Abstract
The aim of the present study was to identify agents that reduce the anthracyclines-induced cardiovascular and renal damage and have the potential to enhance their therapeutic effects. Three fractions of Lycium barbarum (Goji berry), pectin-free, polysaccharide and a combination of the two (1:1, w/w), were tested on rat models of doxorubicin (DOX)-induced cardio- and nephrotoxicity. They were administered p.o. as doses of 2 mg/kg. DOX was applied at a cumulative dose of 20 mg/kg (i.p.). Different biomarkers for cardiotoxicity (creatine kinase, creatine kinase-MB fraction, aspartate aminotransferase, lactate dehydrogenase), nephrotoxicity (creatinine, blood urea nitrogen, uric acid) and the serum potassium levels were evaluated. Histological analysis of hearts and kidneys was performed. The male Wistar rats treated only with DOX showed a drastic increase in all biomarkers for toxicity. Meanwhile, in all cohorts receiving simultaneously any of the plant fractions, the biomarkers for heart and kidney tissue damage were significantly reduced (p < 0.001 for uric acid; p < 0.01 for other parameters). Intriguingly, the pectin-free fraction and the combined one showed the most pronounced decrease in the indicators for toxicity. Histological findings also confirmed these observations, suggesting that the fractions in question need to be investigated as potential enhancers of anthracyclines’ pharmacodynamic effects.
Introduction
Adverse effects caused by the classical anticancer drugs in many cases limit their therapeutic use. Anthracyclines, such as doxorubicin (DOX), daunorubicin, epirubicin, etc., are used alone or in combination as a first-line treatment for different haematological and solid tumors [Citation1]. Their intercalation in the structure of DNA and RNA, leading to inhibition of nucleic acids synthesis, appears to constitute a principal anticancer mechanism of action. Chain breaks are present due to topoisomerase II suppression, as well as reactive oxygen species formation. The latter events cause oxidative stress, membrane and DNA damage, endothelial cell injury, an elevation of intracellular iron and an activation of the apoptotic cascade [Citation2, Citation3]. All these changes in cellular oxidative status are considered to be related to dose-dependent cardiotoxicity and nephrotoxicity. They are life-threatening and limit the use of anthracyclines, compromising the effectiveness of the antitumor therapy [Citation4, Citation5]. Therefore, new approaches to addressing health problems arising from their use are constantly being sought. There are numerous studies on the potential of various natural sources of bioactive substances, which prevent the occurrence of anthracycline-induced cardio- and/or nephrotoxicity [Citation3, Citation6–10].
Recently, there has been a growing interest in the pharmacological activity and organoprotective properties of various substances obtained from natural sources [Citation11–13]. Goji berry (Lycium barbarum, Solanaceae) is an ancient food used in many Asian countries for thousands of years [Citation14]. Due to its rich composition of biologically active substances, this plant is an important component of Chinese traditional medicine [Citation14, Citation15]. In fact, Goji berry has long been а subject of interest in conventional medicine as well, proving its antioxidant [Citation16, Citation17], anti-inflammatory [Citation14, Citation18], anti-tumor [Citation19], lipid lowering [Citation20], immunostimulatory [Citation21] and many other pharmacological effects. The most researched constituents of L. barbarum are its water-soluble polysaccharides, which play a major role in the biological activity of the plant [Citation15, Citation22]. Fruits are also rich in many other useful substances that contribute to the beneficial effects of their consumption [Citation23]. Examples of such valuable substances are carotenoids (mainly zeaxanthin dipalmitate), vitamins, phenolic compounds (such as flavonoids), etc. [Citation24] The antioxidant activity observed with the use of L. barbarum is mainly due to the content of polyphenols. When their amount in the extract increases, there is also an increase in antioxidant activity [Citation25–27].
Bearing in mind the proposed mechanism of anthracycline-induced toxicity, the investigation of the drug’s safety in the presence of antioxidants of natural origin, such as Goji berry, seems reasonable. Hence, the aim of the present study was to evaluate the cardio- and nephroprotective potential of different fractions isolated from L. barbarum. For this purpose, models of DOX-induced cardio- and nephrotoxicity in male Wistar rats were used. We compared the biological effects of fractions containing polysaccharides with those rich in polyphenols. Treatment with a combination of both fractions was also carried out in order to investigate the possibilities of synergism between them.
