Abstract
WRKY transcription factors (TFs) are primarily involved in regulating the response of plants to different types of abiotic stress and related physiological processes. WRKY genes have yet to be characterized in garlic (Allium sativum L.), which is in the family Alliaceae. Here, 78 WRKY TFs were identified based on the garlic transcriptome and genome. They were divided into seven subfamilies based on Arabidopsis thaliana WRKY domains. Cis-element analysis revealed that the WRKY family members have ABRE and MBS cis-response elements that respond to salt stress, gibberellins, and drought stress. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis revealed that the expression of nine WRKY genes was induced under heat and salt stress.
Introduction
Plants experience various types of abiotic stress during growth and development, such as high and low temperatures, drought, and high salinity [Citation1]. Salt stress occurs when the concentration of salt ions, especially sodium and chloride ions, in the soil is high; such conditions inhibit the absorption of water and nutrients by plants in the soil and can affect normal growth and development [Citation2]. High temperatures can increase the content of reactive oxygen species in cells and reduce the activity of superoxide dismutase and peroxidase [Citation3], thus altering the permeability of plant cell membranes and negatively affecting plant growth [Citation4].
Many transcription factor (TF) families in plants are involved in stress responses [Citation5]. These TFs regulate the responses of plants to stress by specifically recognizing and binding to cis-acting elements in the promoters of downstream genes [Citation6]. TFs can also regulate the growth and development of plants by regulating RNA transcription and expression [Citation7]. WRKY TFs comprise one of the largest families of TFs in plants. WRKY TFs control various processes, including growth and development, physiological metabolism, and stress responses [Citation8]. WRKY proteins have a domain with 60 amino acids, and the transcriptional regulatory role of this domain is mediated by the highly conserved WRKYGQK motif at the N-terminus, which can specifically bind to the W-box [Citation9]. The W-box is the shortest sequence required for WRKY TFs to bind to DNA; it includes the highly conserved C/TTGACT/C sequence, and TGAC is a part of the core conserved region [Citation10]. The C-terminus of the WRKY TF family has a typical zinc finger structure and is typically composed of CX4-7CX22-23HXH/C [Citation11].
The first WRKY gene was isolated from sweet potato [Citation12]. WRKY family TFs have been identified and characterized in several plant species, including 74 WRKY genes in Arabidopsis (Arabidopsis thaliana) [Citation10], 136 in maize (Zea mays) [Citation13], 55 in cucumber (Cucumis sativus L.) [Citation14], 109 in rice (Oryza sativa L.) [Citation15], and 182 in soybean (Glycine max) [Citation16].
Garlic (Allium sativum L.) is one of the most economically important vegetables in the family Liliaceae. The garlic planting area globally is nearly 1.58 million hectares [Citation17]. Garlic has been used in traditional and modern medicines for its bioactive compounds [Citation18]. The release of the garlic genome has greatly aided the identification of WRKY gene family members [Citation19]. The aim of this study was to identify and characterize WRKY family members from the garlic genome. Conserved motifs were identified, and phylogenetic relationships among WRKY TFs were analysed. The expression patterns of some AsWRKY genes under abiotic stress treatment were determined. Generally, the results of this study will aid future studies examining the role of WRKY genes in the response of garlic to high temperature and salt stress.
Materials and methods
Plant materials, growth conditions, and stress treatments
The garlic cultivar ‘Xusuan No.6’ was used as the experimental material. This variety was obtained from the Institute of Horticulture, Xuzhou Institute of Agricultural Sciences in Jiangsu Xuhuai Area, Xuzhou, China. Garlic plants were grown in a mixture of organic soil and vermiculite in a greenhouse in the Xuzhou Institute of Agricultural Science. After 20 days, the garlic plants were placed in a growth chamber at 38 °C for high temperature treatment; plants were treated with 250 mmol·L−1 NaCl solution for salt stress treatment. Treatment with ddH2O was used as the control. The leaves of garlic plants were sampled at 2, 8, 24, and 48 h after treatment. The samples were frozen in liquid nitrogen and stored at −80 °C for RNA extraction.
RNA extraction and cDNA synthesis
Total RNA of garlic was extracted using a total RNA extraction Kit (Tiangen, Beijing, China). The extracted total RNA was reverse-transcribed into cDNA using a reverse transcription kit (Primer RT Reagent Kit, TaKaRa, Dalian, China). The cDNA was diluted 18-fold for subsequent RT-qPCR assays.
