Abstract
Many species of genus Astragalus have been extensively studied for their wide range of biological activities, including antioxidant, cytoprotective, anti-inflammatory, antitumour, anti-infective, immune-boosting, antihypertensive and antidiabetic effects. This study presents an in vitro evaluation of the anticancer and immunomodulating properties of saponins obtained from aerial parts of Astragalus glycyphyllos L. In a series of standard MTT experiments we examined the antiproliferative effects of a total saponin mixture, the purified saponin subfraction obtained from it, and the isolated triterpenoid saponin (AGOS1), against different bladder carcinoma and cutaneous T-cell lymphoma tumour models. The in vitro immunomodulating activity of AGOS1 was further examined on murine isolated spleen lymphocytes and peritoneal macrophages. Results showed greatest overall cytotoxicity of the purified subfraction and prominent immune stimulating effects of the isolated saponin on both cell lineages.
Introduction
More than 70% of bladder carcinomas are non-invasive at the time of initial diagnosis, the majority of them being of urothelial type, known as transitional cell carcinomas (TCC) [Citation1,Citation2]. Treatment options depend on the type, stage and location of the tumour, as well as various factors on patient’s part. The intravesical BCG instillation is a standard immunotherapy for an early-stage superficial urothelial carcinoma, however, there is no gold standard local treatment in case of BCG failure [Citation3,Citation4]. Despite the aggressive surgical, radiation and chemotherapy treatment approaches, the tumour recurrence rate is as high as 70% even when properly treated and the increase in survival is quite modest [Citation5,Citation6].
Primary cutaneous T-cell lymphomas (CTCLs) are a group of extranodal, non-Hodgkin’s lymphomas that are characterized by infiltration of malignant monoclonal Th lymphocytes in the skin, which is clinically manifested as rashes, plaques and tumours [Citation7,Citation8]. The most aggressive form known as Sézary syndrome is closely related to mycosis fungoides and is associated with generalized erythroderma, the presence of specific malignant lymphocytes (Sézary cells) in the blood, lymphadenopathy and/or visceral organ involvement [Citation9,Citation10]. Novel targeted therapies have recently emerged, however cost and efficacy issues remain of concern [Citation11,Citation12]. Skin-directed therapies including topical corticosteroids, topical chemotherapy, bexarotene gel, phototherapy, or local radiation are still first-line treatments for patients with limited stage disease. Yet, despite aggressive systemic therapy, late-stage CTCL continues to be a life-threatening disease with high mortality and the current primary goal of treatment is to prevent progression and to control of the disease rather than cure [Citation12–14].
Many experimental and clinical studies report on the proapoptotic and anticancer properties of various saponin sources and preparations against tumour types of different tissue origin, including CTCLs and bladder carcinoma [Citation5,Citation15]. Their biocompatibility, lack of toxicity and immunogenicity as well as the easy availability make them particularly suitable for local application in these malignancies, whether as transdermal therapeutic systems or intravesical instillations. Furthermore, intravesical saponin pre-treatment is an effective approach to increase the anthracyclines absorption through the bladder mucosa and the local bioavailability of the drug [Citation16,Citation17]. Similarly, topical treatment of CTCL lesions with saponin formulations may hold benefit due to synergistic pharmacokinetic or pharmacodynamic interactions [Citation18].
Aside from their multimodal antitumour efficacy, there is mounting in vitro/in vivo experimental evidence on the immunomodulating properties of saponins. Moreover, both biological activities complement one another as various immune cells are strongly engaged in induction of antitumour immunity, tumour suppression and/or regression, and even play a vital role in altering patients’ response to treatment [Citation19].
The presence of cycloartane-type triterpene saponins has been reported in species of genus Astragalus (Fabaceae). These secondary metabolites have been shown to possess a wide range of bioactivities including cytoprotective, anti-inflammatory, antioxidant, antitumour, anti-infective, immune-boosting and antidiabetic effects [Citation20].
