Abstract
Laryngeal tumours with multifactorial etiopathogenesis constitute ∼1% of all body cancers. The imbalance between oxidative and antioxidative systems affects redox-homeostasis. The oxidative stress generated by the continuous formation of reactive oxygen species results in the oxidation of cellular molecules. The present study investigated the protein oxidation levels and the distribution of receptors for advanced glycation end products (RAGE) variants in the risk of laryngeal carcinoma (LC). RAGE gene polymorphisms were determined by restriction endonuclease-based assay in 120 controls and 120 LC patients. Spectrophotometric methods were used to determine oxidant and antioxidant parameters including protein-carbonyl-groups (PCO), advanced-oxidation-protein-products (AOPP), lipid-hydroperoxides (LPH), thiol-fractures, superoxide-dismutase (SOD) activity. The distributions of rs1800624 and rs2070600 genotypes differed non-significantly among the study groups, however, the rs2070600-Ser allele had a higher frequency among the patients. While rs1800624-A allele carriers had higher frequency of perineural and lymphatic invasion, rs2070600-Ser allele frequency was higher in advanced-stage patients and in patients with muscle and perineural invasion. PCO, AOPP, LPH levels, and SOD activity were significantly higher in the patients. According to AUCs all of them are of diagnostic importance, therefore, cut-off values were determined. The analysis of the combined effects of RAGE polymorphisms and the oxidative stress parameters showed that LPH, thiols, and SOD activity differ among RAGE variants. Our results suggest that high levels of serum PCO, AOPP, LPH, and SOD activity and rs2070600-Ser allele may have effects on LC risk individually and both polymorphisms of RAGE may affect the progression of the disease by interacting with the oxidant–antioxidant system.
Introduction
Laryngeal carcinomas (LC), which constitute approximately 1% of all body cancers, are the most common malignant tumours among head and neck cancers with a high metastatic rate and poor prognosis. LC has lower mortality and morbidity compared to other cancer types. Indeed, the early-stage patients can reach 80–90% 5-year survival rates while this is <50% with advanced stage. The treatment strategies involve surgical, radiation or chemo mono-therapy for early-stage patients, which generally allows larynx-preservation, and the combined therapies for advanced-stage patients, which are less likely to preserve the larynx [Citation1, Citation2]. Smoking and alcohol addiction are the most common independent risk factors in laryngeal tumours. Occupational exposure to chemicals such as coal dust, asbestos, and chlorinated solvents, human papillomavirus (HPV) especially in young people, and genetic factors such as family history, polymorphisms in some genes especially involved in tobacco and alcohol metabolism (e.g. encoding GST1, CYP1A1, ADH, ADLH) as well as mutations in DNA repair system and tumour suppressor genes have been reported among other risk factors [Citation3–5].
It is well-known that oxidative stress and deficiency in antioxidant capacity are involved in various pathological states including diabetes, atherosclerosis, neurodegenerative diseases, and cancer. Indeed, the imbalance between the continuous formation of reactive oxygen species (ROS) and the anti-oxidant system affects the redox homeostasis, which later triggers cancer development via damage to cellular components including lipids, proteins, and DNA [Citation6, Citation7].
Protein carbonyl groups (PCOs) and advanced oxidation protein products (AOPPs) are generated by protein oxidation, which leads to the loss of functions or renders proteins more prone to proteolytic degradation. PCO occurs due to the interaction of ROS with amino acid residues such as histidine, proline, arginine, and lysine, and/or with the peptide backbone of proteins. PCO levels are accepted as a chemically stable marker of protein oxidation in biological samples. On the other hand, AOPPs are formed by a high degree of oxidation of proteins [Citation8–10].
Lipid peroxidation is the oxidative degradation of lipids. As an early-phase biomarker, lipid hydroperoxides (LPHs) are formed as a result of free radical reactions from phospholipids in the membrane structure and cause widespread oxidative damage with Fenton reaction in cellular proteins and DNA [Citation9, Citation11].
Substances that prevent oxidative damage caused by free radicals and have the ability to capture and stabilize ROS are called ‘antioxidants’. Thiols are biomolecules containing sulfhydryl (–SH) groups and are considered as anti-oxidant redox sensors that control anti-oxidant defence. Total thiols exist as both intracellular and extracellular thiols in the free oxidized form or bound to reduced glutathione or proteins. In addition to defence against free radicals, they also have roles in detoxification, signal transduction, and apoptosis [Citation12, Citation13]. Another important mechanism in the anti-oxidant system is copper, zinc superoxide dismutase (Cu, Zn-SOD). The Cu, Zn-SOD enzyme (E.C. 1.15.1.1), which catalyses the dismutation of superoxide into oxygen and hydrogen peroxide, is one of the most prominent antioxidant mechanisms that protect the cell [Citation12–14].
Recent studies, interestingly, emphasize the interaction of AOPPs with receptors for advanced glycation end products (RAGE), a scavenger receptor with multiligand binding capacity [Citation15–17]. Guo et al. [Citation15] reported AOPP-induced vascular endothelial dysfunction through RAGE-mediated signalling in endothelial cells. Another study by Zhou et al. [Citation16] demonstrated both in vitro and in vivo models of kidney disease that blocking or silencing RAGEs prevent cells from AOPPs-induced apoptosis. More recently, Wu et al. [Citation17] showed AOPP-RAGE mediated chondrocyte apoptosis in vitro. The RAGE gene is located on chromosome 6p21.3 in the MHC (‘major histocompatibility’) complex. There are various polymorphic regions in the RAGE gene. These polymorphisms, which can affect the expression and function of RAGE, occur especially in the exons and regulatory regions of the gene. The RAGE −374 T > A (rs1800624) variation, a functional polymorphism in the promoter region of the RAGE gene, has marked effects on transcriptional activity. Another potential variation of the RAGE gene, the RAGE Gly82Ser (G > A; rs2070600) variation, occurs in the third exon and leads to a Gly-to-Ser substitution at position 82 of the RAGE protein, which hits the ligand-binding domain of the receptor and thus changes its ligand-binding affinity [Citation17–19].
