plasma cells. It accounts for about 1% of all malignant diseases and 10% of haematological malignancies and takes the second place after non-Hodgkin lymphoma [Citation1]. There are around 60–100 newly diagnosed cases per year in the Latvian population [Citation2].
There are some serological and clinical criteria that help to detect the stage of MM and predict the patients’ prognosis. These criteria include the level of M protein, calcium, haemoglobin, albumin, creatinine, β2-microglobulin and glomerular filtration rate (GFR) [Citation3].
Myeloma cells with plasmablastic morphology are medium to large in size with a central nucleus and one or more nucleoli. These cells are found in 10–15% of all patients with newly diagnosed MM or primary patients [Citation4]. MM with plasmablastic differentiation correlates with poor prognosis, worse clinical features and shorter survival [Citation5].
Antigen p21. Short lived protein p21 is a member of the cyclin-dependent kinase inhibitors; p21 is regulated through p53-dependent and p53-independent pathways. It can act both as a tumour suppressor and as an oncogene; it depends on the cellular context. As p21 lacks defined tertiary structure, it can interact with different kinds of proteins. When functioning as a tumour suppressor, p21 controls the cell cycle, especially, the check point in G1 phase. The other functions include differentiation, apoptosis and migration [Citation6,Citation7]. There are few studies that prove the correlation between p21 over expression and shorter survival of patients with MM [Citation7].
Antigen p53. The tumour suppressor protein p53 is a TP53 gene product. TP53 is a tumour suppressor gene, on chromosome 17p13.1. In normal cells, under normal physiological conditions p53 is expressed at low levels; the p53 protein pathway switches on when cells undergo cellular shock, for example DNA damage, heat shock or hypoxia. Accumulation of p53 protein in cells activates different pathways. The aim of these pathways is to keep the cell genome intact and to stabilize the homeostasis of the cell [Citation8].
MM patients with aberrant p53 nuclear expression usually receive diagnosis in advanced clinical and histological stages and have significantly shorter overall survival than patients without this abnormality [Citation9,Citation10].
The overall survival of MM patients depends on multiple criteria, including the stage of MM, concomitant diseases and different methods of chemotherapy [Citation11].
Antigen Cyclin D1. Cyclin D1 overexpression has been reported in 50–60% cases of MM [Citation12]. Cyclin D1 is a product of the CCND1 gene. CCN family proteins are associated with many important processes in cells such as proliferation, adhesion, angiogenesis, differentiation and survival [Citation13]. Aberrant expression of Cyclin D1 in MM is associated with genetic abnormalities like translocation t (11;14) (q13; q32), polysomy for chromosome 11, amplification of CCND1 and other genetic abnormalities; this feature is associated with longer overall survival [Citation12, Citation14]. Overexpression of Cyclin D1 in MM results in increased proliferation of plasma cells. The studies about Cyclin D1 and its prognostic value are controversial: while Athanasiou et al. [Citation15] demonstrate that Cyclin D1 overexpression correlates with higher histological grade and better prognosis, others such as Padhi et al. [Citation12] and Dunphy et al. [Citation16] have not found any correlation.
Studies have shifted their attention to Cyclin D1 and CCD1 expression to help provide prognostic information of possible response to bortezomib therapy [Citation17]. The aim of this study was to seek correlation between the morphological, immunohistochemical data and clinical parameters, as it could be helpful to determine the prognosis of patients with MM.
Materials and methods
Ethics statement
All respondents gave informed consent for participation in research study and use of biologic material. The research protocol for consent and participation as well as the questionnaire comply with the Declaration of Helsinki Protocol, Ethical Principles for Medical Research Involving Human Subjects, and received approval by Riga Stradins University (Latvia) Ethics Committee (Review Board), permission No. 26-4/7/5 of 04.09.2014.
Study population
The study was retrospective. Bone marrow biopsies of 122 patients from Riga East Clinical University Hospital’s Haematology Centre with MM diagnosed within five years were included in the study.
Material fixation
Bone marrow biopsies were fixed in 10% neutral (pH 7) buffered formalin solution, decalcified and processed in automatic tissue processor. All bone marrow biopsies were stained with routine haematoxylin and eosin, Periodic acid-Schiff (PAS), Giemsa stain and Gordon and Sweet’s reticulin silver staining method using standard protocols (Bio-optica, Italy).