Astragalus glycyphyllos L. (sweet milk vetch) is a perennial herbaceous plant which is widespread in Bulgaria. The species is commonly used in folk medicine as: an antihypertensive, diuretic, anti-inflammatory agent, against cardiac insufficiency, renal inflammation, calculosis, to relieve increased blood pressure, tachycardia, etc. Phytochemical investigation of the plant revealed that it contains triterpenoid saponins and flavonoids [Citation21–24]. An in vivo pharmacological study of a defatted extract from its overground parts showed it exhibited antioxidant and hepatoprotective activity [Citation25]. A recent study also demonstrated that saponins’ mixture induced dose- and time-dependent in vitro/in vivo antiproliferative/antitumour effects on Graffi myeloid tumour [Citation26]. In in vitro experiments, triterpenoid saponin isolated from this mixture showed cytoprotective and neuroprotective properties and suppressed the activity of the human recombinant MAO-B enzyme [Citation24].
In the interest of potential therapeutic applications of saponin-based formulations for topical or intravesical instillation, and as a part of our ongoing research on Astragalus glycyphyllos, we aimed to: (1) evaluate the antiproliferative activity of both saponin fractions and the saponin isolated thereof, against in vitro tumour models of CTCL and bladder carcinoma; (2) investigate the immunomodulating properties of the saponin in murine spleen lymphocytes and peritoneal macrophages in an in vitro setting.
Materials and methods
Plant material and obtaining of saponin samples
A. glycyphyllos herbs were collected from Vitosha Mountain, Bulgaria, in June 2020. The species was identified by Dr. D. Pavlova from the Faculty of Biology, Sofia University, where a voucher specimen was deposited (SO-107613). The plant material (200 g) was dried, pulverised, sieved, and then defatted with dichloromethane. The defatted substance was extracted by percolation with 80% methanol (MeOH; 1400 mL). The resulting extract was separated by column chromatography (CC) on Diaion® HP-20 (Supelco, USA) (gradient H2O/MeOH) and 60 fractions (100 mL) were collected. After TLC analysis, they were combined and eight main fractions were obtained. One of them, named Dia80, contained the majority of the saponins. This fraction was further separated by CC over silica gel (gradient CHCl3/MeOH/H2O) to give 70 main fractions. Subfraction D-010 was found rich in the cycloartane saponin 17(R),20(R)-3β,6α,16β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-O-β-D-glucopyranoside, named AGOS1. This was confirmed by ultra-high-performance liquid chromatography (UHPLC) coupled with high resolution electrospray ionization mass spectrometry (UHPLC-HRESI/MS) [Citation27]. The saponin was isolated from subfraction D-010 and structurally elucidated as reported [Citation24]. Aliquots of Dia80, D-010 and AGOS1 were subjected to pharmacological evaluation.
Quantitative analysis of saponins
An UHPLC-HRESI/MS method was used to quantify the saponin content in the samples and to verify the identity of AGOS1 as reported before [Citation27].
Cell lines
The following cell lines were used in the experiments: T-24 (bladder carcinoma), CAL-29 (bladder transitional cell carcinoma); MJ (cutaneous T-cell lymphoma of the mycosis fungoides type); and HUT-78 (cutaneous T-cell lymphoma of the Sézary syndrome type). The cells were purchased from DSMZ GmbH (Braunschweig, Germany) and cultured under standard conditions: 90% RPMI-1640 culture medium enriched with 10% thermally inactivated foetal calf serum and 2 mmol/L glutamine at 37 °C in a Heraeus incubator, at 5% CO2 and maximum humidity. To maintain the cell lines in the logarithmic growth phase, the cultures were provided with fresh culture medium two or three times a week.
Animals
Healthy male mice (18–20 g, 6–8 weeks old, provided by the National Breeding Centre, Sofia, Bulgaria) were housed under standard conditions of temperature (20°C ± 5 °C), fed with standard pellet diet and free access to water as described in [Citation28].
Ethics statement
The experimental procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of Sofia (KENIMUS), Bulgaria.