This study aimed to determine the levels of oxidant (AOPP, PCO, LPH) and anti-oxidant parameters (thiols and Cu, Zn-SOD), and the distributions of RAGE −374 T/A (rs1800624) and RAGE Gly82Ser (G > A; rs2070600) variations in patients with laryngeal cancer and healthy volunteers, and to investigate their contribution to cancer development and prognosis. To our knowledge, functionally important genetic variations in the RAGE gene, which will be investigated within the scope of our project, have not yet been investigated in the development of LC. Here we demonstrate the association of these genetic variations and the serum oxidant and antioxidant levels with the development of LC. The results obtained may shed light on providing a different perspective on the pathogenesis of the disease and determining the values of RAGE variations and serum oxidant and anti-oxidant parameters as potential molecular biomarkers in the monitoring of disease progression and treatment.
Subjects and methods
Ethics statement
The study protocol was consistent with the principles of the Declaration of Helsinki and approved by both the Ethical Committee of the Istanbul Faculty of Medicine and the Research Fund of Istanbul University, Project number: TSA-2020-32575. All participants provided written informed consent before they participated in the study.
Participants
This study included 120 primary LC patients (114 men and 6 women) and 120 healthy volunteers (51 men and 69 women) followed by the Department of Otorhinolaryngology/Head and Neck Surgery, Haydarpasa Numune Education and Research Hospital (Istanbul, Turkey) and Department of Otorhinolaryngology, Faculty of Medicine, Istanbul University (Istanbul, Turkey). The age distribution between patients and controls was not significant (57.12 ± 8.45→57.55 ± 4.59, respectively; p > 0.05). All patients were newly diagnosed with histopathologically confirmed primary laryngeal cancer. Pathological grade information for patients was determined according to a manual review of the pathology reports and clinical charts. Healthy volunteers with no signs of malignancy and preferably no family history of cancer were included as controls. Exclusion criteria were LC with previous chemotherapy, radiotherapy, and surgery, and any vitamin or antioxidant drug supplementation within 12 months before the sample collection.
Analysis of oxidative stress markers (protein oxidation and anti-oxidant capacity)
Fasting venous blood samples were obtained from LC patients and healthy individuals. After the serum isolation, total protein levels were measured by the biuret method. All individual serum specimens were stored at −80 °C until the analysis.
Determination of PCO
PCO levels were analysed by the previously defined colorimetric method by Reznick and Packer [Citation20]. In the acidic condition, PCO groups of protein samples react with 2,4-dinitrophenylhydrazine (DNPH) reagent (1:4 ratio) and form chromophoric dinitrophenylhydrazones. After the precipitation process with 20% (w/v) trichloroacetic acid, the pellets underwent extensive washing with 400 μL of an ethanol/ethyl acetate mixture (1:1) three times. After centrifugation, the protein pellets were dissolved in guanidine-HCl (6 mol/L) solution, and the absorbance values of the samples were measured at 360 nm (Biotek Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek US, Winooski, VT, USA). The PCO levels were then calculated via the molar extinction coefficient of DNHP (ε = 22.000 L/(mol cm)).
Determination of AOPP
AOPP levels were analysed by the modified Hanasand method [Citation21]. After 1 min of mixing 10 μL of the sample, 40 μL of phosphate-buffered saline, and 200 μL of a citric acid solution (20 mmol/L) in a microplate, 10 mL of 1.16 mol/L potassium iodide was added, and the absorbance values were read at 340 nm. The chloramine-T standards with the range between 0 and 100 μmol/L were used to prepare a standard curve to express AOPP levels as micromoles per litre of chloramine-T equivalents.
Determination of LPHs
LPH levels were analysed by the Wolff method [Citation22] using ferrous oxidation with xylenol orange, version 2 reagent (FOX2). The absorbance values of the supernatants were read at 560 nm after 30 min of incubation of 5 μL samples with 950 μL FOX2 reagent.
Determination of total, nonprotein, and protein thiol fractions (–SH groups)
Total (T-SH), nonprotein (NP-SH) and protein thiol (P-SH) levels were measured according to the Sedlak and Lindsay method with optimizing small volumes [Citation23]. T-SH groups were determined by mixing 20 mL of the sample with 400 μL of 0.2 mol/L Tris buffer, pH 8.2, and 20 μL of 0.01 mol/L 2-nitrobenzoic acid. After the precipitation of the samples, tubes were centrifuged for 15 min at 3000g. Then the absorbance values of the supernatant fractions were read at 412 nm. The molar extinction coefficient of the thiol groups at 412 nm was ε = 13.100 L/(mol cm). The NP-SH groups were detected by mixing 20 μL of the sample with 400 μL of 50% trichloroacetic acid, and the P-SH groups were calculated by subtracting the values of NP-SH from T-SH.
Determination of Cu, Zn-superoxide dismutase (Cu, Zn-SOD) activity
Cu, Zn-SOD activity was measured by the method of Sun and Oberley [Citation24], which uses xanthine oxidase as a superoxide generator to inhibit nitro blue tetrazolium reduction. The enzyme activity was assessed by measuring the inhibition rate of substrate hydrolysis of assay mixture containing 0.3 mmol/L xanthine, 0.6 mmol/L ethylenediaminetetraacetic acid disodium, 150 μmol/L nitro blue tetrazolium, 400 mmol/L sodium carbonate, and 1 g/L bovine serum albumin (BSA). The pH of the assay mixture was adjusted to 10.2. After the incubation of 972 μL of assay mixture and 13 μL of xanthine oxidase (167 U/L) with 25 μL of sample for 20 min, 250 μL of 0.8 mmol/L copper (II) chloride was added to the vial to stop the reaction. The final absorbance was read at 560 nm against a reagent blank. The percent of inhibition rate was calculated according to the equation of Ablank – Asample/Ablank × 100, where A represents the absorbance. A single unit of Cu, Zn-SOD is defined as the amount of enzyme needed to exhibit a 50% dismutation of superoxide radical anion.
