?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The use of native microalgal strains, which are well adapted to local environmental conditions, for sustainable biofuels production has largely been marred by photonics-related challenges. To date, most photobioreactor systems make use of artificial sources of illumination thus increasing the overall costs of biomass production. Solar energy, although sustainable and cost-effective, is difficult to manage and control. It also contains other wavelengths which are detrimental to microalgae. Thus, this study sought to make use of spectral filters for optimal outdoor algaculture. Hence, solar energy was used in wastewater-mediated algaculture of native and imported Chlorella sp. under blue, green, red and yellow coloured spectral filters. The native Chlorella sp. had the highest growth rate of 0.892 d−1 and 0.754 d−1 under green and blue coloured filters, respectively. In comparison, the imported Chlorella strain had a growth rate of 0.379 d−1 and 0.267 d−1 under green and blue filters, respectively. Both strains produced high lipid yields under the blue coloured filter, with the native and imported Chlorella strains managing lipid yields of 41.87% dry cell weight (dcw) and 32.29% dcw, respectively. The native Chlorella strain also significantly lowered (p < 0.05) the levels of total nitrogen and ammonium from wastewater with removal efficiencies of 92.17% and 44.60%, respectively, whereas the imported Chlorella strain managed a removal efficiency of 80.81% total nitrogen and 26.10% ammonium under the blue coloured filter. The results indicate that light filtration technology can be used, sustainably, in the simultaneous algaculture of native strains and remediation of wastewater.
Introduction
The use of microalgal biomass as feedstock for biofuels production has drawn considerable attention due to their robustness, rapid growth, high lipid yield and efficient use of non-arable land [Citation1–3]. The use of native microalgal species, which are well adapted to local environmental conditions and cheaper algal growth media, further presents an opportunity to generate more biomass and lipids for the biofuels production process. Photobioreactors (PBRs) have been identified as the ideal algaculture vessels, generating biomass yields as high as 4300 mg L−1·d−1 [Citation4]. PBRs also offer a controllable culture microenvironment, limit culture contamination by invasive microalgal species and limit water loss through evaporation [Citation5]. Despite all the advantages of PBRs, the use of these systems for optimum algal biomass production is still yet to be realised due to photonics related challenges.
Light is the most critical factor governing optimum biomass production [Citation6]. Different algal species have different spectral quality requirements. The type of photosynthetic pigments concentrated in the cells determine the wavelengths essential for that particular species’ proliferation [Citation5]. Variations in light wavelengths have also been noted to have a huge impact on the quality and quantity of lipids generated by cells [Citation7, Citation8]. To date, most commercial PBRs make use of artificial lighting as an energy source [Citation9]. Artificial lighting systems are easier to manipulate and control. However, dependence on artificial lighting increases immensely the overall biomass production costs. Solar energy is a readily available alternative that is free and contains Photosynthetic Active Radiation (PAR). PAR is a narrow range of wavelengths in the solar spectrum. Algae can only utilise this range for photosynthesis, with blue and red portions being the most efficiently used for maximum biomass and lipid production [Citation10]. Although free and readily available, solar energy is not easy to manage given that its intensity can be very high during summer and very low during winter. The solar spectrum also contains other wavelengths, such as the infrared (IR) and near-infrared (NIR), which are detrimental to algae [Citation11–13]. There is a need, therefore, for cost-effective technologies that only allow the optimised use of solar PAR in algaculture.
Studies have recommended the use of light filtration technologies in algaculture for optimised biomass and lipid yield [Citation5, Citation6, Citation12]. Solar light filters are expected to allow the ideal spectral quality (PAR region) to reach the algae under culture. In countries such as Zimbabwe which experience high insolation and a climate favourable for solar energy utilisation [Citation14], light filtration technology can be used for the optimised production of microalgal biofuel biomass. Sero et al. [Citation5] further highlighted three types of filters which can be employed in this optimised solar radiation use, namely; glass absorption filters, reflective/thin-film filters and thermochromic filters. These filters can ensure that only the PAR region of the solar spectrum is made available to algae. Glass absorption filters operate by allowing specific wavelengths to pass through whilst absorbing the undesired wavelengths. Reflective filters, on the other hand, operate by allowing specific wavelengths to pass whilst reflecting the undesired wavelengths. Reflective filters are more accurate and efficient in their ability to isolate a narrow region of the spectrum as compared to glass absorption filters [Citation15]. Thermochromic filters operate by regulating the amount of light transmitted or reflected, based on atmospheric temperature [Citation16–18]. They are a new form of light filtration technology in algaculture and thus, are still relatively expensive.