Sequence database searches
A total of 78 genes were obtained through comparative analysis of the annotated garlic genome sequences. After Conserved Domain Database analysis, all sequences were found to contain the conserved WRKY conserved domain. Sequences of Arabidopsis WRKY TFs were acquired from the Arabidopsis information resource (http://www.arabidopsis.org/). We obtained WRKY TF sequences of other species from the plant TF database (http://planttfdb.cbi.pku.edu.cn/index.php)
Motif recognition and phylogenetic analysis
Multiple alignments of WRKY protein sequences were performed by Clustal X 1.83 software [Citation20], and a phylogenetic tree was constructed with MEGA 7.0 [Citation21]. MEME (http://meme-suite.org/) was used to predict the conserved motifs of WRKY TFs [Citation22]. The functional interacting networks of proteins were integrated in STRING software with the confidence parameter set at 0.15 [Citation23].
Cis-element analysis of AsWRKY genes
The complete coding sequences and transcriptome sequences of the seven WRKY TFs obtained from garlic were blasted against the local NCBI Genome (garlic). The distances between the seven WRKY genes and upstream genes of garlic were analysed. For WRKY TFs longer than 2000 bp, a sequence of 2000 bp upstream of the translation initiation codon was obtained to predict cis-acting elements. PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to analyse the promoter region of 7 AsWRKY genes (As36680, As40049, As31693, As46742, As39900, As29182, and As21947).
RT-qPCR analysis
RT-qPCR assays were conducted for 9 AsWRKY TF genes (As20748, As29182, As31693, As36680, As38227, As39900, As40049, As46742, and As91121). Primer Premier 6.0 software was used to design primers for these AsWRKY genes. The sequences of all primers are shown in . Primers were synthesized by Nanjing Genscript Corporation (Nanjing, China). RT-qPCR was performed per the instructions of the SYBR Premix Ex Taq kit (TaKaRa). The 20-μL reaction system consisted of 10 μL of SYBR Premix Ex Taq, 7.2 μL of sterile deionized water, 0.4 μL of forward and reverse primers, and 2 μL of diluted cDNA. The reaction conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s for annealing and extension. Three independent biological replicates were performed, and the relative expression level was calculated using the 2−ΔΔCt method [Citation24].
Table 1. Gene primers for RT-qPCR.
Statistical analysis
The data used for expression profile analysis were the means of three biological replicates. Standard error of the mean was calculated based on the replicated data.
Results
Phylogenetic analysis of WRKY TFs in garlic
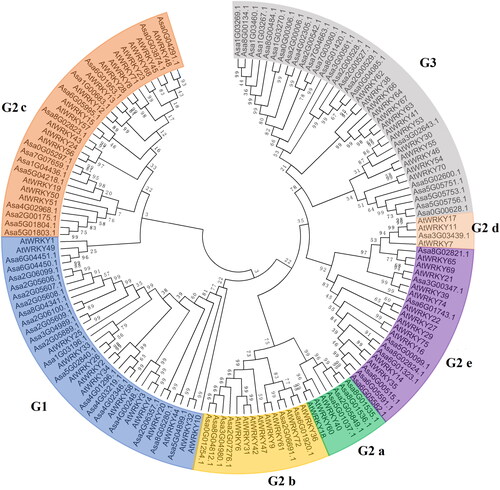
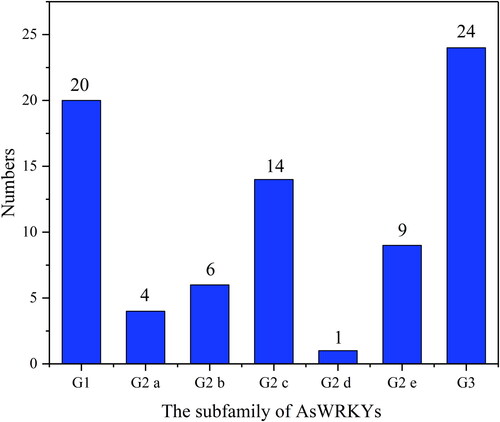
A phylogenetic tree was constructed using the amino acid sequences of 75 and 78 WRKY TF family proteins from A. thaliana and garlic, respectively. An unrooted phylogenetic tree was constructed based on sequence alignment of the WRKY domains of 153 WRKY TFs in garlic and A. thaliana. A total of 78 AsWRKY TFs were grouped into G1, G2 a, G2 b, G2 c, G2 d, G2 e, and G3 groups, which are shown in different colours in . The highest number of AsWRKY proteins (34) were in group G2, followed by group G3 (24) and group G1 (20) (). There were 20 AsWRKYs in group G1, which had two WRKY domains and a C2H2 zinc-finger motif. The number of AsWRKYs in each subgroup of group G2 was as follows: G2 a (4), G2 b (6), G2 c (14), G2 d (1), and G2 e (9). Group G2 possess 34 AsWRKYs, which can be further divided into five subgroups. Group G3 members had one domain and a C2HC zinc-finger motif, which contains 24 AsWRKYs.
