?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pertussis, commonly known as ‘whooping cough’, can affect all age groups and occurs at any time during the year. The aim was to estimate the current health and economic burden of pertussis-associated disease in Bulgaria. We also analyzed the cost-benefit and financial consequences of introducing (since 1st January 2020) an acellular pertussis (acP) booster dose at the age of 12 into the immunization schedule of Bulgaria. A combined pharmacoeconomic (cost-benefit and budget impact analysis) and a population-based screening cohort study was performed. It included 202 women in reproductive age (20 to 39 years). Fifty-one percent had only one child and 49% had two or more children. Over 70% of all the 202 women had an average of 3.75 IU/mL IgG-anti-PT (indication for waning pertussis protection) and 1.5% had >100 IU/mL IgG-anti-PT, which indicate a recent infection. Bulgaria has low pertussis morbidity, as the reported cases are limited, compared to the the average in the European Union and there have been no death cases in the last 10 years. However, significant pertussis infections remain unreported but still occur in the adult population. An acP booster dose, at the age of 12, was projected to give significant potential savings from the reduced number of physician and hospitalization visits, ambulatory care and indirect costs. The budget impact of a booster dose was expected to decrease from 4.45 million BGN to 3.9 million BGN. The acP booster dose would lead annually to around 4.2 million BGN and avoid the active cases with seven.
Keywords:
Introduction
Pertussis, commonly known as ‘whooping cough’, can affect all age groups and occurs at any time during the year. It is contagious, serious and even lethal, for infants who are either un- or incompletely vaccinated [Citation1,Citation2]. Its incidence rates vary from country to country [Citation3].
In Bulgaria, universal vaccination was introduced in 1957, with whole-cell pertussis (wcP) vaccine. As a result, the reported incidence of pertussis-related morbidity and mortality fell by a dramatic 115-fold. Since the year 2000, the annual incidence of pertussis in Bulgaria has varied from 0.49 to 4.34 per 100,000 population with the number of reported cases, per year, varying from 35 to 335. Children under 4 years old accounted for around 50% of these cases [Citation4]. When a pre-school acellular pertussis (acP) booster, at the age of 6, was introduced in 2008 followed by a complete transition from wcP to acP vaccines, in 2010, the annual reported incidence of pertussis, in Bulgaria, was generally low, compared to other European countries [Citation3]. The primary vaccinations are given at 2, 3, 4 months with a booster not earlier than 12 months after the 3rd dose (3 + 1; the complete primary immunization schedule is part of DTaP). At the age of 6, the pre-school acP booster is given and, since 1st January 2020, another acP booster (as part of Tdap) became mandatory at the age of 12 [Citation5]. DTaP is a vaccine that helps younger children to develop immunity to diphtheria, tetanus and whooping cough, while Tdap is a booster immunization given to adolescents and adults.
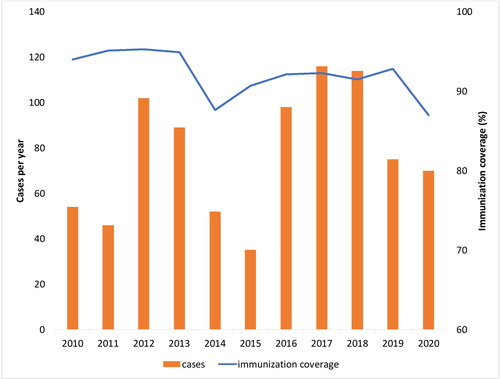
The immunization coverage (completed primary immunization, 3 + 1) in Bulgaria, has been gradually declining from 1990, when it was nearly 99% (only 26 pertussis cases were reported in 1990 with an incidence rate of 0.3/100 000). The immunization coverage reached the lowest rate in 2014 (86.7%). After that, it levelled at around 92% () [Citation6–8]. It should be noted that, in the last decade (2010–2019), many parents hesitated to vaccinate their children. Generally, the immunization coverage is over 92% in infants at 2, 3, and 4 months, dropping to 90% in 6-year-old, 84% for 12-year-old, and below 77% in adults [Citation9]. In the first six months of 2020, due to the COVID-19 lockdown, the mandatory immunizations were postponed, resulting in a dramatic drop in DTaP-3 coverage to 65.3%, but reached 91.2% at the end of the year [Citation9]. At the same time, 24 of all the 27 pertussis cases for 2020 were reported for the period of the first six months (January–June 2020) [Citation10].