Isolation of murine spleen lymphocytes and peritoneal macrophages
Isolation of murine spleen lymp-hocytes and peritoneal macrophages was performed as previously reported [Citation28]. The animals were sacrificed by cervical dislocation and the skin over the abdominal region was removed. Then all procedures were carried out under aseptic conditions. Peritoneal macrophages were harvested by peritoneal lavage using 10 mL sterile RPMI. The cell suspension was washed twice in RPMI-1640 medium and the cells were counted by a hemocytometer. Cells were seeded in 96-well microplates (3 × 104/well) with RPMI containing 10% FBS. Spleens were taken and placed in tissue culture dishes containing around 5 mL RPMI cell culture medium, and finely chopped with scissors. Tissue fragments were filtered and the cell suspension was layered on histopaque density gradient and centrifuged at 2000 g for 20 min at 4 °C. The layer containing lymphocytes at the interface was collected by a Pasteur pipette and washed twice with RPMI-1640 medium. The cell suspension was centrifuged for the second time at 2000× g for 10 min at 4 °C and resuspended in RPMI-1640 medium at 4 × 105/mL cells. The obtained cells were used to test the immunomodulating properties of AGOS1.
Cell viability assays
The cytotoxic and antiproliferative activity of the purified saponin fractions was studied using a validated methodology for assessing cell vitality known as the Mosmann MTT assay [Citation29]. The method is colourimetric and is based on the biotransformation of the yellow tetrazolium dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to violet formazan crystals via the mitochondrial succinate dehydrogenases in viable cells. Tumour cells were treated with serial dilutions of the saponin fractions in the concentration range 200–12.5 µg/mL, and the isolated murine lymphocytes and macrophages were exposed to 1, 10, 20, 60, 100, 200 µmol/L of the saponin AGOS1. After the exposure period of 72 h, cell survival fractions were quantified (in %) relative to the untreated control (set as 100% viable).
Statistical methods
The experimental data were processed using non-linear regression analysis (GraphPad Prism® software). The values were fitted to semi-logarithmic “dose–response” curves and the corresponding half-maximal inhibitory concentrations (IC50) of the screened compounds against malignant and normal immune cells were calculated. Differences were considered statistically significant at the p ≤ 0.05 level.
Results
After phytochemical investigation of a defatted methanol extract from the aerial parts of A. glycyphyllos, we obtained a total saponin mixture (Dia-80), a purified saponin fraction (D-010) and a pure cycloartane saponin (AGOS1). UHPLC-HRESI/MS analysis showed that Dia80 contained 8% total saponins and 4% of AGOS1, and D-010 had 50% of AGOS1. The purity of the isolated saponin AGOS1 was 98%. These quantified samples were used for pharmacological tests.
In vitro cytotoxicity
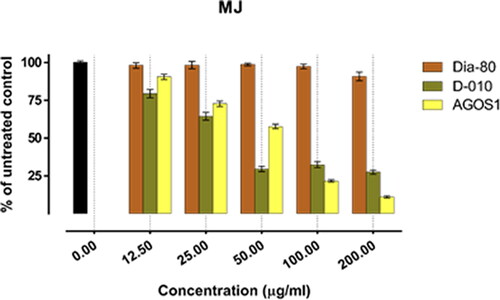
The samples were screened for their cytotoxicity in a panel of human tumour cell lines of different origin and characteristics using a standard MTT assay. The results are summarised in and and reveal some interesting trends.
Figure 1. Inhibition of cell viability by Dia-80, D-010 and AGOS1 in the bladder carcinoma derived CAL-29 cell line.
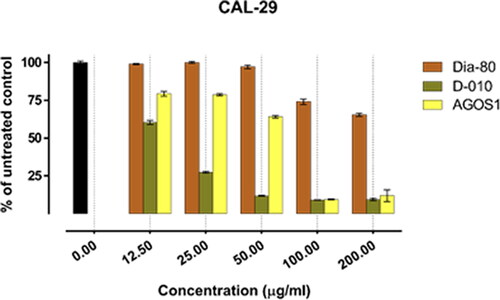
Figure 2. Inhibition of cell viability by Dia-80, D-010 and AGOS1 in the bladder carcinoma-derived T-24 cell line
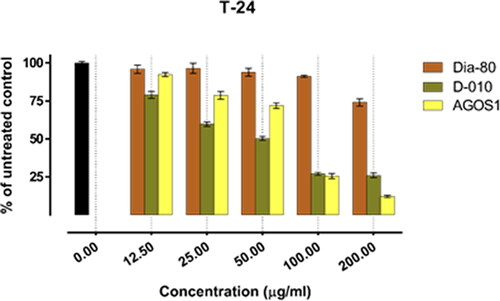
Figure 4. Inhibition of cell viability by Dia-80, D-010 and AGOS1 in the CTCL-derived HUT-78 cell line.
