Abstract
The diagnosis of pulmonary embolism (PE) employs a combination of clinical assessment, D-dimer assay and imaging with pulmonary ventilation-perfusion (V/P) scintigraphy and/or computed tomography pulmonary angiography (CTPA). It is generally accepted that V/P SPECT and CTPA have high diagnostic accuracy. Nonetheless, there are only limited data directly comparing these two modalities. This prospective cross-sectional study included 184 hospitalized patients with clinically suspected PE. Clinical assessment, electrocardiography (ECG), vein ultrasound, echocardiography, arterial blood gas test, D-dimer assay, perfusion single photon-emission computed tomography/computed tomography (P-SPECT/CT) and CTPA were carried out. PE was diagnosed in 109 of 146 patients (74.66%) by P-SPECT/CT and 47 of 89 patients (52.81%) by CTPA. The sensitivity and specificity of P-SPECT/CT were 82.9%, respectively, 64.7%. The positive predictive value of SPECT/CT was 94.7%, the negative predictive value was 33.3% and the validity was 80.8%. For CTPA the sensitivity was 58.2% and specificity 90%. The positive predictive value of CTPA was 97.9%, the negative predictive value 21.4% and the accuracy (performance) 61.8%. There was no significant difference between the two methods regarding the diagnosis of PE at sub-segmental and segmental level. The sensitivity of P-SPECT/CT was significantly higher compared with CTPA, whereas the specificity was significantly higher at CTPA for diagnosis of PE. The P-SPECT/CT showed excellent diagnostic performance with high sensitivity and a very high positive predictive value. Thus, it could serve as first-line imaging for PE in the number of prevalent cases.
Introduction
Acute pulmonary embolism (PE) and venous thromboembоlism (VTE) are severe and potentially fatal complications being the third most frequent acute cardiovascular syndrome behind myocardial infarction and stroke on a global scale [Citation1, Citation2]. According to the 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism [Citation2], the annual incidence rates for PE range from 39 to 115 per 100 000 people, with an upward trend over time, and the incidence of VTE is almost eight times higher in individuals aged ≥80 years than in the fifth decade of life. Epidemiology modelling has ranked PE high among the causes of cardiovascular mortality in the United States and VTE in some European countries [Citation2].
The diagnosis of PE usually involves a combination of clinical assessment, D-dimer testing and imaging techniques with computed tomography pulmonary angiography(CTPA) or ventilation/perfusion single photon emission computed tomography (V/P SPECT) [Citation3, Citation4]. In recent years, both imaging methods have improved [Citation5]. In the diagnostic algorithm the first step is the use of a clinical prediction score for PE [Citation6] or a predictive model [Citation7].
CTPA has shown a higher diagnostic accuracy and specificity than conventional ventilation-perfusion scintigraphy [Citation4, Citation8, Citation9]. CTPA is suitable for imaging the pulmonary vasculature in cases of suspected PE, because it can adequately visualize the pulmonary arteries down to the subsegmental level [Citation2, Citation10, Citation11]. CTPA has the advantages of speed, possibility for suggesting an alternative diagnosis and high interobserver agreement [Citation4, Citation9, Citation12, Citation13].
Ventilation/perfusion (V/P) scintigraphy has seen a decreasing role in the diagnosis of PE over the last few years owing to some drawbacks [Citation4]. For example, there are high proportions of equivocal studies [Citation14] and the interobserver agreement is average when it is performed as traditional planar scintigraphy using Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) interpretation criteria. However, as already pointed out [Citation4, Citation15], the introduction of three-dimensional (3 D) V/P SPECT promises a high diagnostic accuracy for SPECT.
In addition to clinical probability scores and other diagnostic procedures, there are two main imaging modalities that are used in the diagnosis of PE. These are contrast-enhanced CT and ventilation/perfusion (V/P) scintigraphy. While contrast-enhanced CT is quite common [Citation16–18], V/P scintigraphy is more suitable for patients with renal dysfunction, a critical illness or a contrast-medium allergy [Citation18, Citation19].