There is, however, a dearth of literature on the practical application of these light filtration technologies in algaculture. Considering this research gap, the aim of this study was to evaluate the effectiveness of light filters in enhancing the outdoor cultivation of a native Chlorella sp. under wastewater mediated algaculture. Considering that urban wastewater in most developing countries is discharged containing high levels of nutrients [Citation19–22], it was used in this study as a cheap growth medium. As the microalgae proliferate under spectral filters, they can effectively sequester nutrients from wastewater. Thus, light filtration technology carries the potential to enhance the performance of microalgae in simultaneous wastewater treatment and algal biomass production. This study evaluated the performance of a native Chlorella strain under different solar light filters against that of an imported Chlorella vulgaris. The use of this imported species in phycoremediation and biofuels production has been reported before in literature [Citation23–27], thus making it an ideal candidate for comparative purposes.
Materials and methods
Wastewater collection and nutrient determination
Urban wastewater used as a growth medium in this study was collected (in January 2020), in clean 10 L plastic containers, from a discharge point at Fernlea Treatment Plant (FTP) in Chinhoyi, Zimbabwe. The wastewater was then transported within 3 h of collection, at 4 °C, to Chinhoyi University of Technology (CUT), Biology Laboratory. Upon arrival at the laboratory, the collected wastewater was filtered using a 0.45-µm vacuum filtration system (VWR International, Belgium) to remove suspended solid particles which interfere with uniform light distribution in algaculture vessels. To destroy any living microorganisms present, filtered wastewater was sterilised by autoclaving at 121 °C for 25 min. After cooling, it was then stored at 4 °C pending further nutrient determination.
A 300-mL sample of the sterilised wastewater was then used to measure Total Nitrogen (TN), Ammonium (NH4-N), Total Phosphates (TP) and Reactive Phosphates (RP). These parameters were measured using calorimetric based techniques (DR 850 calorimeter, Hach, USA). Duplicate measurements were made for each parameter.
Inoculum preparation
The native Chlorella sp. used in this study was collected from CUT, Biology Department algae repository and had been isolated from Manyame River. The C. vulgaris used for comparative purposes in this study was purchased from Carolina Biological Supply (Burlington, North Carolina, USA, Catalogue No. 15–2075). Numerous studies on wastewater-based algaculture using this species have been reported before, thus making it an ideal comparative candidate [Citation23–27].
To prepare seed cultures, 50 mL of the unialgal stock cultures were transferred to 500-mL Schott bottles containing 400 mL of Bold Basal Medium (BBM). The seed cultures were then exposed to white fluorescent lighting at 25 °C for 21 days and were considered ready for use as inoculum for subsequent experiments due to the progression of their colour intensities.
Solar filters for the algaculture system
To evaluate the effect of different solar wavelengths on algal biomass production and ultimately wastewater treatment, Poly Vinyl Chloride (PVC) films with reflective photoselectivity (Le’Stat, South Africa) were used as solar filters. Rectangular support frames of 30 × 20 x 20 cm were fabricated and then covered by the PVC films of different photoselectivity (blue, green, red and yellow) including a transparent one that was used as the control. Thus, the experiment included five treatments ().
Within each treatment, seven Erlenmeyer flasks measuring 250 mL containing 200 mL of filtered and autoclaved wastewater were used as miniature PBRs for culturing algae. These miniature PBRs had the interior of their necks fitted with loosely packed sterile cotton wool lightly sprayed with ethanol (70% v/v). The ethanol sprayed cotton wool allowed the cultures access to atmospheric air whilst giving protection against microbial contaminants. For each of the strains to be evaluated (native and imported Chlorella strains), 10% (Vinoculum/Vmedia) was inoculated into different PBRs at an initial optical density (OD) value of 0.08. The inoculated miniature PBRs were then placed, in triplicate for each species, in each of the light filtering boxes. Within each treatment, a seventh miniature PBR containing only the autoclaved wastewater was included for the measurement of daily temperature. Temperature readings were recorded from only these PBRs to minimise the risk of contaminating the microalgal cultures.
The miniature PBRs under each treatment were then exposed to solar radiation during the summer month of February until the cells had reached the death phase. To prevent cells from clumping, the miniatures PBRs within each treatment were gently shaken by hand twice daily. After every two days, 5-mL samples were withdrawn from each miniature PBR for assessing the growth performance of algae. The withdrawn samples were replaced with equal amounts of filtered and autoclaved wastewater.
Microalgal growth and nutrient removal assays
Growth patterns of the native and imported Chlorella sp. were determined by measuring OD of algae using a Cecil (CE 1021) UV/Visible Spectrophotometer (Cecil Instruments, England) at a wavelength of 680 nm and a light path of 1 cm [Citation28, Citation29]. The growth rate (GR, per day) was calculated as suggested by Wang et al. [Citation29], by fitting the OD observed during the log phase of growth to an exponential function:
(1)
(1)
where N1 and N0 are the OD680 values at early and late exponential phase, respectively, whilst t1 and t0 are the corresponding days [Citation29]. Algaculture was carried out until conclusive death phases were observed after which algal biomass was harvested through centrifugation (BHG ROTO UNI II, Germany) at 4500 rpm for 20 min. The harvested biomass was dried in an oven at 60 °C for 48 h as recommended by Storms et al. [Citation30]. This dry biomass was then stored at room temperature pending lipid quantification.