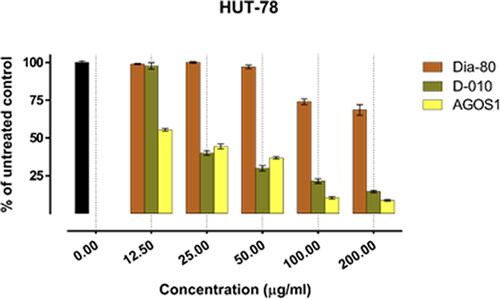
Table 1. In vitro cytotoxicity of the samples against tumour cell lines. IC50 ± SD, [µg/mL].
In all of the tested tumour cell lines, Dia-80 showed a marginal effect on cell viability even at the highest concentration applied. The strongest activity was observed in the bladder carcinoma cells CAL-29 (IC50 = 293.4 µg/mL), and a nearly two-fold drop in cell viability was seen in the least responsive MJ cells of CTCL (IC50 > 500 µg/mL).
On the other hand, D-010 and AGOS1 obtained thereof both inhibited tumour cell growth in a concentration-dependent manner. They exhibited far superior cytotoxic activity (about 10 times higher) than the total saponin mixture Dia80 and their estimated equi-effective concentrations were in a close range. However, except for the HUT-78 cell line, sustainably greater overall cytotoxic activity was determined for D-010 (which was found to predominantly contain AGOS1) as compared to the pure saponin itself. The lowest half-inhibitory concentration of D-010 was obtained in the CAL-29 cell line, where it was about three times more potent than the isolated saponin.
The lowest IC50 value for AGOS1 (18.4 µg/mL) was estimated in the tumourigenic (HUT-78) of the CTCL cell lines (MJ, HUT-78) that grow in suspension. In the solid tumour cells (CAL-29, T-24) and the non-tumourigenic MJ cell line, the pure saponin produced lower and closely comparable tumour-inhibiting effects (half-inhibitory concentrations of 51.8, 65.9 and 52.1 µg/mL, respectively).
Immunomodulating properties of AGOS1
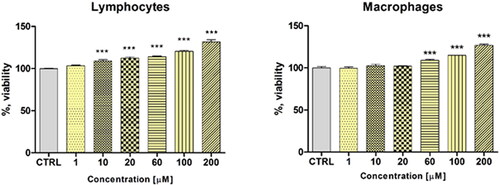
Proliferative responses of the murine spleen lymphocytes and peritoneal macrophages treated with different concentrations of AGOS1 (1, 10, 20, 60, 100, 200 µmol/L) are presented in . There was noticeable dose-dependent stimulation on both immune cell types in the concentration points beyond 10 µmol/L (corresponding to 5.78 µg/mL). It was slightly more prominent in the lymphocyte lineage.