Hybrid SPECT/CT devices seek to improve the diagnostic accuracy by enabling an anatomical characterization of scintigraphic abnormalities. Non-contrast-enhanced thoracic CT imaging is useful in differential diagnosis, as it can reveal structural changes characteristic of abnormal pulmonary perfusion resulting from PE, pneumonia, emphysema or tumour [Citation18]. As suggested [Citation18], combined P-SPECT/CT scanning may replace V/P-SPECT especially when ventilation imaging is contraindicated due to clinical instability, dyspnoea, incompliance, neurological deficit or reduced consciousness. In addition, the examination time is cut by approximately half [Citation18].
In this prospective cross-sectional study the aim was to evaluate in clinical conditions sensitivity, specificity and accuracy of CTPA, on the one hand, and perfusion SPECT/CT, on the other, to perform early detection of PE in patients with clinical probability of PE.
Subjects and methods
Ethics statement
Written informed consent was obtained from all patients.
Subjects
The study included hospitalized patients suspected of PE in Pulmonary Department at UMHAT Alexandrovska (third-line referral hospital) from September 2016 to February 2020.
Inclusion criteria
Men or women over 18 years of age were included in the clinical examination. Similar to Gutte et al. [Citation4] the eligibility criteria were suspicion of PE, defined as an acute onset of new (or a worsening of) shortness of breath or chest pain without any obvious cause, cough, syncope, pain, oedema and erythema of lower extremity, combined with a clinical assessment with a Wells score.
Exclusion criteria
Patients with the following diseases were excluded:
Chronic lung diseases that lead to secondary reduction of microcirculation in the lungs such as COPD, severe asthma, bronchiectasis, pulmonary fibrosis;
Significant cardiovascular diseases - unstable angina; recent myocardial infarction, uncontrolled arrhythmias, congestive heart failure with severe systolic dysfunction.
Liver diseases as liver cirrhosis and liver failure;
Chronic renal failure requiring haemodialysis;
Patients after organ transplantation; congenital or acquired immune deficiency;
Systemic connective tissue diseases;
Psychiatric diseases – schizophrenia, severe bipolar disorder, severe depressive episode with or without psychotic symptoms; manic episodes, etc.;
Dependence on alcohol, drugs, psychotropic drugs and other substances with similar effects;
Pregnant women;
Patients who are not able to understand the written informed consent and patients who do not agree to participate in the study.
Among 212 patients referred to our departments, 27 patients were excluded from the study because of impaired renal functions (3 patients), severe COPD (13 patients) or congestive heart failure (8 patients). Six patients were also excluded because they did not decide to participate.
Clinical examination
All patients who consented to diagnostic testing were subjected to physical examination, blood pressure measurement, blood sampling with D-dimer assay, ECG, chest X-ray, Doppler sonography of the lower-extremity veins, echocardiography, arterial blood gas test, CTPA, and P-SPECT/CT without contrast enhancement.
As described by Gutte et al. [Citation4], the definitive diagnosis was determined at a side-by-side consensus reading of all lesions detected on CTPA and P-SPECT/CT, using all available data: electrocardiography, transthoracic echocardiography, Doppler sonography of the lower-extremity veins, D-dimer levels, clinical data and Wells score probability.
CTPA
CTPA was conducted with MD 64 CT Toshiba Aquilion during a deep-inspiration breath-hold (120 kV, 230 mAs/slice, 0.75 mm collimator, 0.5-s rotation time and pitch of 0.94). The images were taken in the cephalocaudal axis. Iodine contrast was administered (Iomeron) with an automatic injection pump at a rate of 4 mL/s up to 100 mL iodine contrast. For best opacification of the lung arteries, we obtained the scan using bolus tracking, entering a circular region of interest in the trunk of the pulmonary artery. The threshold for triggering was preset at 100 Hounsfield units.
All scans were evaluated by the same radiologist who is certified and experienced in CTPA. The diagnosis of PE was based on failure to enhance the entire lumen because of a filling defect [Citation4, Citation20].