Materials and methods
Ethics statement
The study was conducted according to the national requirements for the protection and humane treatment of laboratory animals, complying with the European Communities Council Directive 86/609/EEC [Citation28]. For the performed experimental procedures, permission was obtained from the Bulgarian Food Safety Agency (BFSA).
Chemicals
DOX was obtained as doxorubicin hydrochloride concentrate for solution for infusion (2 mg/mL) from Accord Healthcare Limited (United Kingdom). L. barbarum fruits (Lot no. L05042017) were purchased from Paula Fruits Ltd., an official importer of Goji berries for Bulgaria with guaranteed Chinese origin. Before the extraction procedures, all plant materials were identified by Iliya Slavov, Assoc. Prof. in pharmacognosy at the Department of Biology, Faculty of Pharmacy, Medical University of Varna, Bulgaria. Тhe raw plant material was stored at room temperature prior to the experimental work. All other reagents were of analytical grade and were purchased from Sigma-Aldrich (USA).
Preparation of plant extracts
Initially, a liquid polyphenolic fraction of L. barbarum fruits and a dry pectin polysaccharide one were isolated [Citation19].
Preparation of alcohol insoluble solids and pectin-free extract (rich in polyphenols)
Extraction was carried out in a 4-L glass flask. It was placed in an incubator as follows: 500.0 g of sliced and dried L. barbarum fruits were transferred to 3.0 L of ethanol/water 70:30 (v/v) solution (solid–liquid ratio 1:6, w/v) pre-heated at 70 °C. The obtained mixture was kept for 1 h and 30 min at 70 °C and vigorously shaken (every 10 min). The resulting material was allowed to cool down and was incubated overnight at room temperature; then, the mixture was filtered through a nylon cloth to remove solid particles.
The same procedure was repeated with incubation for 1 h. After filtration, the insoluble residue was washed with ethanol/water 70:30 (v/v) (70 °C) solution (solid–liquid ratio 1:5, w/v). Finally, the solid part was incubated with acetone (solid–liquid ratio 1:4, w/v) at 30 °C for 1 h. The solid material was vacuum-filtrated and additionally squeezed from excess of solvent through a cloth. The obtained alcohol insoluble residue was vacuum-dried (40 °C, −0.1 mbar) to a constant weight. The alcohol-water solution obtained after the treatment of alcohol insoluble solids was vacuum concentrated for the full removal of ethanol. The volume of the extract was adjusted to 2 L with distilled water and the extract was denoted as pectin-free extract.
Isolation of pectic polysaccharides
Alcohol insoluble solids were extracted with distilled water (1:25, w/v) at 82 °C for 1 h with continuous stirring and then filtered. The solid residue was re-extracted in the same way with distilled water and then filtered. The combined filtrates were vacuum-concentrated and centrifuged (4000×g, 30 min, 15 °C). After additional filtration of the obtained supernatant through a Büchner funnel, it was precipitated by adding two volumes of cold 96% (v/v) ethanol to one volume of extract. After storage at 4 °C for 24 h, the coagulated polysaccharide was separated by filtration, washed with excess of 70% (v/v) ethanol, and finally with 96% (v/v) ethanol. Then, the precipitate was re-dissolved in water, freeze-dried (Christ® Alfa 1-4 LD plus) to a constant weight, and stored in a desiccator until use.
After analysis of the content of biologically active ingredients that was previously reported [Citation19], a mixture of both fractions was prepared with a ratio of polysaccharides and polyphenols 1:1 (w/w). The doses used for the three fractions were 2 mg/kg body weight (b. w.)—determined relative to available polyphenols, polysaccharides and polyphenols/polysaccharides, respectively. The three resulting fractions were dissolved in distilled water. Then each of them was tested to determine the presence of protective effect against DOX-induced cardio- and nephrotoxicity.
Animals
For the purposes of the study, 48 mature male albino Wistar rats (220–250 g) were used. The animals were obtained from the vivarium of the Medical University of Varna, Bulgaria. They were housed in a controlled environment (temperature 23 ± 2 °C, humidity 50 ± 10% and 12 h light/12 h dark cycle) with unrestricted access to food and water. The animals were acclimated for a week prior to the experimental work.