Plasmablastic differentiation
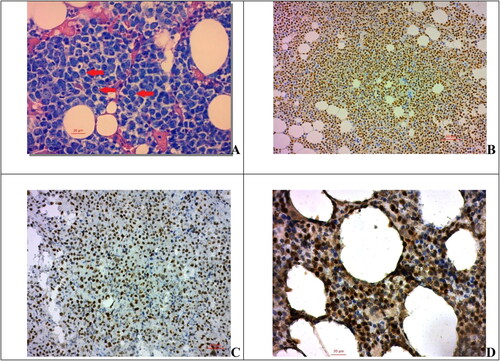
Plasmablastic differentiation was determined by certified pathologists based on cell morphology in routine haematoxylin–eosin and PAS staining of trephine biopsies ().
Figure 1. Expression of cyclin D1, p53 and p21 in myeloma cells. (A) Myeloma cells with plasmablastic morphology, PAS, 400×; scale bar = 20 µm; (B) Cyclin D1 nuclear expression in myeloma cells, 200×; (C) expression of p53 in myeloma cells, 200×; scale bar = 50 µm (D) protein p21 nuclear expression in myeloma cells, 400×; scale bar = 20µm.
Immunohistochemistry
Immunohistochemistry was used to detect the expression of cell cycle proteins. Immunohistochemical antigen expression was determined by a standard polymer-based visualization system (EnVision method by Dako/Agilen, Denmark/USA). Slides were stained using the manufacturers’ protocols.
We used Dako/Aligent monoclonal antibody for p53 (clone Do-7), which labels both mutant and wild-type p53 protein. Immunohistochemistry for p53 with strong nuclear positivity over >10% of myeloma cells was considered as overexpression.
Other immunohistochamical antigens were p21(Clone – DCS-60.2, dilution 1:50, Cell Marque), p53 (Clone-Do-7, Ready-to-Use, Dako), Cyclin D1 (Clone-SP4, Ready-to-Use, Dako).
The expression of p21, p53 and Cyclin D1 in the cytoplasm of myeloma cells was considered positive if more than 10% of the cells were stained with a strongly positive reaction, and negative if less than 10% of the cells were stained ().
Clinical-laboratory data
The patients’ clinical data included laboratory data and stage by Classification of MM according to the Salmon–Durie clinical staging system (without subclassification) and the International Staging System for MM, presence of osteolytic lesions. All slides were examined and photographed with a Zeiss microscope Lab.1a.
The normal, aka, reference values of the studied clinical-laboratory parameters are shown in brackets: β2-microglobulin (0–3 mg/L), albumin (34–52 g/L), haemoglobin (male: 126–175 g/L, female: 118–161 g/L), platelet count (150–410 × 109 L), GFR > 90 mL/min/1.73 m2, creatinine (male: 30–106 μmol/L, female: 30–80 μmol/L), calcium level (2.2–2.6 mmol/L), M-protein.
Statistical analysis
The results are expressed as mean values (M) with standard deviation (±SD) or percentage and range. Statistical analysis was performed using Microsoft Excel 2010 and Graph Pad Prism 5 software. Normality was assessed using the D’Agostino–Pearson omnibus normality test. Correlation between histological, clinical and laboratory parametric data were determined by Pearson’s or Spearman’s tests. Student’s unpaired t-test or Mann–Whitney U test was used to compare the results between groups. Differences were considered statistically significant at a level of p < 0.05.
Results
The distribution of the patients according to the Salmon–Durie clinical staging without subclassification system was: I – 20% (n = 24); II – 45% (n = 55), III – 35% (n = 43). The patients’ mean age was 65 ± 10.81 years (range 36–81). The male-to-female ratio was 1:1.3. The mean bone marrow cellularity in the whole group was 57.87% ± 20.64% (range 15–95%). The mean percentage of atypical plasma cells in BM biopsies was 52.14% ± 21.1% (range 15–90%).
MM with plasmablastic morphology
Percentages of myeloma cells with plasmablastic morphology in the bone marrow of our study population was from 0 to 74% (mean reference 9.87 ± 17.34%; 95% CI 6.76–12.98%). We found correlation between myeloma cases with high percentage of myeloma cells with plasmablastic morphology and increased β2-microglobulin level (r = +0.34, p = 0.0001), increased M-gradient level (r = +0.35; p < 0.0001) and advances stages by Salmon–Durie (r = +0.4; p < 0.0001). Weak statistically significant correlation was found between increased plasmablastic myeloma cells and elevated creatinine level (p = 0.015; r = +0.22) and M protein level in peripheral blood (p = 0.0078; r = +0.24).
Decreased levels of haemoglobin (p = 0.0006; r = −0.31), albumin (p = 0.001; r = −0.29) and GFR (p = 0.0007; r = −0.3) showed negative correlation with an increased amount of plasmablastic cells in the bone marrow (p < 0.05) ().
