Abstract
Asthma-COPD overlap (ACO) is a disorder that combines clinical signs of bronchial asthma and COPD – fixed airways obstruction and/or significant reversibility of the bronchial obstruction, rapid progression, poor quality of life, higher mortality, frequent and severe exacerbations compared to COPD and asthma alone. The aim of our study is to determine the neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-6 (IL-6) plasma levels and to analyze their association with peripheral eosinophilia and exacerbations of patients with asthma-COPD overlap (ACO), Chronic obstructive pulmonary disease (COPD) and asthma. Our results, based on 81 patients, indicate that the plasma levels of NGAL and IL-6 are significantly elevated in ACO and are associated with blood eosinophilia and exacerbations. NGAL and IL-6 may be useful biomarkers for differentiation of patients with frequent exacerbations and ACO.
Keywords:
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are the most common chronic respiratory diseases, affecting over 500 million people worldwide with related costs exceeding €56 billion per year in the European Union [Citation1]. In the classic cases of asthma and COPD, the differentiation of patients is relatively easy, but in real clinical practice there is an overlap between the individual characteristics of the two diseases (asthma-COPD overlap).
The exact prevalence of patients with asthma-COPD overlap (ACO) is difficult to assess because of the lack of a commonly accepted definition. Population-based studies have attempted to evaluate the prevalence of ACO worldwide, especially in the United States and Europe [Citation2–5]. There is a significant difference between the studies depending on the diagnostic criteria used. The prevalence of ACO varies greatly. According to Mendy et al., the prevalence of ACO was from 0.96 to 1.37% in the general population [Citation6], from 26.5% in asthma patients in a study of Mostafa et al. [Citation7], and 17.4% in COPD patients according to Miravitlles et al. [Citation8]. The global prevalence of ACO remains unknown, as there are no systematic reviews and meta-analyses of population-based studies yet [Citation7].
The ACO bears a combination of the clinical signs of both diseases, associated with fixed airways obstruction and/or a significant bronchodilator reversibility, frequent exacerbations, rapid progression, poor quality of life and higher mortality than in asthma or COPD separately [Citation9, Citation10]. The presence of ACO still remains controversial, and it is unclear if these are patients with both asthma and COPD or one of the two sharing some common features and clinical characteristics [Citation11].
We believe that our study of biomarkers associated with airway inflammation, in combination with clinical and lung functional features, would be useful in understanding the pathophysiology of ACO, and also in the definition and the personalization of ACO treatment.
NGAL (neutrophil gelatinase - associated lipocalin) or Lipocalin 2 is a 25-kDa protein of the acute phase of inflammatation secreted by neutrophils, epithelial cells of the airway and the intestinal mucosa, the endothelial and renal tubular cells [Citation12]. NGAL is believed to play a role in neutrophil inflammation and epithelial airway damage by inhibiting the inactivation of matrix metalloproteinase 9 (MMP9) secreted by neutrophils [Citation13, Citation14].
Interleukin 6 (IL-6) is a multifunctional cytokine produced by various cells. The molecular cloning of the cDNAs encoding B cell stimulatory factor 2 (BSF-2), interferon β2 and 26 kDa protein showed that all these molecules are identical. Furthermore, hybridoma/plasmacytoma growth factor (HPGF) and hepatocyte-stimulating factor (HSF) are also identical to this molecule and, therefore, this molecule has been called IL-6 [Citation15, Citation16]. The functionality of IL-6 is used with a wide variety of health-related diseases by addressing inflammation, pathogenesis of pulmonary diseases such as asthma [Citation17], COPD [Citation18] and idiopathic pulmonary fibrosis (IPF), including diabetes mellitus and systemic juvenile rheumatoid arthritis.
Aims
The aim of this study was to investigate the plasma levels of NGAL and IL-6, and to explore their association with the pulmonary function, the bronchodilator response and the peripheral eosinophilia in patients with asthma, COPD and ACO.
Subjects and methods
Ethics statement
All participants signed an informed consent forms for participation in the study.
Subjects
The study was conducted at the Department of Pulmonary Diseases, Medical University of Sofia, the General Hospital for Active Treatment of Pulmonary Diseases “St. Sofia”. We examined 81 patients with broncho-obstructive disease, in a stable condition, with no signs of exacerbation, with no history or clinical evidence of kidney disease: 26 patients with asthma, 25 patients with COPD and 30 patients with ACO.