Discussion
The findings of this study are in accordance with our previous reports on the in vitro bioactivities of various saponin sources as well as with existing literature data on this subject. Purified saponin mixtures, along with isolated saponins of different types and species have been shown to exert anticancer effects both in vivo and in vitro by inhibiting malignant cell proliferation, on the one hand, and boosting the immune system, on the other. A mixture of two saponins derived from Astragalus hamosus was found to effectively inhibit the growth of T-lymphoblastic cells (SKW-3) in a dose-dependent manner [Citation30]. The same mixture, however, was reported to be non-toxic against the normal MCF-10A mammary cell line, suggesting that its cytotoxicity is tumour-specific [Citation31]. This phenomenon has been reported by other research groups as well and has been taken a step further to attest the cytoprotective properties of saponins on normal immune cell populations. As demonstrated before, a purified saponin fraction of A. corniculatus had a protective effect against the myeloid Graffi tumour in hamsters by increasing the number, migration and phagocytic index of peritoneal macrophages and blood polymorphonuclear leukocytes. An increased mitogenic response to phytohaemagglutinin and lipopolysaccharides was observed, i.e. the saponins exhibited a marked immunostimulatory effect [Citation32–34]. In a similar fashion, in vivo treatment of Graffi tumour bearing hamsters with a purified saponin mixture derived from Astragalus glycyphyllos was able to reduce tumour growth, transplantability and animal lethality, and prolong their mean survival time. A pathohistological examination of the animals revealed accumulation of mononuclear cells in tumour stroma and a reduced mitotic index in malignant cells [Citation26]. A stimulatory activity on murine lymphocyte proliferation has also been reported for saponins isolated from Astragalus kahiricus and A. hamosus in the low nano- and micromolar concentration range [Citation35]. Cycloartane and oleanane-type saponins derived from other Astragalus species (A. brachypterus, A. cephalotes, A. microcephalus and A. trojanus) exhibited IL-2 stimulatory activity, with astragaloside VII being the most potent [Citation36]. In fact, the IL-2-inducing effect of some Astragalus plants has strongly been implicated in their immunomodulatory and antitumour activity [Citation37]. Well characterised, for example, is the cytotoxicity profile of the pleiotropic astragaloside IV. Many recent studies give evidence to its modulation of the PKC-αERK1/2-NF-κB, PI3K/Akt/NF-κB, Akt/GSK-3β/β-catenin, JNK/c-Jun/AP-1, p53-dependent and other key signalling pathways in various carcinoma cell lines [Citation38,Citation39]. Furthermore, it has also been shown to possess anti-inflammatory and immunomodulating properties in different experimental designs [Citation40–42] that are assumed to be of particular interest in the immunohistology of CTCL lesions [Citation43–45].
Within this framework, we undertook a study to evaluate the many-sided antitumour effects of saponins derived from the investigated Astragalus. As anticipated, the greatest overall cytotoxicity was determined for the purified D-010 subfraction, which is hardly surprising given the fact that synergism has been known to occur and mediate the biological activities of complex phytochemical mixtures [Citation46,Citation47]. On the contrary, the antiproliferative effect of the total saponin fraction Dia-80 was greatly diminished, possibly due to unfavourable interference and random antagonistic interactions between its many components. Some interesting trends were also seen in the dose-response relationships. For example, in the lower concentration range (12.5, 25, 50 µg/mL) D-010 appears to be more efficient in tumour growth control as compared to AGOS1. Adversely, the cytotoxicity of the latter is more pronounced in the smaller serial dilutions, beyond the concentration point of 50 µg/mL (equivalent to 86.5 µmol/L), i.e. no positive correlation was found between the cytostatic activity of D-010 and AGOS1, on the one hand, and the estimated AGOS1 content (50% in D-010), on the other. Otherwise, the calculated IC50 value for the screened mixture would have had to be twice as high, which was not the case. These considerations only add to the importance of mixture complexity resulting in mutually potentiating effects of the compounds.
A further viability assay was conducted to evaluate the in vitro effects of AGOS1 on the proliferation of isolated murine spleen lymphocytes and peritoneal macrophages. The functional activity of the immune cells can extensively vary, depending on the environment in which they reside and the local stimuli to which they are exposed to. Macrophages are phagocytosing leukocytes and an important part of the innate immune responses to various harmful invaders, including tumours, but can also play a “bridging” role to the acquired immunity due to their antigen-presenting properties. The lymphocyte lineage, on the other hand, is predominantly involved in the adaptive branch of the immune system. The proliferation boosting effect of AGOS1 on the murine isolated normal lymphocytes is particularly interesting since it appeared to have a opposite effect on the malignant lymphocyte populations of both CTCL types. Therefore, the cytotoxic activity of the saponin may be considered cancer-specific and possibly complemented by the immune system on an in vivo level. Further investigations in animal models may be more informative and able to verify these in vitro findings.