Perfusion SPECT/CT
Pulmonary perfusion SPECT/CT included a perfusion SPECT study with simultaneous low-dose CT. Perfusion studies were performed after intravenous injection of 99mTc-macroaggregated albumin during two respiratory cycles. SPECT datasets were corrected for attenuation using the low-dose CT acquisition (without contrast) with iterative reconstruction, as described by Gutte et al. [Citation4]. All P-SPECT/CT images were interpreted by the same nuclear medicine physicians with many years of experience. Diagnostic criteria for PE included the combined findings of a perfusion defect on P-SPECT and corresponding unremarkable chest CT scan [Citation18].
Statistical analysis
Diagnostic performance was calculated as sensitivity, specificity, positive predictive value, negative predictive value and accuracy (performance) as in Gutte et al. [Citation4]. Ninety-five percent confidence intervals were calculated. Differences were considered statistically significant at the p < 0.05 level. All statistical analyses were carried out using SPSS software version 25.0 (IBM SPSS Statistics 25.0).
Results
For the period of this study, 212 patients were screened and 184 met the inclusion criteria. The eligible patients were aged between 24 and 84 years – mean age 57 years and median 58.5 years. Eighty were men (43.5%) and 104 women (56.5%).
The PE diagnosis was confirmed in 47 patients (52.8%) out of the 89 patients by CTPA. The PE diagnosis was excluded in the remaining 42 patients (47.2%). Computed tomography pulmonary angiography assay detected segmental defects in 26 patients (55.3%) and sub-segmental defects in 21 patients (44.7%). The sensitivity of CTPA alone was 58.2% and the specificity 90%. The negative predictive value (NPV) was 21.4%, the positive predictive value (PPV) 97.9%, and the accuracy was 61.8% at 0% non-diagnostic rate. Pulmonary embolism was diagnosed in 109 patients (74.7%) of the 146 patients by P-SPECT/CT. The PE diagnosis was excluded in 33 patients (22.6%) and for P-SPECT/CT alone, 4 of the 146 cases were inconclusive, leading to a non-diagnostic rate of 2.7%. Perfusion SPECT/CT showed segmental defects in 36 patients (33.03%), and sub-segmental defects in 73 patients (67%). Segmental mismatch defects were well visible on the SPECT scans while the distal to sub-segmental mismatch defects could only be detected by SPECT ( and represent our clinical cases). Perfusion SPECT/CT showed a sensitivity of 82.9% and specificity of 64.7%, NPV of 33.3%, PPV of 94.7%, with 80.8% accuracy and non-diagnostic rate of 2.7% ( and ).
Figure 1. CTPA (A) and P-SPECT/CT (B) of Case 1. (A) CTPA of pulmonary artery, main, lobar and segmental branches without visible defects of filling. In the area of left lung lingula parenchyma consolidation and filling defect in the level of subsegmental branches. Small pleural effusion on the left. (B) Perfusion SPECT/CT in the left lung showing perfusion defect in lung lingula with parenchyma consolidation. Small pleural effusion. In the right lung hypoperfusion defects in small branches in lower lobe.
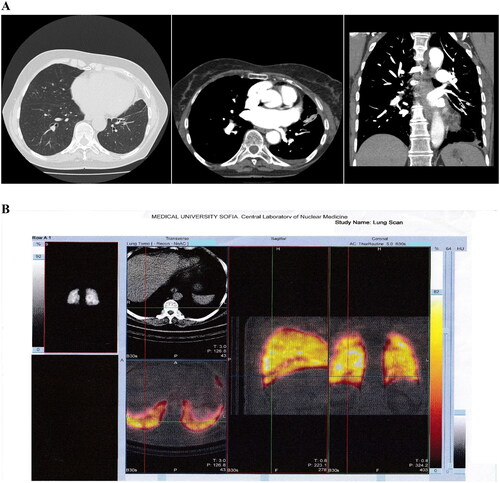
Figure 2. CTPA (A) and P-SPECT (B) of Case 2. (A) CTPA with no filling defects in pulmonary artery, its main, lobar and segmental branches, which are of normal size. (B) Perfusion SPECT/CT showing both side hypoperfusion in the subsegmental level of pulmonary artery.