The supernatant from centrifugation was used to determine TN, NH4-N, RP and TP using a DR850 calorimeter (Hach, USA). The nutrient removal efficiency was then calculated using the following equation:
(2)
(2)
where
is initial (before treatment) nutrient concentration and
is the final day (after treatment) nutrient concentration.
Lipid quantification
Lipid quantification was carried out using the modified version of Bligh and Dyer’s gravimetric technique as described by Storms et al. [Citation30]. Approximately 50 mg of dry algal biomass was measured and then transferred into a mortar pre-washed with hexane. The biomass was ground for 5 min using a pestle. Hexane was then added to the mortar and the resulting slurry homogenised. The resulting mixture was centrifuged (Eppendorf 5804 R, Brinkmann Instruments, Westbury, NY) for 20 min at 10 000 rpm. The supernatant was then pipetted into a pre-weighed metal weighing dish and placed in the fume hood to evaporate the hexane. The mass of the oil extracted was determined gravimetrically after hexane had completely evaporated. The total lipid content of samples was expressed as a percentage using the equation highlighted below:
(3)
(3)
Statistical analysis
The data generated met the assumptions of the two-way analysis of variance (ANOVA) with the treatments and two species used in this study being the two independent factors. Hence, the two-way ANOVA was used to compare mean differences in algal growth rate, lipid yield and percentage nutrient removal. Differences were considered statistically significant at the p < 0.05 level. When mean differences were considered statistically significant, Tukey’s HSD test was carried out for post hoc analysis. Genstat Discovery Edition v4 was used to carry out the statistical analyses for this study.
Results
Wastewater quality
Nutrient levels of wastewater are shown in . The results show a TN value of 1.268 mg L−1, NH4-N value of 0.081 mg L−1, TP value of 1.164 mg L−1 and RP value 0.932 mg L−1. TN and NH4-N values were below the acceptable Zimbabwe National Water Authority (ZINWA) discharge standard of ≤10 mg/L, whereas the TP value was above the acceptable ZINWA discharge standard of ≤0.5 mg/L. The sterilised wastewater had a pH value of 6.7, which fell within ZINWA’s acceptable discharge range of 6 − 9.
Table 1. Means of the physiochemical parameters of the sterilised wastewater analysed prior to its use as medium.
Algal growth performance
shows the growth patterns of the native and imported Chlorella strains under differently coloured light filters. The average mid-day temperature recorded within the PBRs during this experiment was 42 °C. Both strains used in this study exhibited no lag phase under wastewater algaculture. Under all forms of filtered solar radiation, the strains demonstrated an exponential growth phase during the first 4 days (). There was a significant OD difference over time across all the coloured light filters with the native Chlorella strain recording the highest OD value of 0.48 ± 0.04 under the green coloured solar filter. In comparison, the imported Chlorella sp. had its highest OD value of 0.17 ± 0.03 under the same green coloured solar filter. The lowest OD values for both strains were recorded under the transparent solar filter; with the native and imported Chlorella sp. achieving OD values of 0.084 ± 0.01 and 0.071 ± 0.002, respectively. By day 6, both strains across the different filters had reached a decline phase and by day 8, the cells had turned whitish; a sign of cell death ().
Figure 2. Growth profiles of the native and imported Chlorella sp. under green (a), blue (b), yellow (c) and red (d) coloured and transparent (e) solar light filters. Figures (a) - (d) demonstrate an exponential growth phase during the first 2 days and (e) shows no increase in growth within that period. Each data point is a mean value of three replicates. Error bars indicate ± SD of the means.
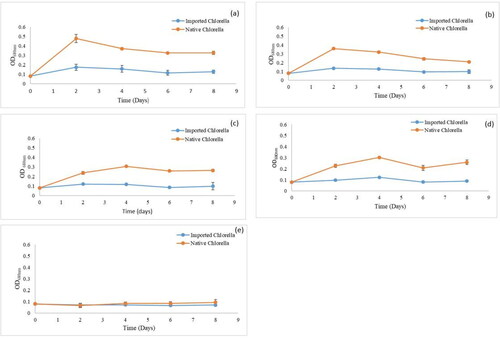
Statistical analyses also showed significant differences in mean growth rates (p < 0.05) of the two strains under different wavelengths of filtered light (). The native Chlorella sp. had a better growth performance across the blue, green, yellow and red coloured light filters. Both strains did, however, exhibit their best proliferation under the green coloured filter (). Under the green coloured light filter, the native Chlorella sp. had the highest exponential growth rate of 0.892 d−1 as compared to the 0.379 d−1 achieved by the imported Chlorella strain. Both strains also attained their second-best growth rates under the blue coloured light filter. The native Chlorella sp. managed an exponential growth rate of 0.754 d−1 as compared to the 0.267 d−1 achieved by the imported Chlorella strains. Pairwise comparisons did, however, prove that differences in mean growth rates of the individual strains under the green and blue coloured light filters were not statistically significant (p > 0.05). The native and imported Chlorella strains failed to proliferate under the transparent light filters, achieving growth rates of 0.13 d−1 and 0.05 d−1, respectively.