Motif analysis of WRKY TFs
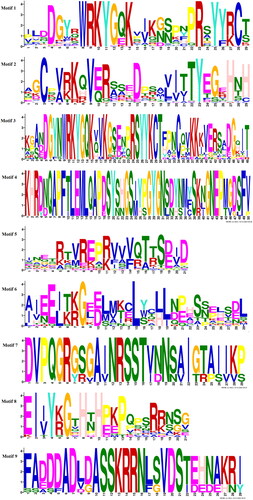
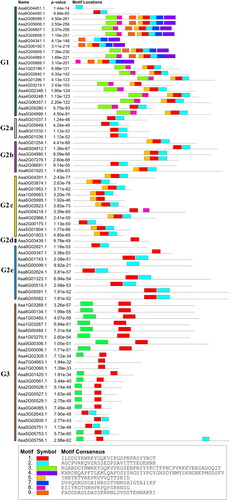
The distribution of AsWRKY protein motifs was determined using MEME software. The symbols for these motifs and the composition of each TF in the WRKY family are shown in and , respectively. Members of groups G1, G2 a, G2 b, G2 c, G2 d, G2 e, and G3 all contained motif 2. Group G1 contained the most types of conserved motifs, including motifs 1, 2, 3, 4, 5, 7, 8, and 9. There was only one TF, As3G03439.1, in subfamily G2 d. Several motifs were only detected in specific subfamilies. For example, motifs 3, 4, 7, and 8 were only detected in subfamily G1, whereas motif 6 was only detected in subfamily G3, indicating that these motifs were highly conserved in all AsWRKY proteins. The composition of motifs for most AsWRKYs members in the same subfamily was similar, suggesting that these proteins might have conserved functions.
Interaction networks of AsWRKYs and related proteins
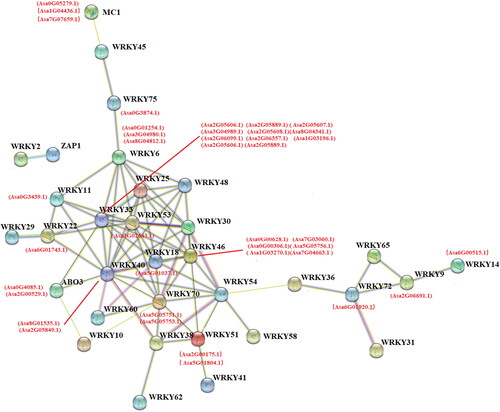
Interactions of AsWRKYs with other proteins were investigated using the Arabidopsis association model in STRING software. A total of 40 AsWRKYs proteins were found to be significantly associated.
The combined score between WRKY70 and WRKY40 protein was the highest (0.941), revealing a strong correlation between Asa5G05751.1 and Asa5G05751.2 and Asa8G01535.1 and Asa2G05849.1 (). Asa5G05751.1 and Asa5G05751.2 belong to group G3 TFs. Asa8G01535.1 and Asa2G05849.1 belong to group G2 a TFs. WRKY6 is a TF involved in the regulation of processes related to senescence and pathogen defense; it can regulate phosphate homeostasis and Pi transport by modulating the expression of PHO1 [Citation25]. A total of 40 AsWRKY TFs were involved in the interaction network, indicating that these TFs might interact. For example, eight factors in group G3 potentially interact with more than 10 proteins, which suggests that group G3 might be more important for transcriptional regulation.