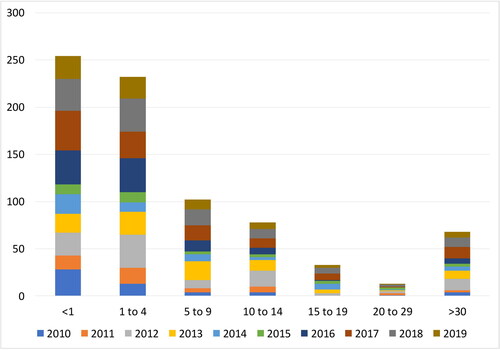
In 2010–2020, the pertussis incidence rates in Bulgaria varied from 0.5 to 1.6 cases per 100,000 and were expected to be stable at around 1.35 in 2021 [Citation4,Citation11]. It is believed that these data only partly represent the whole picture of what occurrs in society. The disease continues to circulate among the population, with many cases going unrecognized, mis- or under-diagnosed, and therefore not reported. This is especially true for relatively mild or atypical infections, occurring in adolescents and adults [Citation12]. Cosnes-Lambe et al. (2008) reported that only 5.5% of positive adults had typical whooping cough and 22.2% had no cough at all [Citation13]. However, these cases can have a significant detrimental effect on public health [Citation14]. In Bulgaria, nearly 10% of confirmed cases during the last 10 years were in people > 20 years old (), which could indicate waning immunity (the longer the duration since vaccination, the higher the attack rate) as well as recent improvements in diagnostic processes [Citation9]. Increased incidence of pertussis in adolescents and adults is an issue, since asymptomatic infections are also transmissible to the most vulnerable group of newborns, un- or partly vaccinated infants [Citation15]. Household members are a presumptive source of transmission to young infants (in over 50% of cases), who have the highest risk of pertussis-related complications (pneumonia, pneumothorax, seizures, encephalopathy), potentially leading to hospitalization and even death [Citation16,Citation17].
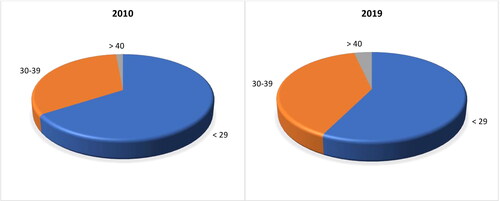
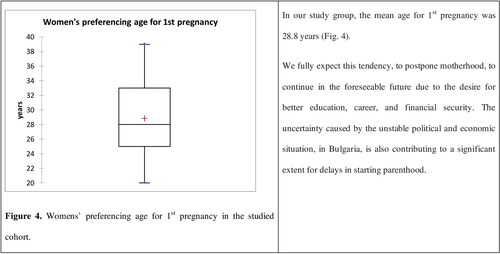
Women of childbearing age are a special concern, since the transplacental transfer of sufficient quantities of antibodies to the neonate is crucial for the temporal protection of a newborn baby until fully immunized. According to Bulgaria’s demographic characteristics, the number of women of childbearing age (15–49) is steadily declining [Citation18,Citation19]. Their number has decreased by 300,000 in 10 years. Consequently, the birth rate is also decreasing. During the observed period (2010–2019), the general fertility rate (GFR) declined from 42.9 in 2010 to 42.02 in 2019. Despite a positive trend for Total Fertility Rate (TFR), increasing from 1.49 (in 2010) to 1.58 (in 2019), it is not enough to compensate for the most substantial factor, i.e. the decline in the number of women of reproductive age in Bulgaria [Citation19]. There is also a growing trend of postponing motherhood. While in 2010, 67.7% of women under 29 years of age gave birth to babies, 33.5% at the age of 30–39, and only 1.5% were over 40 years old, this changed dramatically in the following 10 years, reaching 59.3%, 40.3% and 3.7% in 2019, respectively (). From 2010 to 2019, the majority of Bulgarian women who gave birth were in the age range of 20 to 39 with a steadily increasing average age from 26.8 in 2010 to 28.1 in 2019. Fifty-one percent of them had only one child, 32% had two children, and the remaining 17% had ≥ 3 children.
Because a great number of pertussis cases that occur in young infants are due to pertussis transmitted from household contacts, it was hypothesized that the administration of a dTap dose, to household members, could significantly reduce the risk of pertussis in the first months of life [Citation20]. Parental contact accounts for 55% of infant illness (with mothers being the most common source), followed by siblings for 16–20% and unknown sources for the remaining cases [Citation21–23]. Freitas et al. [Citation21] assumed that booster vaccination of 12-year-old would require 95% coverage to achieve a 20% transmission reduction among infants, while a 90% coverage will result in a lower than 10% transmission reduction. An adolescent booster, at the age of 12, will indirectly protect susceptible infants who surround them, notably their siblings. To provide indirect protection to vulnerable young infants, Tdap for adolescents has been recommended in several countries (France, Germany, Australia, Canada and the USA) for more than 15 years. However, there is only scant information available on its effectiveness to infant morbidity [Citation24–26]. It is evident that the achievement of high vaccination rates would result in a reduction in the incidence in infants [Citation21]. However, high immunization coverage is usually hard to achieve [Citation27].