Figure 3. Growth rate comparisons of the native and imported Chlorella sp. under green, blue, yellow, red and transparent light filters. Values are means ± SD. Different letters above bars indicate statistically significant differences.
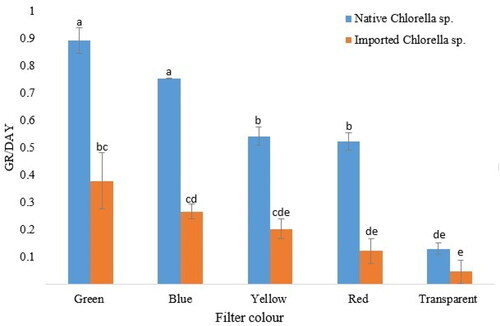
Cells that were cultured under yellow, red and transparent light filters had poor GR. Thus, lipid assays for the native and imported Chlorella strains were carried out for biomass generated under green and blue coloured light filters.
Lipid assays
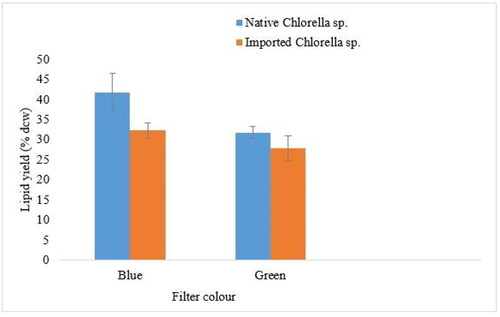
The lipid content of both Chlorella strains under green and blue coloured light filters is shown in . Lipid yield as a percentage of the algal biomass dry cell weight (dcw) showed statistically significant mean differences (p < 0.05) between treatments. A maximum lipid yield of 41.87% dcw was generated by the native Chlorella strain cultured in wastewater under the blue filter. The imported Chlorella strain managed a yield of 32.29% dcw under the blue coloured light filter. Under the green coloured filter, the native and imported Chlorella strains managed lipid yields of 31.81% dcw and 27.98% dcw, respectively.
Phycoremediation capacity of the strains
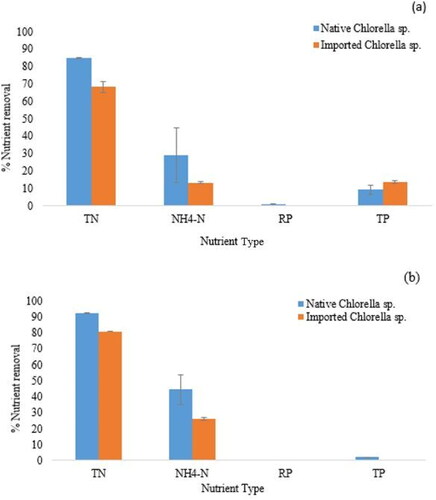
The TN, NH4-N, TP and RP removal efficiencies of the native and imported Chlorella strains after 8 days of wastewater-mediated algaculture under different light conditions are depicted in . The native and imported Chlorella strains failed to efficiently remediate P under blue and green coloured filters. Both strains efficiently remediated TN and NH4-N under blue and green filters. There was a significant difference (p < 0.05) between the removal efficiencies of TN and NH4-N by the two strains, with the native being more efficient. The native Chlorella strain achieved a 92.17% and 44.6% removal efficiency for TN and NH4-N, respectively, under the blue filter. Under the green coloured filter, this strain managed an 84.65% and 28.98% removal efficiency for TN and NH4-N, respectively. In comparison, the imported Chlorella strain managed an 80.81% and 26.10% removal efficiency for TN and NH4-N, respectively, under the blue filter. Under the green filter, the imported Chlorella strain managed a 68.30% and 13.04% removal efficiency for TN and NH4-N, respectively.