Analysis of RAGE −374 T/A (rs1800624) and RAGE Gly82Ser (G > A; rs2070600) variants
Restriction endonuclease-based assay
Genomic DNA was obtained by using a DNA isolation kit (Roche DNA Isolation Kit for Mammalian Blood, Germany), and the polymorphisms were determined by using the polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) method. Genotyping was performed by using Tsp509 I and Alu I endonuclease restriction enzymes for RAGE −374 T/A (rs1800624) and RAGE Gly82Ser (G > A; rs2070600), respectively. The fragments were then visualized under UV light after ethidium bromide staining.
Statistical analysis
Statistical analysis was performed by using the SPSS software package (revision 21.0; SPSS Inc., Chicago, IL, USA). Quantitative data were presented as mean ± SD. The Student’s t test was used to determine the difference in quantitative biochemical parameters for normally distributed data. Categorical data were expressed as numbers and percentages (%).χ2 test was used to compare genotype and allelic comparison, and the demographic distribution of cancer prognosis. Allele frequencies were calculated by the gene counting method. The genotypic and allelic differences of oxidative stress parameters were examined by one-way analysis of variance (ANOVA) and Student’s t test, respectively. Binary logistic regression analysis was used to evaluate the risk factors between groups. MedCalc Statistical Software (MedCalc Software Ltd, Belgium/WEB address: [email protected]) program was used to obtain receiver-operating characteristics (ROC) curve graphs and the cut-off values which are thought to have potential diagnostic value according to AUC values, and the sensitivity and specificity were evaluated by using the SPSS software package. Differences were considered statistically significant at the level of p < 0.05.
Results
Clinical characteristics of the study groups
According to the baseline characteristics of both patients and control groups, the age distribution was balanced between the study groups (p > 0.05). The frequency of classical risk factors, such as male gender, alcohol consumption, and smoking, was higher in the patients’ group than in the controls (p < 0.001 for all).
According to the clinical characteristics of the LC patients (), the localization of the tumour in LC cases was 67.5% in the glottis, and the rest, 32.5% involved in the supraglottis area of the larynx. In the LC patients group, 9.1% of patients had a tumour with grade I, 17.5% with grade II, 56.8% with grade III, and 17.6% with grade IV. Despite the higher incidence of advanced-stage patients, the incidence of perineural invasion and lymph node metastasis were at lower rates. However, the frequency of muscle invasion was 72.8% in the whole group of LC patients ().
Table 1. Clinical characteristics of patients with laryngeal carcinoma.
Genotypic characteristics of the study groups
The distribution of genotype and allele frequencies of RAGE −374 T/A (rs1800624) and RAGE Gly82Ser (G > A; rs2070600) polymorphisms in LC patients and control groups are shown in . Interestingly but similar to the literature, both the patient and control group lacked the RAGE rs2070600 SerSer (AA) genotype. On the other hand, no deviation from HWE (p > 0.05) was detected in both the patient and control group for RAGE rs1800624 and rs2070600 polymorphisms (p > 0.05). However, the LC patients had a higher frequency of carriage of the RAGE rs2070600 Ser allele [GlySer (GA) + SerSer (AA) genotypes] than the control group (p < 0.001; χ2 = 36.2; OR = 12.4; 95% CI: 4.7—32.7) (). Moreover, when the clinical characteristics of the patients were examined in regard to the RAGE variants, the patients with perineural invasion had a higher frequency of RAGE rs1800624 A allele carriage (TA + AA genotypes) than TT genotype (p = 0.008; χ2 = 7.0; OR = 3.6; 95% CI: 1.3—9.5). In addition, the frequency of A allele carriers with lymphatic invasion was higher than that in the patients without lymphatic invasion (p = 0.018; χ2 = 5.6; OR = 2.7; 95% CI: 1.2—6.4). The RAGE Gly82Ser (G > A; rs2070600) polymorphisms showed higher frequencies of the Ser allele in advanced-stage patients (grade III + IV) than in those with early-stage (grade I + II) (p = 0.069; χ2 = 3.3; OR = 2.4; 95% CI: 0.9—6.0) as well as in patients with muscle invasion compared to patients without muscle invasion (p = 0.018; χ2 = 5.6; OR = 2.2; 95% CI: 0.1—0.8). In addition, there was higher frequency of the Ser allele in patients with perineural invasion than the GlyGly genotype (p = 0.007; χ2 = 7.2; OR = 2.9; 95% CI: 1.3—6.4) (data not shown).
Table 2. Distribution of genotype and allele frequencies of RAGE −374 T/A (rs1800624) and RAGE Gly82Ser (G > A; rs2070600) polymorphisms in the study groups.
Oxidant and antioxidant status of the study groups
The levels of protein and lipid oxidation markers and antioxidant markers discussed within the scope of this study are presented in . Accordingly, PCO (p < 0.001), AOPP (p = 0.002), lipid hydroperoxide (LPH) (p < 0.001) and copper, zinc superoxide dismutase (Cu, Zn-SOD) (p < 0.001) levels were significantly higher in the LC patient group when compared with the control group. The correlations of these molecules in the patients’ group are shown in . Although the levels of thiol fractions were not different among the groups, a slight and positive correlation was found between LPH and T-SH (r = 0.359, p < 0.001) levels or P-SH (r = 0.379, p < 0.001) levels. In addition, there was a slight negative correlation between LPH and Cu, Zn-SOD (r = −0.390, p < 0.001) levels, and a strong positive correlation between T-SH and P-SH (r = 0.966, p < 0.001) levels ().