Table 1. Correlation of p21, p53 and Cyclin D1 expression with clinical and laboratory data of multiple myeloma patients.
CyclinD1 expression in myeloma cells
Cyclin D1 protein expression was detected in 62 (51%) cases. Positive correlation was observed between cyclin D1 expression and Salmon–Durie clinical stage, hypercalcemia, β2-microglobulin level and osteolytic lesion in bones (p < 0.05) (). The group of patients with positive Cyclin D1 expression in PCs had significantly higher β2-microglobulin (p = 0.034) and calcium (p = 0.0096) levels, compared to those in the Cyclin D1 negative group. The Cyclin D1 positive patients had a more advanced Salmon–Durie stage and had significantly more pronounced bone osteolytic lesions, as compared to these parameters in the Cyclin D1 negative patients (p < 0.05) ().
Table 2. Clinical parameters in patients’ groups with (positive) and without (negative) antigen expression.
p21 antigen expression in myeloma cells
There were 34 (28%) cases with p21 expression. Weak positive correlation was found between p21 expression and increased M-gradient level in peripheral blood (p = 0.022; r = + 0.21) and more advanced Salmon–Durie stages (p = 0.013; r = +0.22). Weak negative correlation was observed between p21 expression and lower albumin levels (p = 0.033; r = −0.19), see .
The p21 positive patient group had more advanced Salmon–Durie stage (p < 0.0135), lower albumin levels (g/L) (38.89 ± 13.62 in p21 positive patient group vs. 41.33 ± 22.18 (p = 0.0334) and higher M-gradient levels (g/L) (34.74 ± 19.86 vs. 25.62 ± 17.11 (p = 0.014), see .
p53 antigen expression in myeloma cells
p53 protein was detected in 44 (36%) cases. Significant positive correlation (p < 0.05) was observed between p53 protein expression in plasma cells and Salmon–Durie MM clinical stage, elevated β2-microglobulin and serum creatinine level. Furthermore, significant negative correlation was observed between p53 expression and decreased haemoglobin level, GFR and platelet (PLT) count. Patients with p53-positive myeloma cells had a more advanced Salmon–Durie MM stage (p < 0.0001), significantly higher β2-microglobulin (p = 0.029), serum creatinine (p = 0.0192) levels in comparison with these parameters in the p53-negative group ().
The p53-positive group had a significantly lower haemoglobin level (p < 0.0001) and PLT count (p = 0.0188) than in the p53-negative group. In addition, the p53-positive group had a lower GFR in comparison with that in the p53-negative patients (p = 0.0215) ().
Discussion
Our study is the one of the few morphology-based studies and included 122 patients. We found statistical correlation between more than 10 clinical and biochemical markers and the expression of p21, p53 and Cyclin D1 in myeloma cells of bone morrow. These data will be used as predictive indicators of MM progression.
The results of bone marrow histological and immunohistochemical examination are one of the main keys of in the diagnosis of MM. It provides such prognostic information as degree of bone marrow infiltration, grade of tumour cell atypia – plasmablastic morphology, expression of different kinds of antigens in cells.
The results of the present study are relevant to the Latvian population of MM patients. We have analyzed and compared world data of other studies on MM as well. Indeed, the exact role of plasmablastic differentiation of myeloma cells in the pathogenesis of MM is still unclear, but there are several studies that suggest correlation between plasmablastic differentiation and shorter survival and poor prognosis [Citation5, Citation18,Citation19]. One theory is that plasmablastic myeloma cells produce cytokines which stimulate the proliferation of myeloma cells and the progression of the disease. Greipp et al. [Citation3] found correlation between increased levels of Interleukin-6 and increased amount of plasmablasts. Srija et al. [Citation19] proved that the poor prognosis in cases of MM with plasmablastic differentiation was related with decreased renal functions. It is probably related with higher proliferative activity and pathological protein synthesis, which leads to the damage of renal parenchyma and is the reason of shorter survival. Myeloma cells produce monoclonal immunoglobin light chains (kappa, lambda). Immunoglobin light chains have a low molecular weight, and these proteins can be easily filtered through renal glomeruli and then reabsorbed in proximal tubules through endocytosis. The reabsorption limit of renal tubules proximal cells is exceeded because there is overproduction of immunoglobin light chains in cases of MM. The overproduction is more severe if there is high amount of CD138+ myeloma cells or plasmablastic cells in the bone marrow [Citation19]. This pathogenetic chain leads to tubulointerstitial nephropathy [Citation20]. In our study population we found plasmablastic differentiation in 9.87 ± 17.34% cases. Our data show that patients with higher plasmablastic myeloma cell counts had elevated β2-microglobulin and M-gradient levels, decreased haemoglobin and decreased GFR and advanced Salmon–Durie stage.