Discussion
The best growth performance of both strains was observed under green and blue coloured light filters (). To achieve maximum microalgal proliferation, especially when culturing chlorophyll-rich chlorophytes, blue and red light are more ideal, since chlorophyll absorbs mainly these light wavelengths [Citation31]. Chlorophytes generally possess a sizeable amount of chlorophylls a and b and all these forms of chlorophyll are good absorbers of light in the blue and red colour regions of the solar spectrum. Previous studies using photoselective polyester plastic filters indicate that green coloured light filters are capable of transmitting 40 − 42% of the blue portion of the solar spectrum [Citation32, Citation33]. They are also capable of transmitting about 6% of the red portion of the solar spectrum [Citation32]. Photons in the blue region of the solar spectrum are generally high energy carriers and hence more efficient in photosynthesis, ultimately improving biomass productivity [Citation34]. It has also been reported that blue light promotes gene transcription and enhances the regulation of activated enzymes, hence improving microalgal growth [Citation35]. A study by Chi et al. [Citation36] using similar coloured polyethylene (PE) light filters indicates that the blue coloured filter transmits about 84.4% of solar PAR. More than 29% of the PAR transmitted by this filter falls in the 400 − 500 nm range which is basically made up of violet/blue wavelengths [Citation5, Citation36]. As mentioned earlier, blue light has been reported in literature as an ideal wavelength for efficient algal proliferation [Citation31, Citation34, Citation35]. In addition to transmitting a large portion of the essential PAR region, the blue coloured filter is also capable of blocking ultraviolet and near IR regions of the solar spectrum [Citation36] thus allowing efficient microalgal proliferation. According to Chi et al. [Citation36], the ultraviolet and near IR region transmitted by blue coloured light filters can be as low as 2.1% and 13.5% of the total solar radiation, respectively. Therefore, efficient use of light transmitted by the blue coloured filter resulted in improved proliferation by the two cultured chlorophytes in this study.
Low microalgal growth rates of 0.52 d−1 for the native Chlorella sp. and 0.12 d−1 for imported Chlorella sp. were observed under the red coloured filter in this study. A study by Gautier et al. [Citation32], which made use of photoselective polyester filters, suggests that red coloured plastic light filters are capable of transmitting 64% of the red light available in the solar spectrum. All forms of chlorophyll are good absorbers of light in this 630–675 nm (red light) region of the solar spectrum. Thus, high microalgal growth rates were expected under the red light filter in this study. However, lower algal growth rates were observed. Although red coloured photoselective light filters are efficient transmitters of the red wavelength, they are also efficient transmitters of the IR portion of the solar spectrum. Red coloured light filters can transmit up to 94% of the IR available in the solar spectrum [Citation32]. IR is detrimental to algal cells [Citation11, Citation12] and hence, poor algal proliferation was observed in this study under the red coloured filter.
Poor growth rates were also observed for both strains under the yellow light filter. The native and imported Chlorella sp. attained growth rates of 0.54 d−1 and 0.20 d−1, respectively, under the yellow filter. A previous report which made use of PE coloured photoselective filters suggests that the yellow coloured filter is capable of transmitting 53% of the blue portion of the solar spectrum [Citation33]. Chi et al. [Citation36] also reports that these yellow coloured plastic light filters are capable of transmitting as much as 86.5% of solar PAR. Thus, high growth rates under the yellow coloured plastic light filter were also expected in this study. The poor growth rates which were, however, observed in this study were likely as a result of the yellow filter transmitting more of the less useful portion of solar PAR. Of the 86.5% of PAR transmitted by yellow coloured filters, a major share of 36.2% is made up of green light, whereas violet/blue light accounts for 15.5% only [Citation36]. Green light is the least preferred PAR region since it is weakly absorbed by photosynthetic pigments [Citation37]. Photosynthetic organisms appear green because they reflect green light and hence, this light wavelength often leads to poor biomass production [Citation38]. As such, lower growth rates were also observed under the yellow coloured filter in this study.
The lowest algal growth rates were observed under the transparent solar filter. The maximum growth rates of the native and imported Chlorella sp. under the transparent light filter were 0.13 d−1 and 0.05 d−1, respectively. Although capable of high transmission efficiencies of 96% and 98% for the available blue and red solar wavelengths, respectively, transparent light filters also allow 98% of the available IR radiation to reach cells [Citation32]. This high proportion of IR made available to the PBR system eventually leads to cell death. Thus, poor microalgal growth rates were observed in this study under the transparent solar filter.
It is also important to note that in addition to the wavelength quality, intensity also plays a crucial role in microalgal proliferation. Light intensity influences the photosynthetic capabilities of microalgae and, consequently, their proliferation. Although the intensity of filtered light was not assessed in this study, a basic biophotonics principle dictates that increasing light intensity leads to increased photosynthesis right up to a certain point where other parameters (such as CO2 concentration and water availability) become limiting [Citation39]. Continued exposure of microalgae to this excess light will, however, lead to the development of reactive oxygen species and cause damage to the photosynthetic apparatus of the microalgae [Citation40]. It is on this backdrop that this study concludes that there were ideal light intensities under the green and blue coloured light filters since maximum proliferation was observed under these.
In brief, the native Chlorella sp. exhibited total dominance in growth over its foreign counterpart across all the coloured solar filters. This is probably due to the native strain’s good adaptability to local environmental conditions. The native strain was also isolated from urban wastewater draining Manyame River, hence its exquisite proliferation in urban wastewater. Unlike the native strain, the source of the imported Chlorella strain is unknown. It might have been isolated from a clean freshwater source, thus rendering it less adaptable under wastewater environments. Both the Chlorella strains, however, had lower growth rates under the red, yellow and transparent light filters. Since growth rate is an important selection criterion for microalgal strains in biofuels biomass production, only biomass generated from the blue and green coloured filters was used for further biochemical analyses.