The purpose of this study was to develop a dynamic transmission model calibrated to Bulgarian incidence data from 2010–2019, case-coverage effectiveness data of 202 women of childbearing age as well as data from other countries that have already implemented an adolescence acP booster about 10 years before Bulgaria.
This study aimed to estimate the current health and economic burden of pertussis-associated disease and to analyze the the economic aspects of introducing an acP vaccine booster dose at the age of 12, into the immunization schedule of Bulgaria.
We performed a systematic analysis of the available evidence for the effectiveness of the acP adolescent vaccine booster in countries that have already implemented it, concerning infant morbidity. We also performed a cost-benefit analysis to assess the profitability of an acP vaccine booster dose, from the Bulgarian societal perspective, and a budget impact analysis from the perspective of the Ministry of Health.
Model inputs were retrieved from local epidemiology and medical literature. A sensitivity analysis was performed to test the robustness of the results.
Methods
Ethics statement
All patients signed written informed consent forms at their admission authorizing the use of their anonymised (pseudonymised) data for scientific purposes. The data were collected at the Neonatology Clinic, Department of Pediatrics, Faculty of Medicine, Medical University of Sofia, Bulgaria regarding a PhD thesis (Approval No. РК36-3200/27.12.2016 and РК36-774/03.05.2017).
Patients
This study included only women (n = 202; mean age: 29.04 years) born before 2002. All participants were patients in delivery department of ‘St. Sofia’ Hospital and relatively healthy (without any acute illness). Blood samples were drawn between January 2018 and February 2019. According to the immunization schedule of Bulgaria, all subjects should have received wcP at 2, 3, 4 and 18 months and a booster at 4 years of age. All of them confirmed they had not received any pertussis vaccination after that. As it is estimated that the immunization response wanes by 50%, 4 years after the last injection, and is minimal after about 10 years [Citation28], we expected that all the studied women were likely unprotected against pertussis infection unless they had meanwhile regained protection via having contracted a natural infection.
Enzyme-Linked immunosorbent assay (ELISA)
Enzyme-Linked Immunosorbent Assay (ELISA), according to the manufacturer’s instructions (NovaTec Immundiagnostica GmbH, Germany), was used for the determination of IgG-anti-PT in a single sample serology. Diagnostic specificity was reported as 98% and sensitivity as 98%, according to the specification provided by the manufacturer. For quantitative assessment:
IgG-anti-PT levels were classified as negative and positive based on the cut-off values of the manufacturer’s kit.
IgG-anti-PT levels of ≥100 IU/mL were considered a highly specific criterion for recent or active B. pertussis infection (seropositive).
If a participant had not received a pertussis booster vaccine within 12 months prior to the study, values in the range of 40–100 IU/mL were considered to indicate recent contact with B. pertussis within the last year.
A level of 10–40 IU/mL was interpreted as no evidence of recent acute infection and <10 IU/mL indicated seronegativity.
Cost-benefit analysis (CBA)
CBA compares costs and benefits, both of which are quantified in common monetary units.
Costs
Cost-benefit analysis was performed from the societal perspective. Both direct costs (DC) (hospitalization, vaccination, ambulatory care, physician visits) and indirect (IC) costs (days off work for caregivers) were calculated.
Hospitalization costs and visits were taken from the National Contract Framework, 2020–2021 for medical care (clinical path 104). Ambulatory care included mainly antibiotic treatment with macrolides (azithromycin) with an average duration of 5 days. The average number of total days for convalescent period care was considered to be 21 days. To calculate the booster dose vaccination costs for each cohort of 12-year-old children, the number of children was multiplied by the price of the vaccination regimen in the given year. The number of children was taken from the databases of the National Statistical Institute of Bulgaria (NSI) in case of 95% coverage [Citation11].
The vaccination reference value per DDD was taken from the Positive Drug List, Annex 3, where two Diphtheria, Tetanus and Pertussis (acellular, component) vaccines were included as the calculations were presented for both vaccines. The number of pertussis cases for the period 2010–2019 was extracted from the website of the National Center of Infectious and Parasitic Diseases (NCIPD). The indirect costs were calculated using the human capital approach on the basis of average income per day, for the country, and the mean days off work of the parents/caregivers. The income per day for the period 2015–2019 was taken from the publicly available databases of the NSI. All unit costs for hospitalization, ambulatory care, vaccination and income were extrapolated using the TREND function for the next 10 years. An inflation index of 5% was applied.