Table 3. Oxidant and antioxidant levels in the study groups and their correlations in LC patients group.
In , the ROC curve analysis was presented to determine whether the significant oxidative stress parameters have potential diagnostic value to distinguish LC patients and healthy controls. It is well-known that in ROC curve analysis, the size of the area under the curve (AUC) statistically indicates the strength of the discrimination ability of the parameter studied. If AUC ≤ 0.50, the studied parameter has no power to separate the patient and control group. The interpretation of AUCs has been reported to be 0.90–1.00 = excellent; 0.80–0.090 = good; 0.70–0.80 = medium; 0.60–0.70 = weak; 0.50–0.60 = fail [Citation25]. In this scope, AUCs were determined to be 0.825 (p < 0.001) for LPH, 0.731 (p < 0.001) for PCO, 0.705 (p < 0.001) for Zn, Cu-SOD, and 0.683 (p < 0.001) for AOPP. On the other hand, as the non-significant differences of T-SH, NP-SH, and P-SH levels among the study groups and AUCs were <50%, does not include thiol fractures. The cut-off values of oxidative stress parameters, which are thought to have potential diagnostic value according to AUC values by ROC analysis, and the sensitivity and specificity values of these values were also evaluated using the SPSS software package. Accordingly, the optimized cut-off values were found as follows: LPH: 1.73 μmol/mg pr (p < 0.001; 95% CI: 0.8 − 0.9) with 72.6% sensitivity and 74.2% specificity; PCO: 0.955 nmol/mg pr (p < 0.001; 95% CI: 0.6 − 0.8) with 72.4% sensitivity and 66.7% specificity; Cu, Zn-SOD: 6.495 U/mg pr (p < 0.001; 95% CI: 0.6 − 0.8) with 69.4% sensitivity and 66.7% specificity; AOPP: 44.8 μmol/L chloramine-T equivalent (p < 0.001; 95% CI: 0.6–0.8) with 63.3% sensitivity and 63.3% specificity. After determining the cut-off values, a logistic regression analysis was performed between LC patients and healthy controls for testing these parameters in an LC risk model. In the independent variables of binary logistic regression analysis were significant quantitative levels of oxidant and anti-oxidant parameters as LPH, PCO, SOD, and AOPP levels, and shows the results when these parameters are above the optimal cut-off values as risk. Accordingly, the binary logistic regression analysis confirmed that LPH, PCO, SOD, and AOPP levels were associated with increased risk of LC () as well as in the modelling performed according to the cut-off values in .
Figure 1. ROC Curve analysis of significant oxidant and antioxidant parameters.
MedCalc Statistical Software (MedCalc Software Ltd, Belgium/WEB address: [email protected]) program was used to obtain ROC curve analysis. AUC, area under curve; PCO, protein carbonyl; AOPP, advanced oxidation protein products; T-SH, total thiol; NP-SH, non-protein thiol; P-SH, protein thiol; LPH, lipid hydroperoxides; Cu, Zn-SOD, copper, zinc superoxide dismutase. P shows statistical significance as p < 0.05.
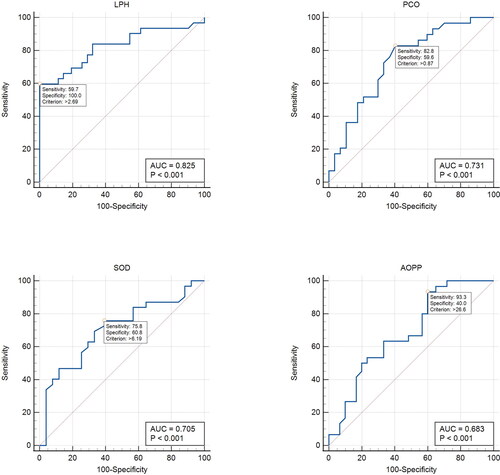
Table 4. Logistic regression analysis of risk factors that may affect the pathogenesis of laryngeal cancer between the patient and control groups.
Combined effects of RAGE variants and oxidative parameters among the study groups
Finally, the effects of oxidant and antioxidant parameters were examined in the prognosis of LC. Interestingly, as seen in , although there was no significant difference in thiol fractures in terms of disease risk, significant differences were detected for disease prognosis. also shows the levels of oxidative and anti-oxidative parameters among RAGE rs1800624 and rs2070600 variants in LC patients. The differences between genotypes were analysed with one-way ANOVA, and the differences between alleles were analysed with Student’s T-test. For RAGE gene rs1800624 genotypes the NP-SH level was significantly higher in the heterozygous TA genotype (p = 0.010), and the LPH level was significantly higher in the homozygous AA genotype (p = 0.001) compared to other genotypes. In the multiple comparisons of NP-SH levels, which were in the order of TA > TT > AA in the patient group, the significance values were AA < TA (p = 0.011), TT < TA (p = 0.013) and AA < TT (p = 0.622); and of LPH levels, which were in the order of AA > TT > TA, it was calculated as TA < AA (p = 0.001), TA < TT (p = 0.022) and TT < AA (p = 0.101). For the RAGE gene rs2070600 genotypes, there was no difference in terms of the levels of oxidative stress parameters; however, the Cu, Zn-SOD levels were close to statistically significantly different: GlySer(GA) > GlyGly(GG) (p = 0.051). On the other hand, when these parameters are examined based on alleles, no significant difference was observed between the patients carrying the RAGE rs1800624 A allele (AA + TA genotypes) and patients with the TT genotype (p > 0.05). However, when the patients with the RAGE rs1800624 T allele (TT + TA genotypes) were compared with the patients with the AA genotype, the LPH levels were higher in the patients with the homozygous mutant AA genotype (p = 0.001). In addition, low PCO (p = 0.062) and NP-SH (p = 0.086) levels were observed in these patients. For RAGE gene rs2070600 alleles, the differences in the levels of oxidative stress parameters between the Gly (G) allele [GlyGly (GG) + GlySer (GA) genotypes] and the SerSer (AA) genotype could not be obtained due to the absence of individuals with the SerSer (AA) genotype in the study group. However, when the patients with the Ser allele [SerSer (AA) + GlySer (GA) genotypes] were compared with the GlyGly (GG) genotype carriers, the Cu, Zn-SOD levels were higher in the patients with the normal GlyGly (GG) genotype at the statistical significance limit (p = 0.051) ().