There was a statistically significant difference (p < 0.05) in the lipid content observed under the blue and green coloured filters (). The highest mean percentage lipid yield was observed in cultures under the blue coloured filter. The native Chlorella strain did, however, produce the highest lipid yield of 41.87% dcw under this filter. The maximum lipid yield generated by the native Chlorella strain under the blue filter is higher than the percentage lipid yield reported by Ansari et al. [Citation41] when they cultured their Chlorella sp. in urban wastewater. It is important to note that both the green and blue coloured filters transmit a higher proportion of violet/blue light. Blue light enhances the activity of the enzymes carbonic anhydrase and ribulose bisphosphate carboxylase/oxygenase (Rubisco). These enzymes play a crucial role in the microalgal carbon cycle and ultimately, lipid yield [Citation6]. Thus, high lipid yields were observed for both strains under the green and blue coloured filters. Higher lipid yield performances under the blue filter may be attributed to the intensity or proportion of blue light being more optimal under this filter than under the green coloured light filter. The native strain may have also generated a higher lipid yield than its foreign counterpart due to it being better adapted to wastewater and subtropical climate in Zimbabwe.
Since microalgal growth rates and lipid yields were narrowed down to the green and blue coloured light filters for both Chlorella strains, variations in N and P removal were also analysed for these two light filters. There were significant reduction efficiencies (p < 0.05) in TN and NH4-N by the two strains with the local strain being the most efficient in N recovery from urban wastewater. The best NH4-N and TN removal efficiencies were achieved by the native Chlorella strain cultured under the blue coloured filter. The native Chlorella strain was isolated from a wastewater polluted water source, hence its ability to efficiently thrive in an urban wastewater environment. Coupled with that, this strain is also well adapted to subtropical environments, hence its ability to assimilate nutrients more efficiently than its foreign counterpart. Microalgae utilise N in its inorganic form as nitrates (NO3), nitrites (NO2) and ammonia (NH4-N). However, NH4-N is the most preferred form since less energy is required for its uptake [Citation42]. The removal of NH4-N during algaculture follows two main pathways which are direct utilisation by algal cells and NH3 stripping [Citation42]. Nutrient stripping occurs when the pH value is over 9. The pH of the wastewater used in this study was 6.7, hence NH4-N removal was largely a result of direct assimilation into algal cells. The observed results also highlight that for both Chlorella strains, TN uptake was higher than NH4-N uptake. This signifies that the strains used in this study may have preferred other forms of N such as NO3 and NO2.
There was no significant P utilisation by either the native or imported Chlorella strain under both blue and green coloured filters in this study. P is a very crucial nutrient for the growth of microalgae, since it facilitates energy transfer, formation of cell membranes, generation of nucleic acids and the syntheses of valuable products such as astaxanthin and PUFAs in cells. Therefore, microalgal growth in wastewater not only plays a pivotal role in wastewater treatment but also makes efficient use of P in cells [Citation42]. P removal follows two main pathways in algaculture. One follows the assimilation of P by microalgae and its subsequent conversion into biomass and other intracellular polyphosphate compounds. The other pathway follows precipitation of P due to pH conditions as high as 9 [Citation42, Citation43]. Since the pH of the wastewater used in this study was below 7, P removal was expected to be a result of direct assimilation into microalgal cells. Poor utilisation of P in this study can be attributed to the suboptimal N:P ratio, falling outside the 6.8–10 range. Simultaneous removal of N and P can only be ensured if their ratio falls within the ideal range [Citation44]. Other studies on chlorophytes show that when they are cultured in a low N:P environment, continuous N limitation is observed resulting in a high internal phosphate pool [Citation44, Citation45]. This leads to subsequent N removal rates being higher. Thus, adding the limiting nutrient N to the medium may lead to maximum P utilisation. However, this would increase the overall cost of biomass production.
Overall, the native Chlorella sp. was the best performing strain in this study in terms of growth rate, lipid yield and wastewater nutrient removal. To realise maximum biomass generation, high lipid yield and efficient wastewater phycoremediation, this native Chlorella sp. should be cultured under the blue coloured solar filter. Hence, light filtration technology enhances the outdoor performance of microalgal isolates in simultaneous wastewater treatment and biofuels production. Despite its good performance, the exponential growth phase of the native Chlorella was generally short-lived () hindering the potential for maximum biomass generation and wastewater phycoremediation. The short exponential growth phase was influenced by temperature upsurge with time as the light filters failed to effectively regulate portions of the solar spectrum which are detrimental to cells. An average mid-day temperature of 42 °C was recorded in this study and this value fell way above the 30 °C optima for mesophilic Chlorella [Citation46]. There is, therefore, need for effective light filtration techniques which can regulate the IR and NIR portions of the solar spectrum which are responsible for PBR temperature upsurge. The use of thermochromic filters as proposed by Sero et al. [Citation5] can be a cost-effective method in which spectral quality and PBR temperature can be effectively regulated for optimum solar-based algaculture. These filters are reflective and absorptive under high and low temperatures, respectively, making them more ideal in solving the overheating challenge faced by algaculture systems during this study.