All experimental procedures were performed between 8:00 and 10:00 a.m.
Experimental design
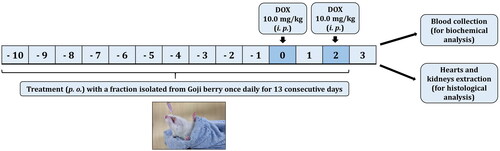
The experimental animals were randomly divided into eight groups (n = 6). All of them were treated with the intended fraction by an oral gavage as follows:
Group 1 (control group) received 1 mL of 0.9% saline once daily for 13 days;
Groups 2, 3 and 4 received 1 mL of pectin-free fraction, polysaccharide fraction and combination of pectin-free extract plus polysaccharide fraction (2 mg/kg, b. w.), respectively, once daily for 13 days;
Groups 5, 6 and 7 received pectin-free fraction, polysaccharide fraction and combination of pectin-free extract plus polysaccharide fraction (2 mg/kg, b. w.), respectively, once daily for 13 days and DOX intraperitoneally (i.p.) (10 mg/kg, two doses) at day 11 and day 13 (cumulative dose: 20 mg/kg, b. w.);
Group 8 (DOX group) received 1 mL of 0.9% saline once daily for 13 days and DOX (10 mg/kg, b. w. - two doses, i.p.) on days 11 and 13 of the experiment (cumulative dose: 20 mg/kg, b. w.).
No deaths were observed in the groups during the experiment.
On day 14, 24 h after the last dose of DOX, all rats were anesthetized by inhalation of diethyl ether. Blood was collected from the postorbital plexus and plasma fractions of all animals were separated by centrifugation at 6000×g for 5 min. Then the resulting specimens were frozen at −20 °C for estimation of the biochemical parameters.
The hearts and the kidneys of all rats were immediately extracted. The organs were washed with ice-cold saline and fixed in 10% neutral buffered formalin for histological analysis. The study design was based on an established and published experimental protocol, which is shown schematically in [Citation3].
Biochemical analysis
The serum biochemical analytes such as biomarkers for cardiotoxicity (creatine kinase, CK; creatine kinase-MB fraction, CK-MB; aspartate aminotransferase, AST; lactate dehydrogenase, LHD) and nephrotoxicity (creatinine, SCr; blood urea nitrogen, BUN; uric acid, UA), as well as potassium levels, were assayed with biochemical analyzer Cobas® 6000 (F. Hoffmann-La Roche Ltd.). They were analyzed in a licensed laboratory in Varna according to the instructions provided with the respective enzyme kits of commercial reagents. Information on the analytical methods used is available at https://usdiagnostics.roche.com/en/core_laboratory/instrument/cobas-6000-analyzer-series.html#menu.
Histopathology of the heart and kidneys
After the extraction and fixation of the left and right heart ventricles, as well as the kidneys, they were embedded in paraffin and separated using a 4–6 µm thick rotary microtome. Thereafter, they were stained with hematoxylin and eosin (H&E). A minimum of 6 fields from each renal section were examined and the severity of change was determined by a blinded observer. All sections were evaluated for structural changes under a Leica DM 1000 LED light microscope and a Leica MC120 HD camera (Leica Microsystems AG, Wetzlar, Germany) and were photographed at 200× or 400× magnification.
Statistical analysis
A four-parameter logistic curve (4PL) generated by Graph Pad Prism 8.01 software (GraphPad Software, USA) was used to plot the graphs and determine the IC50 with a 95% confidence interval. All results are expressed as arithmetic means with standard deviation (±SD). Differences between groups were analyzed using analysis of variance (ANOVA). Six replicates were used for each group, and differences were considered statistically significant at a p < 0.05 level.