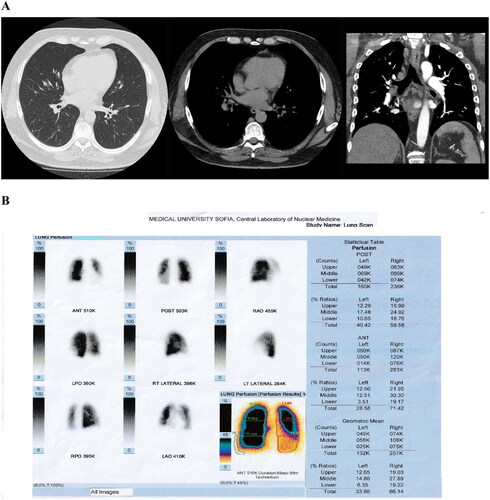
Table 1. Crosstable of imaging methods.
Table 2. Diagnostic value of the imaging methods.
In 13 patients with diagnosed PE in sub-segmental level of P-SPECT/CT, a normal CTPA result was found which accounted for 11.9% of the total diagnosed cases of PE (109 patients) with P-SPECT/CT. The analysis indicated that P-SPECT/CT increased the number of detectable defects on the sub-segmental level by 11.9%.
In 51 patients, head-to-head comparison was made between P-SPECT/CT and CTPA. The results showed that 24 patients (47.1%) had a negative result, and the proven cases of PE were 27 (52.9%). Of these, 11 were segmental in shape and 16 were sub-segmental in shape. For the P-SPECT/CT study, two cases were non-diagnostic. The PE diagnosis was excluded in 11 cases. This diagnosis was confirmed in 38 cases. Of these, 21 cases were at segmental level and 17 cases were at sub-segmental level. In 13 patients (25.49%) with a negative result by CTPA, PE was diagnosed by P-SPECT/CT. Of these, 8 were at sub-segmental and 5 were at segmental level, suggesting that these patients could practically be misdiagnosed. Only one patient with a negative result by P-SPECT/CT had a positive result by CTPA. One patient with a non-diagnostic result by P-SPECT/CT was found positive for PE by CTPA.
Clinical cases
Case 1
Seventy-three-year-old woman after left hip replacement two weeks before presentation. Her complaints were sudden shortness of breath, pain in the left side of the chest in inspiration, tachycardia. Three days before that she had had pain, redness and swelling of the left leg. Comorbidities were arterial hypertension, and permanent atrial fibrillation on the therapy with vit. K antagonists. The diagnosis was established by CTPA and P-SPECT/CT ().
Case 2
Fifty-year-old man with complaints of shortness of breath, palpitations and low blood pressure. Comorbidities were class III obesity, obstructive sleep apnoea, heart failure, arterial hypertension and documented trombophily. The diagnosis was established by CTPA and P-SPECT/CT ().
Discussion
The perfusion single photon emission computed tomography (P-SPECT/CT) is a well-established imaging technique widely used in the modern nuclear medicine diagnostics [Citation4, Citation21]. This nuclear technique is recommended as a “method of choice” for diagnosis in hemodynamically stable patients with suspected pulmonary embolism [Citation22]. As there are no absolute contraindications, this method may be relevant in most patients, especially in the group with contraindications for CTPA [Citation23]. Nuclear medicine techniques are not available at night and on weekends, which makes them not appropriate in the case of emergency [Citation18].
In our prospective study, we compared the diagnostic performance of P-SPECT/CT and CTPA separately and/or in combination in patients with suspected PE. Perfusion SPECT combined with low dose CT had higher sensitivity and lower specificity than that of CTPA.
Only a few studies have compared perfusion SPECT/CT and CTPA diagnostic value. The most recent studies focus on the Ventilation/Perfusion SPECT/CT.
Our findings (sensitivity, specificity and accuracy) showed good agreement with those reported by Gutte et al. [Citation4]. They observed that P-SPECT/CT showed 93% sensitivity, 51% specificity and 68% accuracy, whereas in our study the corresponding values of P-SPECT/CT were 82.9%, 64.7% and 80.8%, respectively. Regarding the CTPA values, Gutte et al. [Citation4] reported sensitivity, specificity, and accuracy of 68%, 100%, and 88%, respectively, as compared to 58.2%, 90% and 61.8%, in our study.