All participants filled out a survey card which includes age, gender, family history, presence of COPD/asthma, duration of the disease, medication taken, other diseases and therapy, atopy and smoking, exacerbation history in the last 12 months. A full clinical examination was conducted. Prior to that, the asthma patients and COPD patients were diagnosed in ambulatory conditions and they met the GINA [Citation9] and GOLD criteria [Citation10]. Patients with ACO were defined by GINA criteria [Citation9] and Sin, et al. [Citation19].
Pulmonary function testing
A pulmonary function test (spirometry) was performed according to the international standards of ERS/ATS with a spirometer (Schiller, Germany). To determine the percentage of the predicted NHANES III values were used. A bronchodilator test with 400 μg Salbutamol was performed and the response was reported after 15 min. The obstruction was classified according to the severity of the airflow limitation by GOLD [Citation10]. The response to the bronchodilator was reported as an absolute value in milliliters (mL) and percentage (%).
Blood sampling and biochemical assays
Peripheral venous blood samples (3 m). were taken for complete blood count (CBC) together with determination of eosinophils number. Machine counting was performed using a Sysmex xn 1000 device and determining the number of eosinophils (Eo). The sample taking was done in standad conditions without food intake in the last 12 h.
To determine the levels of NGAL and IL-6, EDTA vacutainers of 5 mL were used. The plasma was separated using centrifugation for 10-15 min at 3500 rpm and it was stored at −80° C. NGAL levels were measured using a commercially available BioVendor kit, Czech Republic, by the enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions. IL-6 levels were measured using a commercially available Diaclone kit (France), by ELISA, according to the manufacturer’s instructions.
Data analysis
All statistical analysis were performed using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). Differences were considered statistically significant the p < 0.05 level. Because normal distribution was not observed for the NGAL results, we applied a decryption analysis, and the Kruskal-Wallis test. The results are presented as a median and an interquartile range (IQR) in brackets. The biomarkers are additionally presented with a minimum and maximum value.
Results
The clinical characteristics of the studied population are presented in .
Table 1. Clinical characteristics of patients with ACO, asthma and COPD.
The median age of the studied population was 59 years (IQR of 21.35), of which 37 (47%) men and 43 (53%) women. The patients with asthma were significantly younger − 42.50 years of age [Citation20], compared to ACO − 63.50 years (16.25) and COPD patients − 67 years [Citation21] (p < 0.05). There was no significant difference in the mean age between ACO and COPD (p > 0.05).
The patients with ACO (n = 20; 67%) and asthma (n = 18; 70%) were predominantly female, while the group of COPD was dominated by male patients (n = 20; 80%).
The number of smokers and ex-smokers was significantly higher in the ACO (n = 24; 80%) and COPD (n = 22; 84%) groups compared to the asthma group (n = 14; 53%) (p < 0.05).
The atopic patients were observed mainly in the asthma group (n = 21; 82%) and ACO group (n = 19; 65%) compared to COPD (n = 7; 27%) (p < 0.05; for comparison).
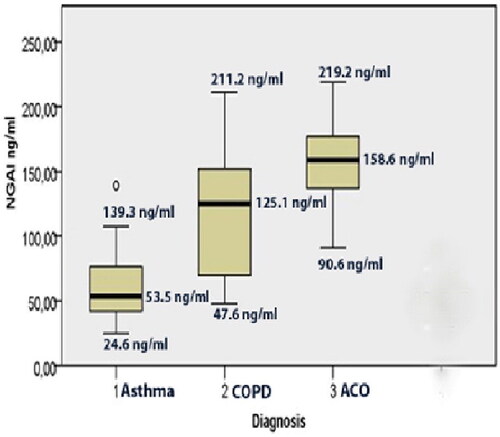
The average exacerbation rate in the previous 12 months was significantly higher (p < 0.05) in ACO (3 (3.75]), compared to COPD (2 (2.50]) and asthma (1 (3.5]). NGAL levels were significantly higher in ACO (158.6 (41.54]) ng/mL compared to COPD (125.1 (85.50] ng/mL) and asthma (53.5 (35.03]) ng/mL (χ2= 46.149; p < 0.05) ().
Figure 1. Plasma levels of NGAL (ng/mL) expressed as a median, in patients with 1. Asthma (53.5 ng/mL; 24.6-139.3); 2. COPD (125.1 ng/mL, 47.6-211.2), 3. ACO (asthma-COPD overlap) 158.6 ng/mL; 90.6-219.2).