Experimental treatment based on immunohistochemical findings was recently introduced for refractory MM, for example, by stimulation of p53-related apoptosis [Citation11]. The current trend of individually tailored treatment implies that in the future, the immunophenotype of each MM patient will have to be taken into consideration, since genetic and immunohistochemical characteristics are very heterogeneous.
In the present study, we retrospectively analyzed Cyclin D1, p53, p21 as prognostic plasma cell antigenic biomarkers in 122 trephine bone marrow biopsies and evaluated their relation to the serological, clinical and laboratory parameters in a large cohort of primary MM patients.
Several laboratory markers, like β2-microglobulin, haemoglobin and albumin levels have been proposed as prognostic factors in MM. It is also known that the overall survival of MM patients is correlated with the creatinine level and GFR [Citation3, Citation21]. Since previous studies have demonstrated the prognostic significance of the immunohistochemically detected phenotype of tumour cells in MM, new data on relations between biochemical and histological prognostic markers could be of particular interest [Citation3, Citation11].
Cyclin D1 is another immunohistochemical prognostic marker associated with genetic abnormalities. Cyclin D1 is necessary for the coordination of the cell cycle G1/S transition, as cyclins act as regulators of cyclin dependent kinases and have a role in tumorogenesis. Some studies have shown it to have a positive prognostic value [Citation12, Citation14].
In our study we detected Cyclin D1 protein expression in 51% of cases, and it was associated with poor prognostic indicators, like high β2-microglobulin and elevated calcium levels. In our analyzed group of patients with Cyclin D1 overexpression there were more osteolytic lesions.
TP53 gene dysregulation in MM occurs through three different segments – deletion of chromosome 17p, monoallelic mutations and biallelic inactivation – each of them having a different prognostic role [Citation22]. p53 was detected in 36% of examined cases using the immunohistochemical method. p53 overexpression correlated with shorter overall survival. p53 plays a role in cell apoptosis. In our study, p53 expression in myeloma cells within a group of patients also correlated with many prognostic clinical and laboratory findings, such as a high β2-microglobulin level, late stage according to the Salmon–Duri classification, low haemoglobin level, platelet count and renal insufficiency indicated by a decreased GFR and elevated creatinine level. The results of our study show that the highest number of significant correlations was in the patients’ group with p53 expression. It is possible that each mechanism of TP53 gene aberration impacts different pathways in cells. That could explain such a wide range of clinical impact.
One of the functions of protein p21 is regulation of the cell cycle. DNA damage activates protein p21, which stops the cell cycle at G1/S phase thus blocking the proliferation of tumour cells. There are a very few studies about role of p21 in MM disease [Citation23]. In our study we found p21 expression in 28% of all cases. There were some correlations between p21 and higher levels of M-gradient, hypoalbuminemia and more advanced stage by Salmon–Durie.
We found that overexpression of Cyclin D1, p21 and p53 in myeloma plasma cells showed a statistically significant correlation with various clinical and laboratory characteristics of MM. Positivity for p21, p53 and Cyclin D1 antigen in myeloma cells are negative predictive factors for MM patients, associated with an advanced Salmon–Durie stage and higher β2-microglobulin levels. Chronic renal failure progression, which was also associated with poorer prognosis in MM patients, was related to expression of p53 in neoplastic plasma cells; in these patients there was an increased creatinine level in blood and decreased GFR. Many studies have evaluated Cyclin D1 as a factor of favourable prognosis and indicator of longer survival time in patients treated with standard and new generation medicine. In our study, Cyclin D1 overexpression was more typical for graver Salmon–Durie stages and higher β2-microglobulin level, thus showing progression of MM. It is possible that patients with Cyclin D1 antigen expression in myeloma cells may have had improved responsiveness to chemotherapy treatment, which may explain higher survival rate in previously published studies [Citation12, Citation14]. Statistical analysis of our study results showed that expression of Cyclin D1 did not correlate with the indicators of renal function and that these patients did not develop chronic renal failure, one of the most common complications of MM.
Thus, further studies in a larger cohort could explore p21, p53 and Cyclin D1 expression for making more precise morphological staging of MM and as a predictive factor for indicating a more aggressive therapy regimen.