In the most comprehensive study (PIOPED II) evaluating the sensitivity of CTPA the value of this parameter was as high as 83% [Citation12], which is in contrast with our observation (58%). This discrepancy in the sensitivity rate could be due to differences in the examined populations, specific technical implementation, staff experience, etc.
Overall, our study on P-SPECT/CT performed on patients with clinical suspicion for PE demonstrated a high sensitivity but a significantly lower specificity (82.9%, respectively, 64.7%).
The CTPA technique has replaced lung scintigraphy in most hospitals for the diagnosis of PE over the last 20 years. It has been well evaluated and has become the new "gold standard" for detecting pulmonary embolism. This method is a faster, widely available, low cost, with a high frequency of conclusive results. It also reproduces more detailed images with high resolution that make the result easier to interpret [Citation4]. Moreover, CTPA is the tool of choice because of its accuracy and its additional diagnostic capabilities for imaging other conditions which clinically mimic PE, such as acute pneumonia, lung abscess, pleural or pericardial effusion, aortic dissection, cardiovascular disease, rupture of oesophagus and malignant outcome, etc. Such conditions have been reported in 11% to 70% of cases with subsequent CTPA performed in cases with suspected embolism. CTPA directly visualizes the presence of a thrombus as a defect in the filling of blood vessels. It also successfully distinguishes acute from chronic form of PE. Multi-detector CTPA is the primary imaging modality in patients suspected of acute PE. After administration of intravenous contrast, CTPA can be performed for 4 to 6 s, which makes it suitable for hemodynamically nonstable patients. The limitations of the method are related to high radiation dose, namely high breast exposure being not recommended for young women or contraindications (renal failure, severe allergy to contrast, diabetics on metformin), affecting up to 23–31% of patients requiring imaging [Citation12]. In this category of patients, hybrid P-SPECT/CT can replace CTPA because it is associated with less radiation exposure.
Perfusion SPECT/CT is a functional test which does not visualize directly the existing thrombus but indicates a functional impairment as a perfusion defect [Citation4]. Three-dimensional images obtained by single-photon emission computed tomography (SPECT) using gamma isotope transmission can enhance V/Q scintigraphy and have a lower radiation dose. Co-registration of ventilation-perfusion scintigraphy and CT (SPECT/CT) gives precise functional information and reliable morphological data about the lung parenchyma, pleural and mediastinal structures. The complementary CT part to the SPECT provides low-dose exposure and faster performance. In addition, P-SPECT/CT raised the number of detectable perfusion defects at the segmental and sub-segmental levels. The functionality of P-SPECT/CT makes it appropriate to assess defects of perfusion and potentially discover new ones, making it the tracking method of choice.
Some authors considered P-SPECT/CT as an alternative method for primary examination in patients with contrast-related contraindications, as well as for young patients and premenopausal women, due to the low exposure of the whole body. The method can also be used to follow up on recovered patients [Citation24, Citation25].
In case of patients who go through CTPA and do not have PE, the lung perfusion SPECT/CT is characterized by lower exposure and lack of side-effects, and should be promoted as an alternative to the CTPA imaging modality [Citation23].
Conclusions
Our analysis demonstrated that both imaging methods, hybrid perfusion SPECT/CT and CTPA, have high diagnostic value and may be used for the diagnosis of PE. Perfusion SPECT/CT had higher sensitivity than CTPA, while CTPA had higher specificity. Perfusion SPECT/CT demonstrated higher clinical value in detection of small thrombi in sub-segmental level. We concluded that perfusion SPECT/CT is effective in detection of PE and may be alternative first choice in hemodynamically stable patients.
Acknowledgements
We are grateful to the radiologists and technologists at the Departments of Nuclear Medicine and at the Department of Imaging Diagnostics – Medical University Sofia, UMHAT Alexandrovska for the competent assistance.
Data availability statement
The data that support the findings of this study are available from the corresponding author [Sevda Naydenska], upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The work was suppored by Medical University of Sofia - Faculty of Medicine 1 St. G. Sofijski str., Sofia, Bulgaria ID: BG 831385737 8313857370037.