Table 5. Distribution of oxidative and antioxidative parameters in the prognosis of laryngeal carcinoma and among RAGE rs1800624 and rs2070600 variants.
Discussion
Larynx cancer is a common cancer type among squamous cell carcinomas of the head and neck. This type of cancer, originating from the larynx epithelium, has a high metastasis rate and a poor prognosis, with a 5-year survival rate of ∼50% [Citation2, Citation3, Citation26]. Despite the global advantages in cancer treatments (surgery, radiotherapy, and/or chemotherapy), the 5-year survival rate is not sufficient, especially in patients with advanced-stage. Moreover, despite the increase in molecular diagnostic methods as well as clinical studies, laryngeal cancer remains as it was 30 years ago. Therefore, new diagnostic markers are needed to provide more effective and personalized treatment for laryngeal cancer patients. Although the major risk factor in the aetiology of laryngeal cancer is smoking and/or alcohol consumption, the incidence of cancer development is higher in the male gender than in the female gender. Besides, emerging phenotypic and genotypic data also point to the role of genetic predisposition in the development of laryngeal cancer [Citation3, Citation26–28].
In the present study, besides the classical risk factors, we investigated the effects of oxidative stress markers and rs1800624 (−374 T > A) and rs2070600 (Gly82Ser, G > A) polymorphisms of the advanced glycosylation end-product receptor (RAGE), a member of the scavenger receptor family, on the development and prognosis of laryngeal cancer. In accordance with the literature, our results indicated that smoking, alcohol consumption and male gender pose an individual risk for the development of laryngeal cancer [Citation3, Citation4, Citation29, Citation30].
The advanced glycosylation end-product receptor (RAGE), a member of the immunoglobulin superfamily, is also known as a pattern recognition receptor that controls innate immunity. RAGEs have a broad spectrum of ligand recognition. RAGE ligands include prototypes of high mobility group (HMG) proteins, members of the S100/calgranulin protein family, extracellular matrix proteins, β-amyloid peptides, phosphatidylserine, complement C3a and some advanced glycosylation end products (AGEs) [Citation30, Citation31]. To control various cellular processes such as inflammation, apoptosis, proliferation, and autophagy, RAGE interacts with this broad type of ligand, regulating many intracellular signalling pathways. Studies suggest that AGE accumulation and increased RAGE expression play important roles in various pathophysiological processes such as diabetes, cardiovascular diseases, neurodegenerative diseases, and cancer [Citation31]. In addition, RAGE signalling has been shown to contribute to the progression of various types of cancer and other pathological disorders [Citation30–34].
RAGE gene polymorphisms have been studied in several cancers [Citation35–40]. In hepatocellular [Citation35] and oral [Citation40] cancers, RAGE rs1800624 (−374 T > A) and especially RAGE rs2070600 (Gly82Ser; G > A) polymorphisms have been reported to play a role in the risk of carcinoma. However, in the Bedoui et al. [Citation36] study while a low frequency of RAGE rs1800624 A allele, and a high frequency of RAGE rs2070600 Ser (A) allele was found in patients with colorectal cancer, it has been reported that both polymorphisms do not affect the disease development. In a study conducted in a Chinese population, it was reported that the RAGE rs2070600 polymorphism increased the risk and the invasion potential of gastric cancer [Citation37], as well as of cervical cancer in the same population conducted by another study [Citation39]. In pancreatic cancer, although no significant relationship was found with RAGE polymorphisms, a significant decrease in serum RAGE level was reported [Citation38]. In the Uloza et al. [Citation30] study ∼8.4-fold increase was reported for the risk of laryngeal cancer development with the RAGE rs1800625 polymorphism.
In the present study, we observed that the RAGE rs1800624 (−374 T > A) polymorphism did not affect the development of laryngeal cancer individually; however, the rs2070600 Ser (A) allele was associated with a significantly increased LC risk. On the other hand, the data obtained from the results of our study, (i) the high frequency of the RAGE rs1800624 A allele in patients with perineural and lymphatic invasion, and (ii) the higher frequency of the RAGE rs2070600 Ser allele in patients with advanced-stage and muscle invasion, suggests both polymorphisms may have implications for LC prognosis.
A study conducted in 2010 reported that oxidative stress contributes to LC initiation, and the risk was increased especially by smoking and alcohol consumption [Citation41]. Liu et al. [Citation42] also examined the effects of oxidative stress associated with smoking in the development of LC and reported that catalase (CAT), glutathione (GSH), and malondialdehyde (MDA) levels were high, whereas superoxide dismutase and nitric oxide levels were low in smokers with laryngeal cancer. Moreover, Kacakci et al. [Citation43] investigated the oxidative and anti-oxidative parameters in the pathogenesis of LC both in serum and tumour tissue of patients. Higher serum levels of MDA and lower serum levels of GSH, and SOD and CAT activity were reported in the LC patients group when compared to the control group. On the other hand, researchers found similar MDA levels both in serum and tumour tissue; however, unlike other anti-oxidant molecules, only the SOD enzyme activity was lower in the tumour tissue than in the surrounding healthy tissue. Lubinski et al. [Citation44] investigated the levels of selenium and zinc [Citation29] associated with oxidative stress in patients with LC and found that high serum selenium levels play an important role in the treatment of laryngeal cancer by reducing the mortality rates, and zinc levels may be a prognostic factor in survival in patients with LC.