Benefits
Two scenarios were developed: (1) with a booster dose and (2) without a booster dose. The number of averted pertussis cases was calculated taking into account that 95% coverage would achieve a 20% transmission reduction among infants [Citation21]. Benefits were calculated by considering the averted direct and indirect costs as a result of booster dose vaccination among the observed patient groups. Net benefit, benefit/costs ratio and return of investment were calculated and compared for both scenarios. Net benefits in the given year (t) were found by extracting the generated benefits (number of averted costs due to booster dose vaccination) from the total costs generated.
Costs and benefits were presented in Bulgarian currency (Bulgarian lev (BGN), 1 euro = 1.95583 BGN) inflated for the next 10-year period by using the following formula in order to have the real values for the observed time-period:
where Y is the parameter (cost or benefit) in the given year,
Yo is the known value in year 1st,r is the inflation index (considered to be 5%).
Budget impact analysis (BIA)
BIA estimates the financial consequences of adopting a new intervention.
Characteristics and size of the target population
The analysis was performed from the perspective of the payer institution (Ministry of Health (MoH)) considering only the direct costs paid by the MoH. The selected time horizon was 3 years. The population selection is the same as that described for the cost-benefit analysis. We assumed that the two available vaccines will have an equal market share.
Resource utilization and cost analysis
The healthcare resources were calculated for two scenarios: ‘World without booster dose’ and ‘World with booster dose vaccination’. Vaccination coverage of 89% was assumed, based on data for second-dose of measles-containing-vaccine (MCV2 at the age of 12) of 92.8% coverage before COVID-19 (2019) and 84.5% in 2020 pandemics [Citation29]. WHO estimates 87% coverage for MCV2 at the age of 12 [Citation30]. In the case of ‘World without vaccination’ the costs for treatment of the affected were zero, because hospitalization costs, visits and medicines use are paid by the National Health Insurance Fund (NHIF). The costs are presented in Bulgarian currency (Bulgarian lev (BGN); 1 euro = 1.95583 BGN).
Data analysis
Prevalence of seropositivity of anti-PT IgG and the estimated incidence rate of recent B. pertussis infection in all subjects and each age group were descriptively calculated. Data were analyzed using SPSS Software version 25 (SPSS Inc., Chicago, USA) and XLSTAT (Addinsoft Inc.). Differences were considered statistically significant at the p < 0.05 level. Percentage distributions of antibody titres in the ranges <10, 10–40, 40–100, and >100 IU/mL were also calculated for each age group. A one-way sensitivity analysis was performed to test the key variable number of vaccinated patients and vaccination price. Vaccination coverage was varied in the range of ±5%. The results were presented in a Tornado diagram. The chi-squared test and K independent samples test for qualitative variable, and the one-way analysis of variance (ANOVA) for quantitative variables were used for comparisons between subgroups. The serological change trends of anti-PT IgG antibodies by age are presented in scatter plots.
Results
IgG antibodies
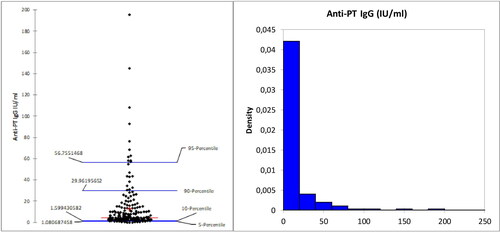
The studied subjects were divided into two groups according to their age (20 to 29 years old and 30 to 39 years old). Of the 202 women included, in this study, 116 were 20–29 years old, 86 were in the cohort of 30 to 39 year olds. The of antibody titres distributed in the ranges of <10, 10–40, 40–100 and >100 IU/mL were calculated for each age group (). In our study group, the mean age for 1st pregnancy was 28.8 years ().
Table 1. Distribution of antibody titres in the ranges of <10, 10–40, 40–100 and >100 IU/mL.
The mean concentration of IgG-anti-PT, in the 202 studied subjects, was 12.669 ± 23.044 IU/mL. In our study group, 144 women (71.3%) had an average of 3.75 IU/mL IgG-anti-PT. This indicates waning of pertussis protection in over 70% of women in the most prevalent childbearing age (20–39 years old) in Bulgaria (). In 2018, there were 820916 Bulgarian women in this age range. We estimate that more than half of them are unprotected against pertussis infection.