Conclusions
Persevering investigations on innovative treatment options with a favourable risk/benefit ratio are a must for curing cancer patients with chronic malignant diseases such as CTCL and TCC. To the best of our knowledge, there are few reports on the chemical and pharmacological profiling of Astragalus glycyphyllos. Newly identified saponin derivatives may be promising lead compounds for further studies. Our screening results ranked the purified fraction D-010 substantially higher in cytotoxicity as compared to the total saponin mixture Dia-80 and the isolated saponin AGOS1. The observed difference in bioactivity can be attributed to the complex phytochemical composition of the fractions and the variety of compounds that are known to act in a synergistic or regulatory manner. Boosting effects of AGOS1 on lymphocyte and macrophage proliferation is another of its tumour-fighting properties that is vital on an in vivo level.
Acknowledgments
The authors are grateful for the financial support from Ministry of Education and Science of the Republic of Bulgaria through contract No. DO1-217/30.11.2018 (BioActiveMed), agreement No. DO1-358/17.12.2020.
Disclosure statement
The authors have no competing interest.
Data availability statement
The experimental data supporting this study are freely available from the corresponding author (I.K.) upon reasonable request.
References
- Singh P, Kumar A, Das S, et al. Management of superficial bladder carcinoma: time to rethink the treatment strategies in the era of orthotopic neo-bladder. Saudi J Kidney Dis Transplant. [Internet]. 2010;21(5):881–885. Available from: https://www.sjkdt.org/article.asp?issn=1319-2442
- Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. JAMA. [Internet]. 2020; 324(19):1980–1991. Available from: https://doi.org/10.1001/jama.2020.17598
- Alhunaidi O, Zlotta AR. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience. [Internet]. 2019; 13:905 Available from: https://pubmed.ncbi.nlm.nih.gov/30915163
- Crabb SJ, Douglas J. The latest treatment options for bladder cancer. Br Med Bull. [Internet]. 2018; 128(1):85–95. Available from: https://doi.org/10.1093/bmb/ldy034
- Guo Y, Liu Z, Li K, et al. Paris polyphylla-derived saponins inhibit growth of bladder cancer cells by inducing mutant P53 degradation while up-regulating CDKN1A expression. Curr Urol. [Internet]. 2018;11(3):131–138. Available from: https://doi.org/10.1159/000447207
- Tanaka MF, Sonpavde G. Diagnosis and management of urothelial carcinoma of the bladder. Postgrad Med. [Internet]. 2011;123(3):43–55. Available from: https://doi.org/10.3810/pgm.2011.05.2283
- Larocca CA, LeBoeuf NR. Overview of cutaneous T-Cell lymphomas. Hematol Oncol Clin North Am. [Internet]. 2019; 133(4):669–686. Available from: https://doi.org/10.1016/j.hoc.2019.04.004
- Peng M, Xiao D, Bu Y, et al. Novel combination therapies for the treatment of bladder cancer. Front Oncol. [Internet]. 2020;10:539527Available from: https://doi.org/10.3389/fonc.2020.539527
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. [Internet]. 2017;37(3):527–546. Available from: https://doi.org/10.1016/j.cll.2017.06.006
- Oka T, Miyagaki T. Novel and future therapeutic drugs for advanced mycosis fungoides and sézary syndrome. Front Med (Lausanne)). [Internet]. 2019;6:116. Available from: https://doi.org/10.3389/fmed.2019.00116
- Geskin L, Malone DC. An exploratory cost-effectiveness analysis of systemic treatments for cutaneous T-cell lymphoma. J Dermatolog Treat. [Internet]. 2018;29(5):522–530. Available from: https://doi.org/10.1080/09546634.2017.1412064
- Guenova E, Hoetzenecker W, Rozati S, et al. Novel therapies for cutaneous T-cell lymphoma: what does the future hold? Expert Opin Investig Drugs. [Internet]. 2014;23(4):457–467. Available from: https://doi.org/10.1517/13543784.2014.876407
- Brouwer IJ, Out-Luiting JJ, Vermeer MH, et al. Cucurbitacin E and I target the JAK/STAT pathway and induce apoptosis in sézary cells. Biochem Biophys Reports [Internet]. 2020;24:100832. Available from: https://www.sciencedirect.com/science/article/pii/S2405580820301424
- PDQ Adult Treatment Editorial Board. Mycosis Fungoides (Including Sézary Syndrome) Treatment (PDQ®): Health Professional Version. PDQ Cancer Inf Summ [Internet]. 2002; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26389288.