Results
Effects on biochemical parameters for cardiotoxicity
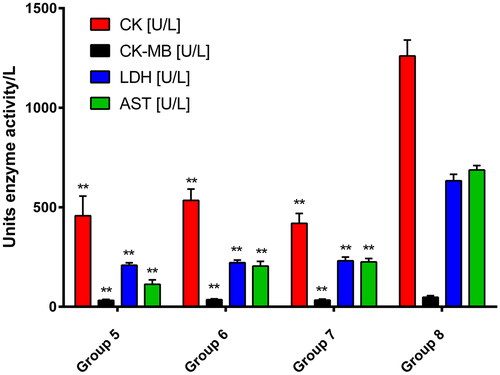
Group 8, treated with the cumulative dose of DOX (20 mg/kg, b.w., i.p.), showed drastically significant increase in the enzymatic activities of CK, CK-MB, LDH and AST compared to the control group 1 (p < 0.001), which received physiological solution only. The simultaneous rise in these values served as a marker for both early and late stages of cardiac tissue damage and disruption of functional integrity and permeability of cell membrane [Citation29]. The anthracycline-induced toxicity was significantly lower in all cohorts with additional intake of any of the plant fractions ().
Table 1. Effects of individual L. barbarum fractions administered p. o. at a dose of 2 mg/kg (b.w.) on the activities of the enzymes CK, CK-MB, LDH and AST in DOX-induced cardiotoxicity (20 mg/kg, b.w., i.p.).
The polyphenol-rich pectin-free fraction (group 5) and the combined one (group 7) showed the most pronounced reduction in markers for myocardial tissue damage. The polysaccharide fraction partially reduced some of the markers, although the reduction was significant compared to the DOX-model (group 8). A graphical comparison between groups of particular importance is illustrated in .
Figure 2. Changes in biochemical markers for cardiotoxicity upon treatment with pectin-free fraction (group 5), polysaccharide fraction (group 6) and combination of both fractions (group 7) compared to the DOX-model (group 8). Values are presented as mean ± SD. One-way ANOVA was performed for comparison between groups. ** p < 0.01 compared to the model.
Effects on biochemical parameters for nephrotoxicity
The same doses of all substances were used to determine the nephroprotective potential of the prepared Goji berry fractions.
The biochemical analysis (; ) showed that the individual fractions of Goji berry managed to reduce the levels of biochemical parameters for renal function and nephrotoxicity when used concomitantly with DOX. The pectin-free fraction (group 5) and the combinated one (group 7) again showed the most pronounced reduction in the indicators, suggesting possible nephroprotection.
Figure 3. Changes in biochemical markers for nephrotoxicity by treatment with pectin-free fraction (group 5), polysaccharide fraction (group 6) and combination of both fractions (group 7) compared to the DOX-model of nephrotoxicity (group 8). Values are presented as mean ± SD. One-way ANOVA was performed for comparison between groups; ** p < 0.01, *** p < 0.001 compared to the model.
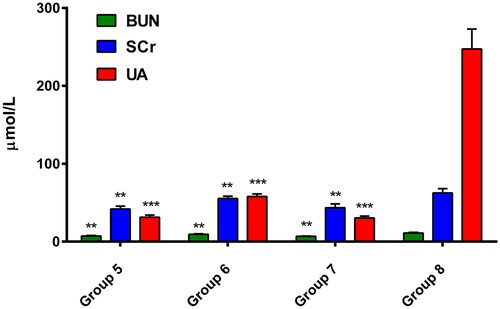
Table 2. Effects of the different L. barbarum fractions administered orally at a dose of 2 mg/kg (b.w.) on serum urea, creatinine and urinary levels in DOX-induced nephrotoxicity (20 mg/kg, b.w., i.p.).