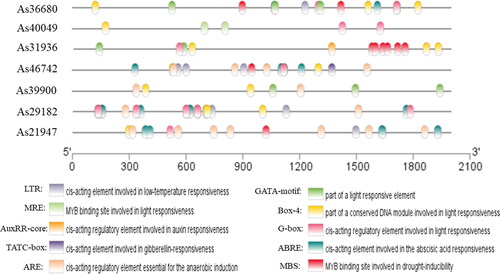
Cis-element analysis of AsWRKY genes
The expression of eukaryotic genes is regulated by many factors, and the promoter plays a particularly important role in transcriptional regulation [Citation26]. Promoter analysis through the PlantCARE database revealed several different types of cis-elements, and the 10 most common cis-elements are shown in . There were many conserved elements in the genes examined, included a cis-acting regulatory element essential for the anaerobic induction, a cis-acting element involved in low-temperature responsiveness (LTR), a cis-acting regulatory element involved in seed-specific regulation (ABRE), a cis-acting element involved in gibberellin-responsiveness (TATC-box), drought-inducibility cis-regulatory elements (MBS), a motif named CAAT-box associated with promoter and enhancer regions of common cis-acting elements, and four light-responsive elements (Box-4, G-box, GATA-motif, and MRE). Almost all WRKY TFs show a binding preference to the cognate cis-acting element [Citation27]. As36680, As31693, As46742, As29182, and As21947 all contained a cis-acting element related to abiotic stress, such as MBS, LTR, or ABRE. This indicates that AsWRKY TFs might be involved in the response of garlic to high and low temperature stress.
RT-qPCR analysis of AsWRKY genes under abiotic stress
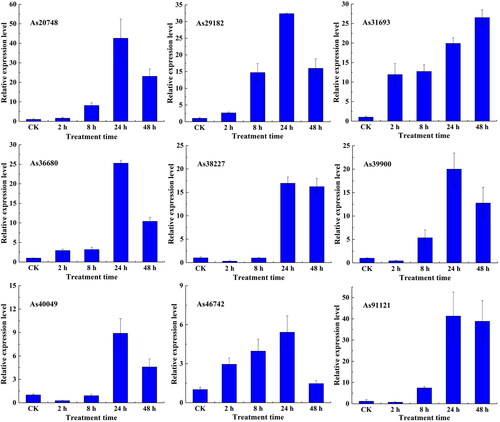
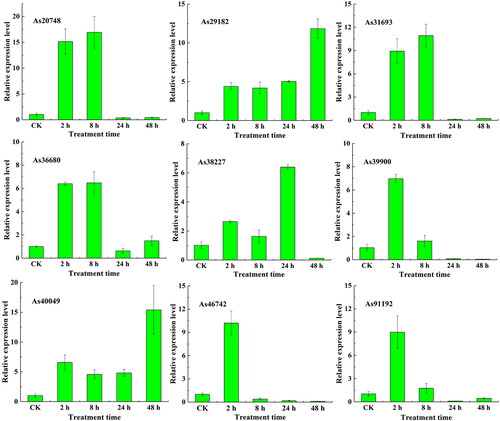
With global warming, heat temperature stress has become an increasingly important abiotic stress affecting plant growth and crop yields [Citation28]. The process by which plants respond to abiotic stress is regulated by various types of TFs [Citation29]. RT-qPCR expression analysis was performed on AsWRKY TF genes (As20748, As29182, As31693, As36680, As38227, As39900, As40049, As46742, and As91121) in response to high temperature and salt stress.
shows the different expression profiles of 9 AsWRKY genes after high temperature treatment. The expression levels of eight genes (As20748, As29182, As36680, As38227, As39900, As40049, As46742, and As91121) peaked after 24 h of treatment; the expression of these genes was 42.62-, 32.37-, 25.28-, 16.93-, 20.00-, 8.90-, 5.42-, and 41.30-fold higher compared with the control (CK), respectively, and began to decrease after 48 h of treatment. This indicates that some genes showed a consistent expression response to high temperature treatment. In addition, the expression levels of As20748, As29182, As31693, As36680, and As46742 increased compared with CK in each period under high temperature treatment, and the expression levels of As31693 increased during all stages. The expression levels of As38227, As39900, As40049, and As91121 decreased slightly compared with CK after 2 h of treatment but began to increase slightly after 8 h of treatment.
The expression profiles of 9 AsWRKY genes varied under salt treatment (). The expression levels of As20748, As31693, and As36680 increased in the early stage of salt treatment and peaked at 8 h of treatment; the expression of these genes were 19.08-, 16.94-, 10.09-, and 6.47-fold higher at 8 h of treatment compared with CK, respectively, and then decreased significantly after 24 and 48 h of treatment. The expression of As39900, As46742, and As91192 peaked at 2 h, was significantly higher at 2 h of treatment compared with CK, and decreased after 8 h of treatment. The expression levels of As29182 and As40049 were higher than CK within 2–8 h of treatment and peaked at 48 h of treatment.