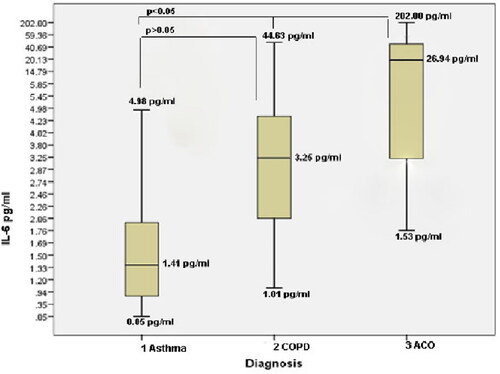
IL-6 levels were significantly higher in ACO group (26.94 (17.66]) pg/mL compared to COPD(3.25 (2.17] pg/mL) and asthma group (1.41 (1.31]) pg/mL ((х2 = 35.054; p < 0.05) ().
Figure 2. Plasma levels of IL-6 (pg/mL) expressed as a median in patients with 1. Asthma (1.41 pg/mL; 0.05-4.98); 2. COPD (3.25 pg/mL, 1.01-44.63), 3. ACO (asthma-COPD overlap) 26.94 pg/mL; 1.53-26.94).
There was no significant difference in the Eo count in ACO (296.1 (325] cell/μL) when compared to asthma (471.8 (322.25] cells/μL) and COPD (283.2 (200] cells/μL) (p > 0.05, respectively), but there was a significant difference in Eo between asthma and COPD (p < 0.05).
There was an increased number of Eo according to the Sin et al. [Citation19] criteria (Eo ≥ 300 cells/μL) in 26 patients with ACO, 20 patients with COPD and 5 patients with asthma.
In the ACO group, we observed association between peripheral eosinophilia and the elevated serum levels of NGAL (χ2 = 16,633; p < 0 .05) and IL-6 (χ2 = 21.853; p < 0.05). Neither increased NGAL, nor IL-6 levels, were associated with the airway limitation in patients with asthma, COPD and ACO (preFEV1/FVC) (χ2 = 0.72, p > 0.786), but the two markers are in association with a positive bronchodilator response in ACO (postFEV1/FVC < 70% and ΔFEV1 ≥ 15% and ≥400 mL or ≥12% and ≥200 mL) (χ2 = 3.999; p < 0.05).
The increased levels of NGAL and IL-6 in ACO corresponded to the increased number of exacerbations for the past 12 months (3 (3.75]) (p < 0.05).We did not find such association in COPD (2 (2.50]) or asthma (1 (3.5]) (p < 0.05, respectively).
The pulmonary function is shown in , including the bronchodilator response, the peripheral eosinophilia and the serum levels of NGAL and IL-6 in patients with asthma, COPD and ACO.
Table 2. Characteristics of patients with ACO, asthma and COPD and their association with NGAL, IL-6, peripheral eosinophilia, positive bronchodilator responses and exacerbations over 12 months.
Discussion
In this research, we aimed to evaluate the plasma levels of NGAL and IL-6, and to correlate their levels with the lung function, bronchodilator response and Eo in patients with asthma, COPD and ACO. Such a study was performed for the first time in Bulgaria.
Our results showed that thepatients with signs of ACO had significantly increased plasma levels of NGAL (158.6 ng/mL) compared to COPD (125.1 ng/mL) and asthma (53.5 ng/mL) (p < 0.05) as well as increased plasma levels of IL-6 (ACO (26.94 pg/mL) compared to COPD (3.25 pg/mL) and asthma (1.41 pg/mL) (p < 0.05).
Although we did not find correlation between NGAL and IL-6 with airflow restriction, elevated levels of NGAL and IL-6 were associated with a positive bronchodilator response (p < 0.05) and corresponded with peripheral eosinophilia in patients with ACO, compared to asthma and COPD.
NGAL is a member of the lipocalin family and is produced and released by neutrophils [Citation22]. The plasma level of NGAL is associated with tobacco smoke, respiratory tract inflammation and pulmonary injury [Citation23]. As a congenital immunostimulatory antibacterial factor released by neutrophils, it is also produced by tubular cells in the kidney and epithelial cells in the intestinal and respiratory tract [Citation20, Citation24]. Urinary NGAL is considered a sensitive marker of acute kidney injury because it can show tubular cell damage [Citation20, Citation25, Citation26,]. In Bulgaria, NGAL is used as a quantitative biomarker for early diagnosis of acute renal impairment [Citation27].
The first study elevating NGAL levels in COPD patients was from Keatings and Barnes [Citation21]. Studies have also shown BAL and plasma NGAL levels to be elevated in patients with COPD [Citation22, Citation23, ] and pulmonary emphysema in smokers [Citation28].