Another point of concern is the number of women with >100 IU/mL IgG-anti-PT, which is indicative of a recent infection. In the studied group of 20 to 39-year-old, this percentage was 1.5%, and an additional 6.4% had 40–100 IU/mL (indicative of suspected infection). We estimate that around 12,313 women (in 2018) could have had a recent infection and 50,579 had been in contact with a Bordetella strain in the last ≥2 years. The estimation yielded an infection incidence rate of 1500 per 100,000 population (for 20–39-year-old women only) for the year 2018 compared to an annual incidence of reported pertussis of 1.6 per 100,000 (whole population) for the same period. This is in agreement with Rendi-Wagner et al. [Citation31], who reported data about the seroepidemiology of B. pertussis in Israel. They estimated 2448 per 100,000 population (≥3 years of age) for the year 2000 compared to an annual incidence of reported pertussis of 5.6 per 100,000 for the year 2000. These data suggest that significant pertussis infections occur in the adult population, but have massively been unrecognized and unreported in Bulgaria. The consequence of this high natural infection rate could potentially result in more transmission to un- or partly immunized infants and children.
To prevent transmission from parents to their babies, an adolescent booster is one of many strategies that should be considered. This strategy was implemented in Bulgaria on January 1st, 2020. The effect is expected to impact starting in 2028 when current 12-year-old (as of 2020) will be in their 20’s and enter the most common childbearing age (20–39) for Bulgaria. The next thirty years (2020 until 2050) will be a transition period when two cohorts of women immunized with wcP and acP will be of childbearing age. On one hand, this will form an impediment to fully assess the effect of adolescence acP, and on the other hand, 30 years is too long a time. Therefore, we must seek the experience of other countries that have already introduced the adolescent booster, like France, Germany, Canada, the USA (some 15 years ago) and the Czech Republic, Greece, Ireland, Hungary, Italy and Slovakia (some 5 years ago) [Citation32]. By the beginning of 2021, only Croatia, Cyprus, Denmark, Latvia, Malta and Romania still did not recommend the pertussis booster for adolescents.
Cost-benefit analysis
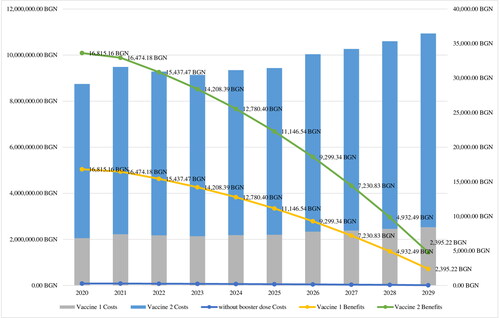
The costs for treatment and vaccination per patient are presented in . Considering a 20% reduction of cases, in the case of a booster dose, the number of affected subjects will decrease every year in the observed period 2020–2029 (). The number of avoided cases is expected to decrease in the case of booster vaccination by around 7 per year (). Vaccination costs per year vary in a wide range depending on the type of vaccine applied: between 2.2 million BGN and 7.4 million BGN for the observed period when considering the population of 12-year-old. Logically, the costs will decrease due to the tendency for reduction in the number of affected subjects, which is a consequence of the effective pertussis vaccine coverage program in the country.
Table 2. Healthcare resources per patient.
Table 3. Number of cases and hospitalizations for both scenarios.
Comparing both scenarios, the costs and benefits for adolescent booster were found to be higher than no vaccination. A booster dose of a more expensive vaccine leads to the same benefits with higher costs ( and ).
Table 4. Cost-benefit analysis.
Budget impact analysis
Total costs were not higher than 4.5 million BGN for every year of the analyzed period 2021–2023, there being a significant reduction (). The inclusion of acP booster dose would lead to around 4.2 million BGN vaccination costs per year ().
Table 5. A world with booster dose – Cost analysis.
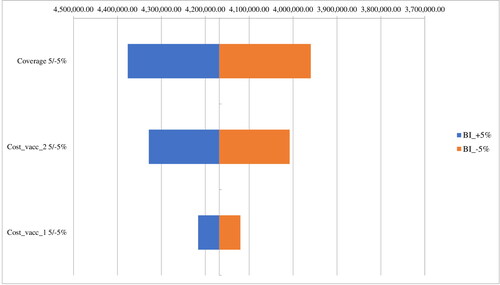
The tornado diagram illustrates that the change in coverage with 5 and −5% and the costs for a more expensive vaccine (Vaccine 2) with 5% would lead to the most significant change in the budget impact for the observed period ().