Effects on histopathological indicators of cardio- and nephrotoxicity
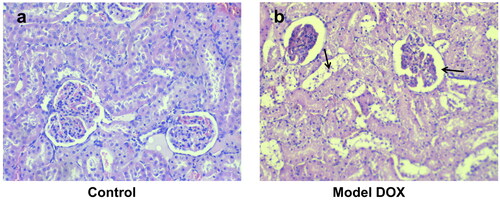
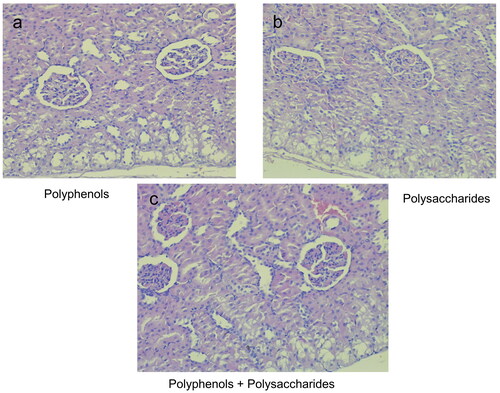
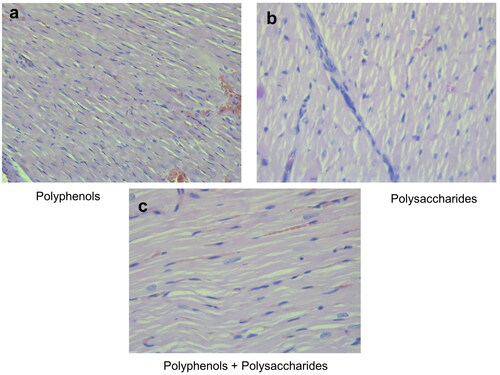
The hematoxylin and eosin (H&E) stained longitudinal sections of group 8 (treated only with DOX) showed significant damage in cardiac cell muscles (). The degree of DOX-induced cardiotoxicity was significantly lower following intake of individual fractions of L. barbarum. In the pectin-free fraction () and the combination of pectin-free extract and polysaccharides (2 mg/kg, b.w., i.p.) (), histological analysis showed a structure similar to that in group 1 (control). There were fibrous changes in animals treated with the polysaccharide fraction (), but there were no myofibrillar ruptures.
Figure 4. Photomicrographs of muscle sections of left ventricular chambers stained with H&E × 400. (a) Control (group 1) - normal myofibrilar architecture. (b) DOX-treated animals (group 8; cumulative dose: 20 mg/kg, b.w., i.p.) - disorganization of cardiac muscle fibres (left arrow), cytoplasmic fading and pyknotic nucleus formation (central arrow) and myofibril ruptures (right arrow).
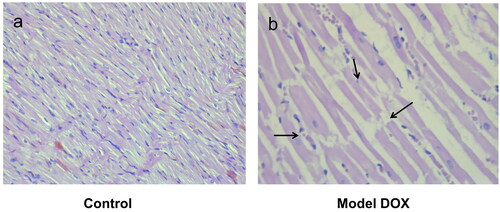
Figure 5. Photomicrographs of muscle sections of left ventricular of rats treated with the individual fractions of L. barbarum - pectin-free fraction (a), polysaccharide fraction (b) and combination of pectin-free extract and polysaccharides (2 mg/kg, b.w., p.o.) (c); H&E × 400.
The architecture of the kidney tissue in the control group animals was normal, whereas the rats treated with DOX only showed renal injuries ( and b, respectively). In contrast, significantly less tissue damage was found in the renal histological incisions in all animals treated simultaneously with either pectin-free fraction, polysaccharide fraction or combination of them (2 mg/kg, b.w., p.o.) and DOX (compared to the DOX-only group). There were minor lesions in the cortical area, but significantly less pronounced than in the model of renal toxicity ().
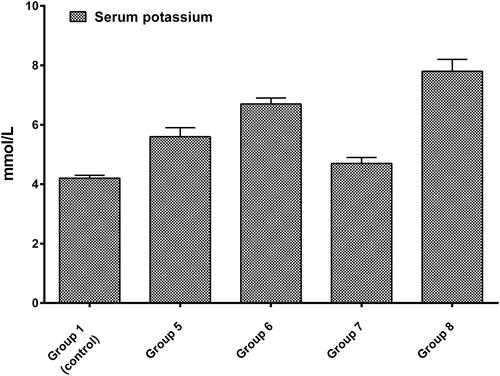
Determination of plasma potassium concentration
As the major intracellular cation, potassium is critical to muscle cell activity. The etiology of its increase could be a kidney disorder similar to that caused by DOX [Citation30]. As expected, animals treated only with DOX (20 mg/kg, b.w., i.p.) showed the highest increase in potassium levels. On the other hand, the rats treated simultaneously with any of the plant fractions and anthracycline demonstrated a decrease in serum potassium, but the results were non-significant. However, it should be noted that it followed the same pattern that was observed in the biochemical assays and the histopathological examination: again, the most satisfactory results were observed in group 5 (which received the pectin-free fraction) and group 7 (which was treated with the combination of pectin-free extract plus polysaccharide fraction; ).