References
- Orenstein B. Diagnosing PE-Is V/Q imaging a better choice, especially for younger women? Radiol. Today. 2009;10(17):14.
- Konstantinides SV, Meyer G, Becattini C, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism, developed in collaboration with the European Respiratory Society (ERS). European Respiratory Journal. Jan 2019. 1901647.
- Bajc M, Neilly JB, Miniati M, et al. EANM guidelines for ventilation/perfusion scintigraphy: part 2. Algorithms and clinical considerations for diagnosis of pulmonary emboli with V/P(SPECT) and MDCT. Eur J Nucl Med Mol Imaging. 2009;36(9):1528–1538.
- Gutte H, Mortensen J, Jensen CV, et al. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med. 2009;50(12):1987–1992.
- Mortensen J, Gutte H. SPECT/CT and pulmonary embolism. Eur J Nucl Med Mol Imaging. 2014;41(S1):81–90.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting, to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98–107.
- Miniati M, Bottai M, Monti S, et al. Simple and accurate prediction of the clinical probability of pulmonary embolism. Am J Respir Crit Care Med. 2008;178(3):290–294.
- Grenier PA, Beigelman C. Spiral computed tomographic scanning and magnetic resonance angiography for the diagnosis of pulmonary embolism. Thorax. 1998;53(Supplement 2):S25–S31.
- Blachere H, Latrabe V, Montaudon M, et al. Pulmonary embolism revealed on helical CT angiography: comparison with ventilation–perfusion radionuclide lung scanning. AJR Am J Roentgenol. 2000;174(4):1041–1047.
- Patel S, Kazerooni EA, Cascade PN. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. Radiol. 2003;227(2):455–460.
- Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8(8):1716–1722.
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317–2327.
- Coche E, Verschuren F, Keyeux A, et al. Diagnosis of acute pulmonary embolism in outpatients: comparison of thin-collimation multi-detector row spiral CT and planar ventilation-perfusion scintigraphy. Radiol. 2003;229(3):757–765.
- PIOPED Investigators Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753–2759.
- Gutte H, Mortensen J, Jensen C, et al. Added value of combined simultaneous lung ventilation–perfusion single-photon emission computed tomography/multislice-computed tomography angiography in two patients suspected of having acute pulmonary embolism. Clin Respir J. 2007;1(1):52–55.
- Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Radiol. 2007;242(1):15–21.
- Strashun AM. A reduced role of V/Q scintigraphy in the diagnosis of acute pulmonary embolism. J Nucl Med. 2007;48(9):1405–1407.
- Palmowski K, Oltmanns U, Kreuter M, et al. Diagnosis of pulmonary embolism: conventional ventilation/perfusion SPECT is superior to the combination of perfusion SPECT and nonenhanced CT. Respiration. 2014;88(4):291–297.
- Hofman MS, Beauregard JM, Barber TW, et al. 68Ga PET/CT ventilation-perfusion imaging for pulmonary embolism: a pilot study with comparison to conventional scintigraphy. J Nucl Med. 2011;52(10):1513–1519.
- Lake DR, Kavanagh JJ, Ravenel JG, et al. Computed tomography and pulmonary embolus: a review. Semin Ultrasound CT MR. 2005;26(5):270–280.
- Reinartz P, Wildberger JE, Schaefer W, et al. Tomographic imaging in the diagnosis of pulmonary embolism: a comparison between V/Q lung scintigraphy in SPECT technique and multislice spiral CT. J Nucl Med. 2004;45(9):1501–1508.
- Bajc M. Potential of hybrid V/P SPECT–low-dose CT in lung diagnostics. Breathe. 2012;9(1):48–60.
- Garcheva M. Impact of single photon emission tomography combined with computed tomography (SPECT/CT) in pulmonary examinations – short review with two case reports. Nucl Med Rev Cent East Eur. 2014;17(2):101–107.
- Kyrle PA, Eichinger S. New diagnostic strategies for pulmonary embolism. Lancet. 2008;371(9621):1312–1315.
- Remy-Jardin M, Pistolesi M, Goodman LR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner society. Radiol. 2007;245(2):315–329.