Conclusions
The importance of biophotonic technologies in solar-based algaculture for biofuels production was investigated in this study. This study showed that the reflective blue coloured light filter enhances biomass production, lipid productivity and nitrogen recovery from urban wastewater. The results obtained in this study also indicate that the native strain is a better performer, in terms of biomass production, lipid yield and nitrogen recovery from wastewater than its foreign counterpart due to its adaptability to local environmental conditions. However, during this study, elevated temperatures within the PBRs remained a challenge indicating that although light filtration is crucial in solar-based algaculture, there is a need for effective light filters that can regulate both the spectral quality and temperature in algaculture systems. The use of thermochromic filters can be a more effective way in which solar radiation can be effectively used in algae-based biofuels production. It is envisaged that the thermochromic filters would regulate solar wavelengths made available to the algae under culture together with temperatures within PBRs.
Data availability statement
The data that support the findings of this study are openly available in [figshare] at [https://doi.org/10.6084/m9.figshare.20359293.v1].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cheah WY, Ling TC, Show PL, et al. Cultivation in wastewaters for energy: a microalgae platform. Appl Energy. 2016;179:609–625.
- Nagi GK, Minhas AK, Gaur S, et al. Integration of algal biofuels with bioremediation coupled industrial commodities towards cost-effectiveness. Front Energy Res. 2021;9:489.
- Sero ET, Siziba N, Bunhu T, et al. Isolation and screening of microalgal species, native to Zimbabwe, with potential use in biodiesel production. All Life. 2021;14(1):256–264.
- Hu Q, Guterman H, Richmond A. A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotech Bioeng. 1996;51(1):51–60.
- Sero ET, Siziba N, Bunhu T, et al. Biophotonics for improving algal photobioreactor performance: a review. Int J Energy Res. 2020;44(7):5071–5092.
- Vadiveloo A, Moheimani NR, Cosgrove JJ, et al. Effect of different light spectra on the growth and productivity of acclimated nannochloropsis sp. (eustigmatophyceae). Algal Res. 2015;8:121–127.
- Duarte B, Feijão E, Goessling JW, et al. Pigment and fatty acid production under different light qualities in the diatom phaeodactylum tricornutum. Appl Sci. 2021;11(6):2550
- Vadiveloo A, Moheimani NR, Alghamedi R, et al. Sustainable cultivation of microalgae by an insulated glazed glass plate photobioreactor. Biotechnol J. 2016;11(3):363–374.
- Płaczek M, Patyna A, Witczak S. Technical evaluation of photobioreactors for microalgae cultivation. E3S Web Conf. 2017;19:02032.
- Kim ZH, Park YS, Ryu YJ, et al. Enhancing biomass and fatty acid productivity of tetraselmis sp. in bubble column photobioreactors by modifying light quality using light filters. Biotechnol Bioproc E. 2017;22(4):397–404.
- Huang Q, Jiang F, Wang L, et al. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering. 2017;3(3):318–329.
- Michael C, Del Ninno M, Gross M, et al. Use of wavelength-selective optical light filters for enhanced microalgal growth in different algal cultivation systems. Bioresour Technol. 2015;179:473–482.
- Nwoba EG, Parlevliet DA, Laird DW, et al. Can solar control infrared blocking films be used to replace evaporative cooling for growth of nannochloropsis sp. in plate photobioreactors? Algal Res. 2019;39:101441
- Chiteka K, Enweremadu CC. Prediction of global horizontal solar irradiance in Zimbabwe using artificial neural networks. J Clean Prod. 2016;135:701–711.
- Hunten DM. Canadian scientists report-XXIII-interference filters. J R Astron Soc Canada. 1960;54:177–187.
- Kalyani VL, Sharma V. Different types of optical filters and their realistic application. J Manage Eng Inf Technol. 2016;3:12–17.
- Kamalisarvestani M, Saidur R, Mekhilef S, et al. Performance, materials and coating technologies of thermochromic thin films on smart windows. Renewable Sustainable Energy Rev. 2013;26:353–364.
- Saeli M, Piccirillo C, Warwick M, et al. Thermochromic thin films: synthesis, properties and energy consumption modelling. In: Mendez-Vilas A, editor. Materials and processes for energy: communicating current research and technological developments. Bodajoz: formatex Research Center; 2013. p. 736–746.