Conclusions
Plasmablastic differentiation of MM cells correlates with the severity of MM disease. Expression of p53 and Cyclin D1 in myeloma cells is a negative predictive factor in cases of MM, as late Salmon–Durie stage and higher β2-microglobulin level are more common in these cases. p21 antigen expression in MM cells correlates with poor prognostic criteria such as increased M-gradient levels, hypoalbuminemia and advanced stage of the disease. Patients ‘group with p53 expression has the highest number of significant correlations of clinical parameters: levels of haemoglobin, β2-microglobulin, creatinine and GFR, PLT counts.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Jurijs Nazarovs, upon reasonable request.
References
- Castaneda O, Baz R. Multiple myeloma genomics – a concise review. Acta Med Acad. 2019;48(1):57–67.
- Pildava S. 2012. Doctus database (in Latvian) on the World Wide Web [last accessed 2020 Jun 10]. Retrieved from https://www.doctus.lv/2012/7/multipla-mieloma
- Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420.
- Subramanian R, Basu D, Dutta TK. Prognostic significance of bone marrow histology in multiple myeloma. Indian J Cancer. 2009;46(1):40–45.
- Møller HE, Preiss BS, Pedersen P, et al. Clinicopathological features of plasmablastic multiple myeloma: a population-based cohort. APMIS: acta pathologica. APMIS. 2015;123(8):652–658.
- Fulciniti M, Martinez-Lopez J, Senapedis W, et al. Functional role and therapeutic targeting of p21-activated kinase 4 in multiple myeloma. Blood. 2017;129(16):2233–2245.
- Ohata M, Nakamura S, Fujita H, et al. Prognostic implications of p21 (Waf1/Cip1) immunolocalization in multiple myeloma. Biomed Res. 2005;26(3):91–98.
- Jovanović KK, Escure G, Demonchy J, et al. Deregulation and targeting of TP53 pathway in multiple myeloma. Front Oncol. 2018;8:665.
- Soussi T. The history of p53. A perfect example of the drawbacks of scientific paradigms. EMBO Rep. 2010;11(11):822–826.
- Chen MH, Qi CX, Saha MN, et al. p53 nuclear expression correlates with hemizygous TP53 deletion and predicts an adverse outcome for patients with relapsed/refractory multiple myeloma treated with lenalidomide. Am J Clin Pathol. 2012;137(2):208–212.
- Lonial S, Durie B, Palumbo A, et al. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia. 2016;30(3):526–535.
- Padhi S, Varghese RG, Ramdas A. Cyclin D1 expression in multiple myeloma by immunohistochemistry: case series of 14 patients and literature review. Indian J Med Paediatr Oncol. 2013;34(4):283–291.
- Yan S, Liu H, Liu Z, et al. CCN1 stimulated the osteoblasts via PTEN/AKT/GSK3β/cyclinD1 signal pathway in myeloma bone disease. Cancer Med. 2020;9(2):737–744.
- Cook JR, Hsi ED, Worley S, et al. Immunohistochemical analysis identifies two cyclin D1+ subsets of plasma cell myeloma, each associated with favorable survival. Am J Clin Pathol. 2006;125(4):615–624.
- Athanasiou E, Kaloutsi V, Kotoula V, et al. Cyclin D1 overexpression in multiple myeloma. A morphologic, immunohistochemical, and in situ hybridization study of 71 paraffin-embedded bone marrow biopsy specimensAm J Clin Pathol. 2001;116(4):535–542.
- Dunphy CH, Nies MK, Gabriel DA. Correlation of plasma cell percentages by CD138 immunohistochemistry, cyclin D1 status, and CD56 expression with clinical parameters and overall survival in plasma cell myeloma. Appl Immunohistochem Mol Morphol. 2007;15(3):248–254.
- Anwer F, Gee KM, Iftikhar A, et al. Future of personalized therapy targeting aberrant signaling pathways in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19(7):397–405.
- Suarez-Londono JA, Rohatgi A, Antoine-Pepeljugoski C, et al. Aggressive presentation of plasmablastic myeloma. BMJ Case Rep. 2020;13(4):e234436.
- Srija M, Zachariah PP, Unni VN, et al. Plasmablastic myeloma presenting as rapidly progressive renal failure in a young adult. Indian J Nephrol. 2014;24(1):41–44.
- Lamb EJ, Levey AS, Stevens PE. The kidney disease improving global outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem. 2013;59(3):462–465.
- Durie BGM, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. ):
- Flynt E, Bisht K, Sridharan V, et al. Prognosis, biology, and targeting of TP53 dysregulation in multiple myeloma. Cells. 2020;9(2):287.
- Dhyani A, Machado-Neto JA, Favaro P, et al. ANKHD1 represses p21 (WAF1/CIP1) promoter and promotes multiple myeloma cell growth. Eur J Cancer. 2015;51(2):252–259.