- Kaufman DS. Challenges in the treatment of bladder cancer. Ann Oncol. [Internet]. 2006;17(5):v106–12. Available from: https://www.sciencedirect.com/science/article/pii/S0923753419382006
- Sasaki M, Hashimoto H, Yachiku S. Studies on enhancement of drug absorption through the bladder mucosa. Nihon Hinyokika Gakkai Zasshi. [Internet]. 1994;85(9):1353–1362. Available from: https://doi.org/10.5980/jpnjurol1989.85.1353
- Giannantoni A, Di Stasi SM, Chancellor MB, et al. New frontiers in intravesical therapies and drug delivery. Eur Urol. [Internet]. 2006;50(6):1183–1193. Available from: https://www.sciencedirect.com/science/article/pii/S0302283806009687
- Zhang C, Li B, Gaikwad AS, et al. Avicin D selectively induces apoptosis and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell lymphoma cells. J Invest Dermatol. [Internet]. 2008;128(11):2728–2735. Available from: https://www.sciencedirect.com/science/article/pii/S0022202X15336708
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. [Internet]. 2018;32(19-20):1267–1284. Available from: http://genesdev.cshlp.org/content/32/19-20/1267.abstract
- Ionkova I, Shkondrov A, Krasteva I, et al. Recent progress in phytochemistry, pharmacology and biotechnology of astragalus saponins. Phytochem Rev. 2014;13(2):343–374.
- Elenga PA, Nikolov S, Panova D. Triterpene glycosides and sterols from Astragalus glycyphyllos L. Pharmazie. 1986;41(4):41–42.
- Elenga PA, Nikolov S, Panova D. Triterpene glycosides from Astragalus glycyphyllos L. a new natural compound of the overgrowing parts of Astragalus glycyphyllos L. Pharmazie. 1987;42(6):422–423.
- Linnek J, Mitaine-Offer AC, Miyamoto T, et al. Two cycloartane-type glycosides from the roots of Astragalus glycyphyllos. Planta Med. 2008;74(09):PB141.
- Shkondrov A, Krasteva I, Bucar F, et al. A new tetracyclic saponin from Astragalus glycyphyllos L. and its neuroprotective and hMAO-B inhibiting activity. Nat Prod Res. [Internet]. 2020; Feb 1634(4):511–517. Available from: https://doi.org/10.1080/14786419.2018.1491040
- Shkondrov A, Simeonova R, Kondeva-Burdina M, et al. Study to evaluate the antioxidant activity of Astragalus glycyphyllos extract in carbon tetrachloride-induced oxidative stress in rats. EJMP. [Internet]. 2015;7(2):59–66. Available from: http://www.sciencedomain.org/abstract.php?iid=1018&id=13&aid=8249
- Georgieva A, Popov G, Shkondrov A, et al. Antiproliferative and antitumour activity of saponins from Astragalus glycyphyllos on myeloid Graffi tumour. J Ethnopharmacol. 2021;267:113519.
- Shkondrov A, Krasteva I, Ionkova I, et al. Production of saponins from in vitro cultures of Astragalus glycyphyllos and their antineoplastic activity. Biotechnol Biotechnol Equip [Internet]. 2019;33(1):1413–1418. Available from: https://doi.org/10.1080/13102818.2019.1671222
- Aluani D, Tzankova V, Kondeva-Burdina M, et al. Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int J Biol Macromol. [Internet]. 2017;103:771–782. Available from: https://www.sciencedirect.com/science/article/pii/S0141813017304798
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Krasteva I, Momekov G, Zdraveva P, et al. Antiproliferative effects of a flavonoid and saponins from Astragalus hamosus against human tumor cell lines. Pharmacogn Mag. 2008;4(16):269–272.
- Dineva I, Krasteva I, Berger M, et al. In vitro antineoplastic activity of some cytoreductive drugs versus new compounds of plant origin. Int J Curr Chem. 2010;1(4):281–290.