Discussion
Adolescents and adults are the main source of pertussis infection for infants, with mothers being the dominant cause [Citation23]. Maternal immunization is probably the best strategy to prevent the disease and has been recommended in an increasing number of countries, incl. Bulgaria [Citation33]. In the present study, we found a waning of pertussis protection in over 70% of the studied women in the most prevalent childbearing age (20–39 years old) in Bulgaria. Fifty-one percent of them had only one child; however, the remaining 49% have two or more children at the time of the study. Since siblings account for around 16–20% of B. pertussis transmission, this emphasizes the need for good protection of un- or not fully vaccinated infants who are at the highest risk of severe pertussis complications [Citation34]. Considering herd protection for adolescents and adults, vaccination would result in fewer cases among infants [Citation35]. To reduce the transmission to newborn babies, an adolescent booster was implemented in Bulgaria starting from January 1st, 2020. There were 60 965 children due for re-immunisation at age 12 in 2020, of whom 21 776 were re-immunised. The immunization coverage was 35.7%. The low immunisation coverage is related to late provision of vaccine - in the last quarter of 2020 as well as the COVID-19 pandemic. Those not covered in time will be re-immunised in 2021 [Citation9].
Bulgaria is one of the countries where an adolescence booster was introduced at a later stage, compared to the USA, Canada, Australia and most other European countries, so we can learn from their experience. Although a adolescence booster would be beneficial for the target groups, it will also have an influence on the circulation of B. pertussis and indirectly on herd immunity. One possible negative effect of the adolescent booster may be a change in the infection peak towards older childbearing age adults. This would be a result of the duration of immunity being shorter after vaccination than that as a result of natural infection [Citation36]. Such a trend has already been observed in many European countries, but not in others incl. Bulgaria, where children between 0 to 4 are still the most vulnerable group accounting for more than 50% of the cases.
The benefits of a strategy of including an additional adolescent booster, in the Bulgarian vaccination program, for children at school, seem to be supported by available data but its effect on pertussis in infants and other age groups is not expected to be marginal [Citation36]. As most pertussis cases are caused by parents, an adolescent booster (at 12 years old) would have a limited effect. It is very important to mention here, that those vaccinated with acP still could be colonized by B. pertussis and transmit the disease [Citation37]. It is more than obvious that we need a better vaccine that induces longer-lasting immunity [Citation38]. However, this is not a priority in the current COVID-19 pandemic so we have to rely on immunity induced by natural infection [Citation39].
The booster effect at the age of 12 will be especially positive in respect of coverage of minority population groups in Bulgaria, who tend to give birth between the ages of 15–19. A 90% vaccination rate is usually considered enough for creating good community-level protection [Citation40]. However, this is not the case when the remaining 10% of persons, without immunity, happen to be concentrated in certain deprived and marginalized communities. This appears to be the case with such groups in Bulgaria. Annually, in Bulgaria, from 5,000 to 10,000 children are not subjected to regular immunizations, for medical and non-medical reasons. As a result, the interplay between the accumulation of non-immune contingents, waning immunity, and boosting of pertussis immunity by natural infection/vaccination, influences the epidemiology of pertussis and its transmission dynamics in the population [Citation23]. The ease with which children are reached by the school healthcare system is an advantage, speaking in favour of a booster vaccination at 12 years of age. It should be borne in mind that in Bulgaria, some children from minorities leave school after finishing primary education at the age of 14. High circulation of B. pertussis among adults may support the idea of introducing adult booster vaccinations at 25 and every 10 years thereafter, adding to the Bulgarian Immunization Calendar. However, the merits of investing in a vast and expensive recurring vaccination program for adults, to boost their immunity, seem debatable. On the one hand, the immunization coverage for adults in Bulgaria is relatively low (< 40%), and high circulation rates of B. pertussis have been observed among the population. On the other hand, the milder course of the disease, in adults, leads to natural boosting of the immune system.
The cost-benefit analysis demonstrates significant benefits in the case of booster vaccination related to less active cases, fewer hospitalized patients, less indirect costs and therapy costs for complications, which is similar to those demonstrated in other studies [Citation35]. The calculated benefits decrease every other year because of the lower expected number of active cases [Citation9]. The latter is probably a result of the effective vaccination program in the country against pertussis. Some published economic evaluations overestimated the vaccine coverage as the tendency for Bulgaria is for a slight decrease. However, we considered a high coverage rate and found that the costs related to vaccination with 89% coverage were still higher than the benefits resulting in a negative net benefit and benefit/cost ratio lower than 1. A lower coverage rate would lead to a decrease in the difference between costs and benefits and converting the booster dose in more cost-beneficial than no vaccination. Most pertussis-related costs are due to lost productivity at work and social activities (i.e. indirect costs) of caregivers, which is in accordance with other published studies [Citation41]. Budget impact analysis performed from the perspective of the Ministry of Health results in a positive impact. It is a logical conclusion due to the fact that MoH budget does not cover hospital admissions costs, ambulatory care and productivity losses. No similar studies for the Bulgarian reality have been published so far, which restrains us from making final conclusions and recommendations for the cost-benefit of the acP booster dose vaccination.