NGAL has two functions of potential importance in the pathogenesis of COPD: inhibition of bacterial growth and enhancement of matrix degradation [Citation29]. In addition, other authors have shown that expression of NGAL in the lung can be induced by reactive oxygen species [Citation30]. As the pathophysiology of both asthma and COPD is related to oxidative stress caused by environmental exposure and inflammation of the airways, ACO may increase oxidative stress, which can lead to airway hyperreactivity, injury and remodeling and may also be related to elevated NGAL levels [Citation26].
Interleukin 6 (IL-6) is a multifunctional 26 kD protein that is directly involved in the immune responses after infection and cellular damage. It is mainly produced in acute and chronic inflammation. IL-6 is secreted in the serum and induces an inflammatory response via the interleukin 6 receptor alpha. Studies have shown increased plasma levels of IL-6 in patients with ACO compared to healthy controls and asthma [Citation31] and increased levels in sputum in patients with ACO compared with asthma and COPD [Citation32]. We did not find any literature data concerning the association between elevated serum IL-6 levels and those of NGAL in patients with asthma, COPD and ACO.
Our results demonstrated that the patients with ACO had higher plasma levels of NGAL and IL-6 than the patients with asthma and COPD alone, which supports our hypothesis that these may be reliable inflammatory biomarkers in the diagnosis of ACO. The findings of other researchers [Citation33] are similar and they recommend the evaluation of plasma NGAL levels for differentiation of ACO from asthma alone, although they find the highest levels for COPD. IL-6 is suggested as a marker for differentiation of patients with ACO from asthma [Citation31]. According to other authors, NGAL levels are higher in women with ACO than in other individuals without ACO.
This result may be remarkable due to the lack of biomarkers other than the Eo blood count and immunoglobulin E (IgE) commonly used in the diagnosis of ACO. Identifying patients with ACO can be important for several reasons. They have more severe symptoms and impaired pulmonary function [Citation4, Citation34]. They have more frequent exacerbations than COPD [Citation35, Citation36] and poor quality of life [Citation37]. Therefore, patients with ACO need more financial resources for health care at a higher cost than patients with COPD [Citation38].
We found no association between airflow limitation and elevated NGAL and IL-6 levels in patients with ACO. According to Juan et al. [Citation31], there is a correlation between elevated serum IL-6 levels and airflow limitation in patients with ACO compared with asthma. We found a connection between high levels of NGAL and IL-6, and a positive bronchodilator response. ACO is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. As asthma and COPD are heterogeneous diseases with a scope of the underlying mechanisms, respectively, ACO is not considered as a single separate disease. Previous studies have shown that asthma, COPD and ACO differ in their biomarker profiles [Citation15, Citation26, Citation32, Citation34]. We hypothesize that biomarker measurements are an important aspect of ACO recognition, which in combination with clinical features and spirometry, will promise to help identify patients with ACO [Citation33]. In general, patients with ACO have more severe clinical symptoms and a relatively worse prognosis compared to COPD. Therefore, it is important to distinguish this subgroup of patients early and to provide appropriate treatment. Several diagnostic criteria have been proposed to improve the differential diagnosis in patients with ACO from non-ACO as well as some useful biomarkers, including Eo counts. However, it is unclear whether a blood Eo threshold exists to diagnosing ACO [Citation39]. Currently, in ACO, eosinophil reference levels have not been established, but they are often applied in studies for ease of measurement and standardization, and their relationship to exacerbation risk [Citation40]. An interesting fact about our study is that peripheral eosinophilia and the levels of NGAL and IL-6 are higher in patients with ACO, compared with asthma and COPD alone.
Conclusions
Plasma levels of NGAL and IL-6 were significantly elevated in ACO patients and were associated with peripheral eosinophilia and frequent exacerbations. These results suggest that the plasma levels of NGAL and IL-6 may be useful biomarkers for differentiation of patients with ACO from asthma and COPD.
Data availability
The data that support the findings of this study are openly available at https://osf.io/rf428/?view_only=3fb71f456d264394a22518a96a9df329
Additional information
Funding
References
- Burrowes KS, Doel T, Brightling C. Computational modeling of the obstructive lung diseases asthma and COPD. J Transl Med. 2014;12(Suppl 2):S5.
- de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, Middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985.
- Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the U.S. population. COPD. 2011;8(6):400–407.
- Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the national health and nutrition examination survey, 1988-1994. Arch Intern Med. 2000;160(11):1683–1689.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Mendy A, Forno E, Niyonsenga T, et al. Prevalence and features of asthma-COPD overlap in the United States 2007-2012. Clin Respir J. 2018;12(8):2369–2377.
- Hosseini M, Almasi-Hashiani A, Sepidarkish M, et al. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and Meta-analysis. Respir Res. 2019;20(1):229.
- Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. GOLD works with healthcare professionals and public health officials around the world to raise awareness of COPD and to improve prevention and treatment of this lung disease. https://goldcopd.org/.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. 2016. https://ginasthma.org/
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. 2020. GOLD works with healthcare professionals and public health officials around the world to raise awareness of COPD and to improve prevention and treatment of this lung disease. https://goldcopd.org/.
- Singer E, Marko L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207(4):663–672.
- Asimakopoulou A, Weiskirchen S, Weiskirchen R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front Physiol. 2016;7:430.
- Bouchet S, Bauvois B. Neutrophil gelatinase-associated lipocalin (NGAL), Pro-Matrix metalloproteinase-9 (pro-MMP-9) and their complex Pro-MMP-9/NGAL in leukaemias. Cancers (Basel). 2014;6(2):796–812.
- Tho NV, Park HY, Nakano Y. Asthma-COPD overlap syndrome (ACOS): a diagnostic challenge. Respirology. 2016;21(3):410–418.
- Matsuda T, Kishimoto T. Interleukin 6. Encyclopedia of immunology. Second Edition ed1998. 1458–61.
- Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16(3–4):249–284.
- Mehrotra N, Freire AX, Bauer DC, et al. Predictors of mortality in elderly subjects with obstructive airway disease: the PILE score. Ann Epidemiol. 2010;20(3):223–232.
- Sin DD, Miravitlles M, Mannino DM, Soriano JB, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673.
- Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52(3):595–605.
- Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med. 1997;155(2):449–453.
- Ekberg-Jansson A, Andersson B, Bake B, et al. Neutrophil-associated activation markers in healthy smokers relates to a fall in DL(CO) and to emphysematous changes on high resolution CT. Respir Med. 2001;95(5):363–373.
- Eagan TM, Damas JK, Ueland T, et al. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest. 2010;138(4):888–895.
- Cowland JB, Sorensen OE, Sehested M, et al. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J Immunol. 2003;171(12):6630–6639.
- Soni SS, Cruz D, Bobek I, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–150.
- Iwamoto H, Gao J, Koskela J, et al. Differences in plasma and sputum biomarkers between COPD and COPD-asthma overlap. Eur Respir J. 2014;43(2):421–429.
- Hristova Z, Tsachev K. Novel structural biomarkers for assessment of renal function and early diagnosis of acute kidney injury. Med Rev. 2013;49(4):5–13.
- Betsuyaku T, Nishimura M, Takeyabu K, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159(6):1985–1991.
- Yan L, Borregaard N, Kjeldsen L, et al. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276(40):37258–37265.
- Roudkenar MH, Kuwahara Y, Baba T, et al. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat Res. 2007;48(1):39–44.
- Fu JJ, McDonald VM, Gibson PG, et al. Systemic inflammation in older adults with Asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. 2014;6(4):316–324.
- Gao J, Iwamoto H, Koskela J, et al. Characterization of sputum biomarkers for asthma-COPD overlap syndrome. COPD. 2016;11:2457–2465.
- Wang J, Lv H, Luo Z, et al. Plasma YKL-40 and NGAL are useful in distinguishing ACO from asthma and COPD. Respir Res. 2018;19(1):47.
- Gelb AF, Christenson SA, Nadel JA. Understanding the pathophysiology of the asthma-chronic obstructive pulmonary disease overlap syndrome. Curr Opin Pulm Med. 2016;22(2):100–105.
- Jo YS, Lee J, Yoon HI, et al. Different prevalence and clinical characteristics of asthma-chronic obstructive pulmonary disease overlap syndrome according to accepted criteria. Ann Allergy Asthma Immunol. 2017;118(6):696–703 e1.
- Menezes AMB, Montes de Oca M, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304.
- Hardin M, Cho M, McDonald ML, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44(2):341–350.
- Rhee CK, Yoon HK, Yoo KH, Kim YS, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014;11(2):163–170.
- Tu X, Donovan C, Kim RY, et al. Asthma-COPD overlap: current understanding and the utility of experimental models. Eur Respir Rev. 2021;30(159):190185.
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974.