- Krasteva IN, Toshkova RA, Nikolov SD. Protective effect of Astragalus corniculatus saponins against myeloid graffi tumour in hamsters. Phytother Res. [Internet]. 2004;18(3):255–257. Available from: https://doi.org/10.1002/ptr.1277
- Toshkova RA, Krasteva IN, Wesselinova DW, et al. Influence of purified saponin mixture from Astragalus corniculatus bieb: on phagocytic cells in graffi-tumor bearing hamsters. J Ethnopharmacol. 2007;109(3):394–399.
- Toshkova RA, Krasteva IN, Nikolov SD. Immunorestoration and augmentation of mitogen lymphocyte response in graffi tumor bearing hamsters by purified saponin mixture from astragalus corniculatus. Phytomedicine. 2008;15(10):876–881.
- Verotta L, Guerrini M, El-Sebakhy NA, et al. Cycloartane and oleanane saponins from egyptian astragalus spp. as modulators of lymphocyte proliferation. Planta Med. 2002; Nov68(11):986–994.
- Nalbantsoy A, Nesil T, Erden S, et al. Adjuvant effects of Astragalus saponins macrophyllosaponin B and astragaloside VII. J Ethnopharmacol. [Internet]. 2011;134(3):897–903. Available from: https://www.sciencedirect.com/science/article/pii/S0378874111000699
- Yesilada E, Bedir E, Çalış İ, et al. Effects of triterpene saponins from astragalus species on in vitro cytokine release. J Ethnopharmacol. [Internet]. 2005;96(1-2):71–77. Available from: https://www.sciencedirect.com/science/article/pii/S0378874104004568
- Zhang J, Wu C, Gao L, et al. Chapter Four – Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. In: Du G, editor. Pharmacological Advances in Natural Product Drug Discovery [Internet]. Academic Press; 2020. p. 89–112. (Vol. 87; Advances in pharmacology). Available from: https://www.sciencedirect.com/science/article/pii/S105435891930064X.
- Cai T, Zhang C, Zhao Z, et al. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. Biomed Pharmacother. [Internet]. 2018;104:291–299. Available from: https://www.sciencedirect.com/science/article/pii/S0753332218304943
- Huang L, Yao Y, Li J, et al. The effect of astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia. [Internet]. 2012;83(8):1514–1522. Available from: https://www.sciencedirect.com/science/article/pii/S0367326X12002420
- Jin H, Wang L, Li B, et al. Astragaloside IV ameliorates airway inflammation in an established murine model of asthma by inhibiting the mtorc1 signaling pathway. Clement Y, editor. Evidence-Based Complement Altern Med [Internet]. 2017. 2017:4037086. Available from: https://doi.org/10.1155/2017/4037086
- Li J, Huang L, Wang S, et al. Astragaloside IV attenuates inflammatory reaction via activating immune function of regulatory T-cells inhibited by HMGB1 in mice. Pharm Biol. [Internet]. 2016;54(12):3217–3225. Available from: https://doi.org/10.1080/13880209.2016.1216133
- Vieyra-Garcia P, Crouch JD, O’Malley JT, et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight. [Internet]. 2019;4(1). Available from: https://doi.org/10.1172/jci.insight.124233
- Nasu K, Said J, Vonderheid E, et al. Immunopathology of cutaneous T-cell lymphomas. Am J Pathol. [Internet]. 1985;119(3):436–447. Available from: https://pubmed.ncbi.nlm.nih.gov/3893149
- Krejsgaard T, Odum N, Geisler C, et al. Regulatory T cells and immunodeficiency in mycosis fungoides and sézary syndrome. Leukemia [Internet]. 2012;26(3):424–432. Available from: https://doi.org/10.1038/leu.2011.237
- Guan Z, Chen L, Zhou Y, et al. The synergistic antitumour effect of multi-components from pulsatilla chinensis saponins in NCI-H460 lung cancer cell line through induction of apoptosis. Pharm Biol. ]. 2020;58(1):427–437. Available from: https://doi.org/10.1080/13880209.2020.1761404
- Herrmann F, Wink M. Synergistic interactions of saponins and monoterpenes in HeLa cells, Cos7 cells and in erythrocytes. Phytomedicine. ]. 2011;18(13):1191–1196. Available from: https://www.sciencedirect.com/science/article/pii/S0944711311003564