Discussion
WRKY proteins are some of the most important TFs in plants for their roles in many biological processes [Citation30]. Previous studies have shown that WRKY TFs are involved in the regulation of various physiological processes, including biotic and abiotic stress responses, leaf senescence, epidermal hair development and plant seed development [Citation31]. The classification of WRKY proteins includes three groups depending on the number of WRKY domains and the characteristics of their zinc-finger-like motifs [Citation32]. The classification of WRKY TFs family in A. thaliana identifies three major groups (G1–G3). Within group G2, there are five subgroups, and almost all group G3 members respond to various types of biotic stress [Citation33]. Few studies of the WRKY proteins in A. sativum L. have been conducted. In this study, the 78 AsWRKYs TFs identified were divided into three groups based on the garlic genome and transcriptome, and group G2 was divided into five subgroups (group G2 a, G2 b, G2 c, G2 d, and G2 e) based on the amino acid sequences outside the WRKY domain. The number of proteins in subgroup G2 c was the highest among all subgroups. These results are consistent with studies of other species [Citation34, Citation35]. Several TFs and their interacting proteins play a role in plant growth processes [Citation36]. Study of proteins interacting with WRKY TFs can provide important insights into the regulation and mode of action of WRKY members [Citation37]. In this study, we constructed the interaction network between the garlic WRKY TFs and A. thaliana. There were a total of 40 garlic TFs involved in the interaction network, which suggests that the WRKY TFs of garlic may be involved in multiple biological processes.
WRKY TFs have been shown to play important roles in the response to different types of biotic and abiotic stress [Citation38]. Although several studies have examined the role of WRKY TFs in the response to different types of biotic stress, the function of WRKY proteins in the response to different types of abiotic stress is less well studied [Citation39]. To clarify the relationship between abiotic stress and garlic growth and development, the responses of AsWRKY under high temperature and salt stress were studied. RT-qPCR assays revealed that the expression of nine WRKY TFs was induced in response to these abiotic stresses. The relative expression level of the four AsWRKY genes was more often up-regulated than down-regulated under abiotic stress. This indicates that AsWRKY genes can respond to both high temperature and salt stress. The results of our study enhance our understanding of the potential physiological roles of individual WRKY genes during high temperature and salt stress and will aid future functional analyses of AsWRKY genes in garlic, and offer a useful resource for understanding the potential physiological role of individual WRKY genes during heat temperature and high salt stress. The key function of WRKI TFs in the response of plants to stress suggests that specific WRKI TFs could be overexpressed or regulated by means of gene engineering to create new varieties of crops or improve the quality of existing ones and/or their resistance to multiple stress factors.
Disclosure statement
No potential conflict of interest was reported by the authors..
Funding
The research was supported by the Basic Research Program General Project of Xuzhou (KC21032), the National Characteristic Vegetable Industry Technology System of China (CARS-24-A-07), and the Jiangsu Modern Agricultural Industry Technology System Construction Special Fund (JATS [2020]043).
References
- Bano C, Amist N, Singh N. Role of polyamines in plants abiotic stress tolerance: advances and future prospects. In: Plant life under changing environment. 2020. p. 481–496.
- Kosová K, Vítámvás P, Prášil IT. Proteomics of stress responses in wheat and barley: search for potential protein markers of stress tolerance. Front Plant Sci. 2014; 5(711):711– 714.
- Balfagón D, Zandalinas SI, Mittler R, et al. High temperatures modify plant responses to abiotic stress conditions. Physiol Plant. 2020;170(3):335–344.
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007; 59(2):206–216.
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51(1):21–37.
- Sakuma Y, Liu Q, Dubouzet JG, et al. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression . Biochem Biophys Res Commun. 2002;290(3):998–1009.
- Balazadeh S, Siddiqui H, Allu AD, et al. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010;62(2):250–264.
- Ding M, Chen J, Jiang Y, et al. Genome-wide investigation and transcriptome analysis of the WRKY gene family in Gossypium. Mol Genet Genomics. 2015;290(1):151–171.
- Rushton PJ, Somssich IE, Ringler P, et al. WRKY transcription factors. Trends Plant Sci. 2010;15(5):247–258.
- Ülker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7(5):491–498.