Limitations of the study
This study has some limitations. First, this is the lack of verified vaccination status, since the method used to collect information was interviews with the participants. However, this is not a big concern, since participants received their last dose of wcP at the age of 4. Another limitation is that pertussis serology was measured by the determination of IgG-anti-PT and antibodies for filamentous haemagglutinin, pertactin and fimbriae were not measured, which attributed to the general difficulties of performing a correct diagnosis.
Another limitation of the cost-benefit analysis is related to the lack of reliable data for effectiveness. We took into consideration only the results of the Freitas et al. (2011) study for the achievement of a 20% transmission reduction among infants [Citation10]. So, further, a more comprehensive cost-benefit study is required. Moreover, our study focussed only on the 12-year-old booster without presenting subgroup analysis and answering what the cost-benefit ratio would be among adults. We have not considered the potential effects that a reduction in adolescents pertussis incidence would have among adults.
Key issues
The inclusion of acP booster dose would lead to around 4.2 million BGN vaccination costs per year and decrease the active pertussis cases with approximately 7 avoided cases per year.
To reduce the transmission to newborn babies, an adolescent booster was implemented in Bulgaria starting from January 1st, 2020.
An acP booster dose is expected to give significant potential savings from the reduced number of physician and hospitalization visits, ambulatory care, and indirect costs.
Conclusions
In summary, our work points to several important conclusions. First, significant pertussis infections have been unreported but still occur in the adult population. The consequence of this high natural infection could potentially result in more transmission to un- or partly immunized infants and children despite boosting the natural immunity in society. Second, there is a strong downward trend in the number of Bulgarian women of reproductive age. This trend is further overshadowed by the current tendency to postpone maternity and childbirth to an older age. Third, a significant waning of pertussis protection in women in the most prevalent childbearing age (20–39 years old) in Bulgaria was detected. Bulgaria is one of the countries with low pertussis morbidity, as the reported cases are limited, compared to the EU average and there have been no death cases in the last 10 years. Globally, although the problem has been worked on for decades, there is still no consensus on exactly which strategy is best suited to control pertussis outbreaks. Further national-based studies are required to demonstrate the real cost-benefit of acP booster among 12-year-old considering local effectiveness data.
Authors’ contributions
The authors provided equally valuable contribution to the manuscript. IM, MK and IN carried out the research and drafted the manuscript. IM and II entered the patients’ data in a database. IM, IN, II and MK performed the statistical analysis. All authors participated in the study design and reviewed the paper. All authors read and approved the final manuscript.
Disclosure statement
No one involved in the manuscript has any potential conflict of interests to declare.
Funding
This work was supported by the Bulgarian Ministry of Education and Science under the National Program for Research Young Scientists and Postdoctoral Students.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [MK], upon reasonable request.
References
- Winter K, Harriman K. Risk markers for pertussis among infants <4 months of age: Understanding the hispanic disparity. Pediatr Infect Dis J. 2018;37(2):126–131.
- Heininger U. Early prevention of pertussis is key. Lancet Infect Dis. 2019;19(7):689.
- Heininger U, André P, Chlibek R, et al. Comparative epidemiologic characteristics of pertussis in 10 Central and Eastern european countries, 2000–2013. PLoS One. 2016;11(6):e0155949.
- Raycheva R, Stoilova Y, Kevorkyan A, et al. Epidemiological prognosis of pertussis incidence in Bulgaria. Folia Med (Plovdiv). 2020;62(3):509–514.
- ECDC Bulgaria: Recommended vaccinations. Date of last update: 27 October 2021. Available from: https://vaccine-schedule.ecdc.europa.eu/.
- WHO Routine immunization profile Bulgaria, 2019. 2021. Available from: https://www.euro.who.int/.
- Anonnymous. Bulgaria. 2021. Available from: https://www.sabin.org/immunization-policy-bulgaria.
- Knoema: Bulgaria - immunization against DPT as a share of children ages 12–23 months, 2019. 2021. Available from: https://knoema.com/atlas/Bulgaria/topics/Health/Health-Service-Coverage/Immunization-against-DPT.
- National Center of Infectious and Parasitic Diseases (NCIPD). Reports on the number of infectious diseases by year. 2021. Available from: https://www.ncipd.org.
- Knoema: Bulgaria - Pertussis reported cases, 2020. 2021. Available from: https://knoema.com/atlas/Bulgaria/topics/Health/Communicable-Diseases/Pertussis-cases.