The relationship between cancer development and oxidative stress in the Turkish population has been reported in many publications. Zengin et al. [Citation45] reported that lipid peroxidation was increased in intracranial tumour tissues compared to tumour-free surrounding tissues. The researchers have shown an increased MDA level and decreased SOD activity and GSH level in advanced tumours, and reported tumoral reduction by enzymatic or non-enzymatic anti-oxidants. The study concluded that low antioxidant levels in tumour tissues might be related to the increased use of antioxidant systems to scavenge lipid peroxides. On the other hand, Inci et al. [Citation46] investigated the levels of lipid peroxidation end-products and endogenous antioxidant components such as Cu, Zn-superoxide dismutase (Cu, Zn-SOD), glutathione peroxidase (GSH Px), glutathione reductase (GSSG Rd), and glutathione (GSH) in patients with laryngeal cancer. They found that laryngeal cancer tissue contains higher lipid peroxidation than the surrounding non-cancerous tissue, and Cu, Zn-SOD, and GSH Px activities and GSH levels are significantly higher and GSSG Rd activity is significantly lower in cancer tissues. Therefore they suggested that the determination of antioxidant status may be beneficial in determining tumour resistance in treatment, choosing the right radiotherapy/chemotherapy, and monitoring the effectiveness of the therapeutic strategy. Yanar et al. [Citation10] examined the serum oxidative stress parameters in patients with laryngeal cancer and reported that PCO, AOPP, LPH levels, and SOD activity were at higher levels in the patient group. Bozan et al. [Citation47] reported that the oxidant/anti-oxidant balance shifted toward oxidative stress in patients with laryngeal cancer. Wigner et al. [Citation48] studied MDA, which is an indicator of lipid peroxidation, some non-enzymatic antioxidant system markers and protein carbonyl (PCO) groups and thiol (–SH) groups, which are indicators of oxidative/nitrative damage in proteins, in patients with bladder cancer, and reported that all parameters were significantly higher in the patients. Wesołowski et al. [Citation49] reported higher oxidant radical absorbance capacity in saliva in patients with head and neck cancers. This finding outlines a natural compensatory mechanism that maintains oxidant and antioxidant homeostasis in response to increased oxidative stress as well as abnormal endogenous cell metabolism. On the other hand, no relationship was reported between the oxidant radical absorbance capacity and tumour spread.
In the present study, similar to our previous report [Citation10], serum PCO, AOPP, LPH levels, and Cu, Zn-SOD activity were significantly higher in the patient group compared to the control group; however, thiol fractions (–SH groups) were not different among the study groups. Thus, it was indicated that these oxidative stress indicators other than –SH groups may have individual effects on the risk of laryngeal cancer. Indeed, when the ROC analysis results were examined, according to AUCs the levels LPH, PCO, Zn, Cu-SOD, and AOPP may have potential diagnostic values for evaluation of LC. Therefore, the cut-off values were determined as 1.73 µmol/mg pr for LPH, 0.955 nmol/mg pr for PCO, 6.495 U/mg pr for Zn, Cu-SOD, and 44.8 µmol/L chloramine T equivalent for AOPP. On the other hand, by logistic regression models created with these parameters, it has been determined that each parameter maintains its significance even if it is above the determined cut-off values. Besides, our results also suggest that these markers including also thiol fractions may also have impacts on LC prognosis, especially by muscle invasion.
The combined effects of these parameters, which were predicted to have diagnostic value in the evaluation of laryngeal cancer risk, with the RAGE rs1800624 (–374 T > A) and rs2070600 (Gly82Ser; G > A) polymorphisms were also investigated in our study. Interesting results were obtained for the RAGE rs1800624 polymorphism in which the order of the genotype frequency was TA > TT > AA in the patient group and RAGE rs1800624 A allele (TA + AA genotype) had no individual effect on the risk of LC. According to our results, the NP-SH and LPH levels were significantly higher in the LC patients with RAGE rs1800624 TA genotype and RAGE rs1800624 AA genotype, respectively. In addition, the LPH and PCO levels were lower in the patients carrying the RAGE rs1800624 T allele (TT + TA genotypes) than in the RAGE rs1800624 AA genotype carriers. This indicates that although the individual effect of the A allele on the risk of disease was not significant, considering the importance of RAGE in metabolism, the RAGE rs1800624 polymorphism may have a potential effect together with oxidative stress parameters in the formation and/or progression of the disease. It is well-known that redox homeostasis is essential for normal balanced metabolism, and disruption of this balance has been associated with many pathophysiological conditions. On the other hand, as some of the oxidative reaction products are removed by RAGE, potential variations in the RAGE gene may manifest with functional disorders in the RAGE protein [Citation30–34]. The results of our study suggested that by carrying homozygous RAGE rs1800624 mutant A allele (AA genotype), oxidative reaction products may not be removed and may contribute to the disease. As for the RAGE rs2070600 polymorphism, homozygous mutant Ser (A) allele [SerSer, (AA) genotype] was not observed in the whole study group. On the other hand, despite the non-significant results, finding of low SOD activity and high oxidative parameters in patients with RAGE rs2070600 homozygous normal Gly(G) allele (GlyGly, GG genotype) carriers, and the GlySer(GA)> GlyGly(GG) order for Cu, Zn-SOD level which was an indicator of antioxidant system, suggested that mutant Ser(A) allele, which may be found as an individual risk factor for LC, creates a physiological response with the increase in SOD activity for maintaining the oxidant-antioxidant system balance. The results of our study indicate that both polymorphisms of RAGE may affect the progression of the disease by interacting with the oxidant-antioxidant system. However, the absence of sRAGE measurement in our study is the limitation of our study. It was reported that serum RAGE levels, which are inhibitors of cell surface receptors, play a role in RAGE-mediated pathological processes. Indeed, lower sRAGE levels were reported in pancreatic cancer and patients with impaired glucose intolerance than in diabetic patients, and this situation was associated with the risk of pancreatic cancer by oxidative processes and then with inflammatory processes with glycosylation and glycoxidation reactions over glucose metabolism [Citation38]. This highlights the importance of the tissue-specific and/or circulating compensatory pathways used by metabolism in maintaining physiological balance.