- Eulgem T, Rushton PJ, Robatzek S, et al. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5(5):199–206.
- Ishiguro S, Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5’ upstream regions of genes coding for sporamin and beta-amylase from sweet potato . Mol Gen Genet. 1994;244(6):563–571.
- Wei K-F, Chen J, Chen Y-F, et al. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012;19(2):153–164.
- Ling J, Jiang W, Zhang Y, et al. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics. 2011;12(1):471– 420.
- Ross CA, Liu Y, Shen QJ. The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol. 2007;49(6):827–842.
- Pasquini M, Bonfanti M, Martinazzo R. Quantum dynamical investigation of the isotope effect in H2 formation on graphite at cold collision energies . Phys Chem Chem Phys. 2016;18(9):6607–6617.
- Kong Q, Mostafa HHA, Yang W, et al. Comparative transcriptome profiling reveals that brassinosteroid-mediated lignification plays an important role in garlic adaption to salt stress. Plant Physiol Biochem. 2021;158:34–42.
- Martins N, Petropoulos S, Ferreira IC. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review . Food Chem. 2016;211:41–50.
- Sun X, Zhu S, Li N, et al. A Chromosome-Level genome assembly of garlic (Allium sativum) provides insights into genome evolution and allicin biosynthesis. Mol Plant. 2020;13(9):1328–1339.
- Thompson JD, Gibson TJ, Plewniak F, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739.
- Bailey TL, Nadya W, Chris M, et al. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34:W369–W373.
- Damian S, Andrea F, Stefan W, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43(D1):D447–D452.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108.
- Ye Q, Wang H, Su T, et al. The ubiquitin E3 ligase PRU1 regulates WRKY6 degradation to modulate phosphate homeostasis in response to low-Pi stress in Arabidopsis. Plant Cell. 2018;30(5):1062–1076.
- Sagar S, DeepikaBiswas DK, et al. Genome-wide identification, structure analysis and expression profiling of phospholipases D under hormone and abiotic stress treatment in chickpea (Cicer arietinum). Int J Biol Macromol. 2021;169:264–273.
- Ciolkowski I, Wanke D, Birkenbihl RP, et al. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol. 2008;68(1–2):81–92.
- Wu Z, Liang J, Zhang S, et al. A canonical DREB2-type transcription factor in lily is post-translationally regulated and mediates heat stress response. Front Plant Sci. 2018;9:243.
- Yamasaki K, Kigawa T, Inoue M, et al. Structures and evolutionary origins of plant-specific transcription factor DNA-binding domains. Plant Physiol Biochem. 2008;46(3):394–401.
- Ding W, Ouyang Q, Li Y, et al. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol. 2020;40(4):557–572.
- Wu Z-J, Li X-H, Liu Z-W, et al. Transcriptome-wide identification of Camellia sinensis WRKY transcription factors in response to temperature stress. Mol Genet Genomics. 2016;291(1):255–269.
- Lei L, Bingyan X, Xiaofeng D, et al. WRKY transcription factors and their roles in plant defense responses. Mol Plant Breed. 2005; 3(3):401–408.
- Yang J, Chen H, Yang C, et al. A WRKY transcription factor WRKY184 from Brassica napus L. is involved in flowering and secondary wall development in transgenic Arabidopsis thaliana. J Plant Growth Regul. 2020;92(2):427–440.
- Song H, Wang P, Hou L, et al. Global analysis of WRKY genes and their response to dehydration and salt stress in soybean. Front Plant Sci. 2016;7(9):9–15.
- Yue H, Wang M, Liu S, et al. Transcriptome-wide identification and expression profiles of the WRKY transcription factor family in Broomcorn millet (Panicum miliaceum L.). BMC Genomics. 2016;17(1):1–11.
- Wu XJ, Li MY, Que F, et al. Genome-wide analysis of PHD family transcription factors in carrot (Daucus carota L.) reveals evolution and response to abiotic stress. Acta Physiol Plant. 2016;38(3):1–15.
- Chi Y, Yang Y, Zhou Y, et al. Protein-protein interactions in the regulation of WRKY transcription factors. Mol Plant. 2013;6(2):287–300.
- Liu X, Song Y, Xing F, et al. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma. 2016;253(5):1265–1281.
- He X, Li JJ, Chen Y, et al. Genome-wide analysis of the WRKY gene family and its response to abiotic stress in buckwheat (Fagopyrum tataricum). Open Life Sci. 2019;14(1):80–96.