- NSI: National Statistics Institute database. 2020. Available from: https://www.nsi.bg/en.
- Dalby T, Harboe ZB, Krogfelt KA. Seroprevalence of pertussis among danish patients with cough of unknown etiology. Clin Vaccine Immunol. 2010;17(12):2016–2023.
- Cosnes-Lambe C, Raymond J, Chalumeau M, et al. Pertussis and respiratory syncytial virus infections. Eur J Pediatr. 2008;167(9):1017–1019.
- Kandeil W, Atanasov P, Avramioti D, et al. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–455.
- Wiley KE, Zuo Y, Macartney KK, et al. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine. 2013;31(4):618–625.
- Schellekens J, von König C-HW, Gardner P. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr Infect Dis J. 2005;24(5 Suppl):S19–S24.
- Vlkova E, Gospodinova M. Severe pertussis in an infant. SSM. 2020;51(2):38–40.
- Koytcheva E, Philipov D. Bulgaria: Ethnic differentials in rapidly declining fertility. DemRes. 2008;19:361–402.
- Traykov T, Tsvetkov K. Trends and specifics in the reproductive behaviour of the bulgarian population at the beginning of the 21-st century. J Bulg Geograph Soc. 2019;41:43–47.
- Forsyth K, Plotkin S, Tan T, et al. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135(6):e1475-82–e1482.
- Freitas AC, Okano V, Pereira JCR. Pertussis booster vaccine for adolescents and young adults in são paulo. Brazil. Rev Saúde Pública. 2011;45(6):1062–1071.
- Gilley M, Goldman RD. Protecting infants from pertussis. Can Fam Physician. 2014;60(2):138–140.
- Wendelboe A, Njamkepo E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–299.
- Quinn HE, McIntyre PB. The impact of adolescent pertussis immunization, 2004–2009: lessons from Australia. Bull World Health Organ. 2011;89(9):666–674.
- Skoff TH, Cohn AC, Clark TA, et al. Early impact of the US tdap vaccination program on pertussis trends. Arch Pediatr Adolesc Med. 2012;166(4):344–349.
- Halperin SA. Canadian experience with implementation of an acellular pertussis vaccine booster-dose program in adolescents: implications for the United States. Pediatr Infect Dis J. 2005;24:141–146.
- Liko J, Robison SG, Cieslak PR. Pertussis vaccine performance in an epidemic year-Oregon, 2012. Clin Infect Dis. 2014;59(2):261–263.
- Schwartz K, Kwong J, Deeks S, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ. 2016;188(16):E399–E406.
- WHO vaccine-preventable diseases: monitoring system. 2020 global summary (Bulgaria). 2020 (data as of October12, 2020). Available from: https://apps.who.int.
- GHO: The Global Health Observatory (Bulgaria). 2020. Available from: https://www.who.int.
- Rendi-Wagner P, Tobias J, Moerman L, et al. The seroepidemiology of Bordetella pertussis in Israel-Estimate of incidence of infection. Vaccine. 2010;28(19):3285–3290.
- Esposito S, Principi N, European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Vaccine Study Group (EVASG). Immunization against pertussis in adolescents and adults. Clin Microbiol Infect. 2016;22(Suppl 5):S89–S95.
- Di Mattia G, Nicolai A, Frassanito A, et al. Pertussis: new preventive strategies for an old disease. Paediatr Respir Rev. 2019;29:68–73.
- Baxter R, Bartlett J, Fireman B, et al. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139(5):e20164091.
- Fernandes EG, Rodrigues C, Sartori A, et al. Economic evaluation of adolescents and adults’ pertussis vaccination: a systematic review of current strategies. Hum Vaccin Immunother. 2019;15(1):14–27.
- Hallander HO, Nilsson L, Gustafsson L. Is adolescent pertussis vaccination preferable to natural booster infections? Expert Rev Clin Pharmacol. 2011;4(6):705–711.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111(2):787–792.
- Chit A, Zivaripiran H, Shin T, et al. Acellular pertussis vaccines effectiveness over time: a systematic review, Meta-analysis and modeling study. PLoS One. 2018;13(6):e0197970.
- Solans L, Locht C. The role of mucosal immunity in pertussis. Front Immunol. 2018;9:3068.
- Zahariev B, Georgieva L. ESPN thematic report: inequalities in access to healthcare. Bulgaria 2018. European Social Policy Network (ESPN). European Commission, 2018. Available from: https://ec.europa.eu.
- Purdy KW, Hay JW, Botteman MF, et al. Evaluation of strategies for use of acellular pertussis vaccine in adolescents and adults: a Cost-Benefit analysis. Clin Infect Dis. 2004;39(1):20–28.