As a result, the risk of smoking, which is among the classical risk factors, in laryngeal cancer was also seen in our study results. In addition, the data obtained from the results of our study draw attention to the individual contribution of the RAGE rs2070600 Ser allele, as well as the oxidative stress parameters including LPH, PCO, Zn, Cu-SOD, and AOPP to the risk of laryngeal cancer. On the other hand, the results of our study suggest that together with both RAGE polymorphisms [rs1800624 (−374 T > A) and rs2070600 (Gly82Ser; G > A)], the oxidative stress parameters which were found to have potential diagnostic value may also have combined effects on the prognosis of LC.
Conclusions
RAGE is a member of the important scavenger receptor family, which is effective in maintaining the physiological balance in the oxidation–glycosylation axis. On the other hand, the importance of oxidant–antioxidant homeostasis in providing physiological conditions is well known. Our study results draw attention to the combined and individual effects of both RAGE and oxidative stress parameters on laryngeal cancer, and point out that these parameters may be important in laryngeal cancer pathology.
Acknowledgements
The present study was supported by a grant from the Scientific Research Projects Coordination Unit of Istanbul University (Project No: TSA-2020-32575).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gamez ME, Blakaj A, Zoller W, et al. Emerging concepts and novel strategies in radiation therapy for laryngeal cancer management. Cancers (Basel). 2020;12(6):1651. PMID: 32580375; PMCID: PMC7352689.)
- Zhang G, Fan E, Zhong Q, et al. Identification and potential mechanisms of a 4-lncRNA signature that predicts prognosis in patients with laryngeal cancer. Hum Genomics. 2019;13(1):36. PMID: 31416476; PMCID: PMC6694645.
- de Miguel-Luken MJ, Chaves-Conde M, Carnero A. A genetic view of laryngeal cancer heterogeneity. Cell Cycle. 2016;15(9):1202–1212. Epub 2016 Mar 3. PMID: 26940775; PMCID: PMC4894505
- Jarvinen AK, Autio R, Haapa-Paananen S, et al. Identification of target genes in laryngeal squamous cell carcinoma by high-resolution copy number and gene expression microarray analyses. Oncogene. 2006;25(52):6997–7008. Epub 2006 May 22. PMID: 16715129
- Makitie AA, Monni O. Molecular profiling of laryngeal cancer. Expert Rev Anticancer Ther. 2009;9(9):1251–1260. PMID: 19761429
- Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. Epub 2014 Jan 7. PMID: 24406211.
- Islam MO, Bacchetti T, Ferretti G. Alterations of antioxidant enzymes and biomarkers of nitro-oxidative stress in tissues of bladder cancer. Oxid Med Cell Longev. 2019;2019:2730896. PMID: 31191796; PMCID: PMC6525891.
- Cai Z, Yan LJ. Protein oxidative modifications: beneficial roles in disease and health. J Biochem Pharmacol Res. 2013;1(1):15–26.
- Yanar K, Aydin S, Simsek B, et al. Intercellular adhesion molecule-1 Lys469Glu polymorphism, systemic redox homeostasis and gestational diabetes mellitus in pregnant women. Can J Diabetes. 2019;43(3):173.e1–178.e1. Epub 2018 Aug 2. PMID: 30297297
- Yanar K, Çakatay U, Aydın S, et al. Relation between endothelial nitric oxide synthase genotypes and oxidative stress markers in larynx cancer. Oxid Med Cell Longev. 2016;2016:4985063. Epub 2015 Nov 22. PMID: 26682008; PMCID: PMC4670686.
- Gueraud F, Atalay M, Bresgen N, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44(10):1098–1124. PMID: 20836659.
- Eryilmaz MA, Kozanhan B, Solak I, et al. Thiol-disulfide homeostasis in breast cancer patients. J Cancer Res Ther. 2019;15(5):1062–1066.
- Erel Ö, Erdoğan S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50(SI-2):1728–1738. PMID: 32233181; PMCID: PMC7672356.
- Moldogazieva NT, Mokhosoev IM, Feldman NB, et al. ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic Res. 2018;52(5):507–543. Epub 2018 Apr 19. PMID: 29589770.
- Guo ZJ, Niu HX, Hou FF, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10(10):1699–1712. PMID: 18576917; PMCID: PMC6464001.
- Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a Central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82(7):759–770. Epub 2012 May 23. PMID: 22622498.
- Wu Q, Zhong ZM, Zhu SY, et al. Advanced oxidation protein products induce chondrocyte apoptosis via receptor for advanced glycation end products-mediated, redox-dependent intrinsic apoptosis pathway. Apoptosis. 2016;21(1):36–50. PMID: 26520447.
- Schröter D, Höhn A. Role of advanced glycation end products in carcinogenesis and their therapeutic implications. Curr Pharm Des. 2018;24(44):5245–5251.
- Ahmad S, Khan H, Siddiqui Z, et al. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin Cancer Biol. 2018;49:44–55. Epub 2017 Jul 13. PMID: 28712719.
- Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363.
- Hanasand M, Omdal R, Norheim KB, et al. Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta. 2012;413(9-10):901–906.
- Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202(2):384–389. PMID: 1519766.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25(1):192–205.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500.
- Zou KH, O’Malley AJ, Mauri L, et al. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657.
- Gong S, Xu M, Zhang Y, et al. The prognostic signature and potential target genes of six long non-coding RNA in laryngeal squamous cell carcinoma. Front Genet. 2020;11:413. PMID: 32411183; PMCID: PMC7198905.
- Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52(Pt 2):228–240. Oct Epub 2018 Jan 31. PMID: 29355614.
- Chan JYK, Zhen G, Agrawal N. The role of tumor DNA as a diagnostic tool for head and neck squamous cell carcinoma. Semin Cancer Biol. 2019;55:1–7. Epub 2018 Aug 3. PMID: 30082187.
- Lubiński J, Jaworowska E, Derkacz R, et al. Survival of laryngeal cancer patients depending on zinc serum level and oxidative stress genotypes. Biomolecules. 2021;11(6):865. PMID: 34200699; PMCID: PMC8228711.
- Uloza V, Tamauskaite T, Vilkeviciute A, et al. Determination of SIRT1 rs12778366, FGFR2 rs2981582, STAT3 rs744166, and RAGE rs1800625 single gene polymorphisms in patients with laryngeal squamous cell carcinoma. Dis Markers. 2019;2019:1–9. PMID: 31781300; PMCID: PMC6875326.
- Xie J, Mendez JD, Mendez-Valenzuela V, et al. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013;25(11):2185–2197. Epub 2013 Jul 6. PMID: 23838007.
- Loyo M, Pai SI. The molecular genetics of laryngeal cancer. Otolaryngol Clin North Am. 2008;41(4):657–672. PMID: 18570952.
- Waghela BN, Vaidya FU, Ranjan K, et al. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem. 2021;476(2):585–598. Epub 2020 Oct 6. PMID: 33025314.
- Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl). 2005;83(11):876–886. Epub 2005 Aug 24. PMID: 16133426.
- Wang D, Qi X, Liu F, et al. A multicenter matched case-control analysis on seven polymorphisms from HMGB1 and RAGE genes in predicting hepatocellular carcinoma risk. Oncotarget. 2017;8(30):50109–50116. PMID: 28187002; PMCID: PMC5564833.
- Bedoui SA, Barbirou M, Stayoussef M, et al. Identification of novel advanced glycation end products receptor gene variants associated with colorectal cancer in Tunisians: a case-control study. Gene. 2020;754:144893. Sep 5 Epub 2020 Jun 13. PMID: 32544495.
- Gu H, Yang L, Sun Q, et al. Gly82Ser polymorphism of the receptor for advanced glycation end products is associated with an increased risk of gastric cancer in a chinese population. Clin Cancer Res. 2008;14(11):3627–3632. Jun 1 PMID: 18519797.
- Krechler T, Jachymova M, Mestek O, et al. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin Biochem. 2010;43(10-11):882–886. Epub 2010 Apr 14. PMID: 20398646.
- Xu Q, Xue F, Yuan B, et al. The interaction between RAGE gene polymorphisms and HPV infection in determining the susceptibility of cervical cancer in a chinese population. CBM. 2012;11(4):147–153. PMID: 23144152.
- Su S, Chien M, Lin C, et al. RAGE gene polymorphism and environmental factor in the risk of oral cancer. J Dent Res. 2015;94(3):403–411. Epub 2015 Jan 12. PMID: 25582438; PMCID: PMC4814014.
- Manjunath MK, Annam V, Suresh DR. Significance of free radical injury in laryngeal and hypopharyngeal cancers. J Laryngol Otol. 2010;124(3):315–317. Epub 2009 Nov 23. PMID: 19930749.
- Liu Y, La C, Wei J, et al. The effect of catalase on smoking related laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(15):1346–1349. PMID: 26685399.
- Kacakci A, Aslan I, Toplan S, et al. Significance of the counteracting oxidative and antioxidative systems in the pathogenesis of laryngeal carcinoma. J Otolaryngol Head Neck Surg. 200938(2):172–177. PMID: 19442365.
- Lubiński J, Marciniak W, Muszynska M, et al. Serum selenium levels and the risk of progression of laryngeal cancer. PLoS One. 2018; 513(1):e0184873. Erratum in: PLoS One. 2018;13(3):e0194469. PMID: 29304040; PMCID: PMC5755727.
- Zengin E, Atukeren P, Kokoglu E, et al. Alterations in lipid peroxidation and antioxidant status in different types of intracranial tumors within their relative peritumoral tissues. Clin Neurol Neurosurg. 2009;111(4):345–351. Epub 2008 Dec 30. PMID: 19117666.
- Inci E, Civelek S, Seven A, et al. Laryngeal cancer: in relation to oxidative stress. Tohoku J Exp Med. 2003;200(1):17–23. PMID: 12862307.
- Bozan N, Demir H, Gürsoy T, et al. Alterations in oxidative stress markers in laryngeal carcinoma patients. J Chin Med Assoc. 2018;81(9):811–815. Epub 2018 May 31. PMID: 29778552.
- Wigner P, Szymańska B, Bijak M, et al. Oxidative stress parameters as biomarkers of bladder cancer development and progression. Sci Rep. 2021;11(1):15134. PMID: 34302052; PMCID: PMC8302678.
- Wesołowski P, Zawada K, Wojtowicz A, et al. Assessment of salivary total antioxidant capacity in patients with primary untreated head and neck squamous cell carcinoma with ORAC. J Oral Pathol Med. 2016;45(10):753–757. Epub 2016 Feb 14. PMID: 26876359.