- Dlamini S, Nhapi I, Gumindoga W, et al. Assessing the feasibility of integrating remote sensing and in-situ measurements in monitoring water quality status of lake chivero, Zimbabwe. Phys Chem Earth. 2016;93:2–11.
- Dube T, Chibanda M, Manhire B, et al. Sewage effluent causes metal pollution of a Sub-tropical river system in Zimbabwe. Bull Environ Contam Toxicol. 2020;104(3):339–344.
- Hamandishe VR, Saidi PT, Imbayarwo-Chikosi VE, et al. A comparative evaluation of carcass quality, nutritional value, and consumer preference of oreochromis niloticus from two impoundments with different pollution levels in Zimbabwe. Int J Food Sci. 2018;2018:7862971.
- Siziba N. Effects of damming on the ecological condition of urban wastewater polluted Rivers. Ecol Eng. 2017;102:234–239.
- Ayatollahi SZ, Esmaeilzadeh F, Mowla D. Integrated CO2capture, nutrients removal and biodiesel production using chlorella vulgaris. J Environ Chem Eng. 2021;9(2):104763.
- Church J, Hwang JH, Kim KT, et al. Effect of salt type and concentration on the growth and lipid content of chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour Technol. 2017;243:147–153.
- Fathi AA, Azooz MM, Al-Fredan MA. Phycoremediation and the potential of sustainable algal biofuel production using wastewater. Am J Appl Sci. 2013;10(2):189–194.
- Fazal T, Rehman MSU, Javed F, et al. Integrating bioremediation of textile wastewater with biodiesel production using microalgae (chlorella vulgaris). Chemosphere. 2021;281:130758.
- Lu Q, Zhou W, Min M, et al. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour Technol. 2016;201:33–40.
- Hu X, Meneses YE, Stratton J, et al. Acclimation of consortium of micro-algae help removal of organic pollutants from meat processing wastewater. J Clean Prod. 2019;214:95–102.
- Wang L, Min M, Li Y, et al. Cultivation of green algae chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol. 2010;162(4):1174–1186.
- Storms ZJ, Cameron E, de la Hoz Siegler H, et al. A simple and rapid protocol for measuring neutral lipids in algal cells using fluorescence. J Vis Exp. 2014;87:51441.
- Blair MF, Kokabian B, Gude VG. Light and growth medium effect on chlorella vulgaris biomass production. J Environ Chem Eng. 2014;2(1):665–674.
- Gautier H, Rocci A, Grasselly D, et al. Effect of adding heating pipes on the temperature and the physical and chemical traits of tomato fruits. In: International Conference on Sustainable Greenhouse Systems-Greensys 2004. Vol. 691. 2004. p. 59–66.
- Rajapakse NC, Young RE, Mcmahon MJ, et al. Plant height control by photoselective filters: current status and future prospects. HortTechnology. 1999;9(4):618–624.
- Kang Z, Kim BH, Ramanan R, et al. A cost analysis of microalgal biomass and biodiesel production in open raceways treating municipal wastewater and under optimum light wavelength. J Microbiol Biotechnol. 2015;25(1):109–118.
- Teo CL, Atta M, Bukhari A, et al. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol. 2014;162:38–44.
- Chi B, Zhang X, Shi Q, et al. Colored plastic films affect demographic characteristics of Aphis gossypii on cucumber plants. Int J Pest Manage. 2019;65(4):338–347.
- Terashima I, Fujita T, Inoue T, et al. Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009;50(4):684–697.
- Johkan M, Shoji K, Goto F, et al. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ Exp Bot. 2012;75:128–133.
- Metsoviti MN, Papapolymerou G, Karapanagiotidis IT, et al. Effect of light intensity and quality on growth rate and composition of chlorella vulgaris. Plants. 2020;9:1–17.
- Lingvay M, Akhtar P, Sebők-Nagy K, et al. Photobleaching of chlorophyll in Light-Harvesting complex II increases in lipid environment. Front Plant Sci. 2020;11:1–14.
- Ansari AA, Khoja AH, Nawar A, et al. Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl Water Sci. 2017;7(7):4151–4158.
- Ding J, Zhao F, Cao Y, et al. Cultivation of microalgae in dairy farm wastewater without sterilization. Int J Phytoremediation. 2015;17(1–6):222–227.
- Mahapatra DM, Chanakya HN, Ramachandra TV. Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J Appl Phycol. 2013;25(3):855–865.
- Cai T, Park SY, Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renewable Sustainable Energy Rev. 2013;19:360–369.
- Lavoie A, De La Noue J. Hyperconcentrated cultures of scenedesmus obliquus. A new approach for wastewater biological tertiary treatment? Water Res. 1985;19(11):1437–1442.
- Ru ITK, Sung YY, Jusoh M, et al. Chlorella vulgaris : a perspective on its potential for combining high biomass with high value bioproducts. Appl Phycol. 2020;1(1):2–11.