Discussion
Anthracycline antibiotics are among the most powerful and commonly used chemotherapeutic agents. However, their cardiotoxicity and nephrotoxicity are the main limiting factors for their widespread use [Citation31]. The exact mechanisms involved in the organ toxicity have not yet been fully elucidated. In this regard, there are two main theories: (1) the formation of iron-bound free radicals and the formation of the metabolite doxorubicinol and (2) the induction of mitochondrial dysfunction with subsequent activation of apoptotic and necrotic processes [Citation32, Citation33]. One of the strongest findings to support the iron hypothesis is that the iron chelator dexrazoxane protects against DOX-induced toxicity in vivo [Citation34]. Details about mitochondrial dysfunction and the exact mechanisms associated with it, can be found in the reviews of Gorini et al. [Citation35] and Varga et al. [Citation36]. Therefore, it can be concluded that substances that reduce oxidative stress and stabilize mitochondrial dysfunction would alleviate the damage to the heart and kidney tissue by anthracyclines, and in particular DOX.
In numerous studies, the ingredients of L. barbarum have shown to have potential antioxidant and anti-inflammatory properties, that contribute to reduced damage and promote repair of mitochondrial function [Citation16, Citation37, Citation38]. In our previous study, we analyzed and examined different fractions extracted from L. barbarum in an attempt to trace which of the ingredients are responsible for the observed effects [Citation19]. The pectin-free fraction, rich in polyphenols, has shown the highest antioxidant and anti-breast cancer cell potential. In vitro studies with the same isolated fractions together with DOX on the same breast cancer cells were performed. The concomitant use of DOX with the pectin-free fraction and combination pectin-free/polysaccharides led to a significant reduction in IC50, confirming their greatest potential to act in combination [Citation39].
Very often, the increase in therapeutic activity is accompanied by an increased incidence of adverse reactions or, conversely, evidence of reduced side effects (in this case, organoprotective activity) correlates with decreased therapeutic activity. To monitor whether fractions of L. barbarum will affect the most common adverse reactions of anthracyclines (in particular DOX), we undertook the test for organ protection in experimental rats. The results showed that the individual fractions of Goji berry could protect against the organ damage induced by DOX, reducing the biochemical markers of organ toxicity, as well as the histological findings. The most pronounced protective effects were observed in the polyphenols rich pectin-free fraction (group 5) and the combined one (group 7). Since the antioxidant activity is most strongly manifested in the pectin-free fraction, we assume that this pharmacological effect is attributed namely to it. The antioxidant capacity of polyphenols has been recently described and confirmed by Yang et al. [Citation40], who evaluated their scavenging activity against 1,1-diphenyl-2-picrylhydrazyl, hydroxyl, superoxide anion and 2,2′-azinobis-di-(3-ethyl-benzothiazolin-6-sulfonic acid) diammonium salt free radicals. These theories are supported by the biochemical and histopathological analysis of other authors, where total extract and L. barbarum polysaccharides have been studied [Citation41, Citation42]. The fraction containing both polyphenols and polysaccharides also showed highly expressed biological activity in the in vitro and in vivo experiments performed in this study. The hypothesis stated here is based on the fact that polysaccharides support and contribute to the action of polyphenolic components in the extract [Citation43, Citation44]. A limitation of the present study is the lack of a positive control. The reason is that there was not a proper match, as recently published studies also did not include positive controls [Citation45, Citation46].
Conclusions
Recently, Goji berry has been recognized not only as a product used in traditional Chinese medicine, but also as an important element of health-promoting diet worldwide. The results of the present study reaffirmed its appellation ‘superfood’, as a significant organoprotective effect was demonstrated against DOX-induced cardio- and nephrotoxicity in rats. The effects were more pronounced in the fractions that contained significant amounts of polyphenols. Thus, the in-depth study of the combination of DOX and Goji berry extracts is encouraged, as this shows potential for enhancing the anticancer efficacy without compromising the safety profile of the anthracycline antibiotics. Monitoring these effects in humans would help to clarify the exact mechanisms, which would contribute to the creation and application of enhancers of pharmacodynamic effects on-target and reduce those off-target.
Compliance with ethical standards ethics approval
The study protocol was approved by the Bulgarian Food Safety Agency (BFSA) and all the requirements for the protection and humane treatment of laboratory animals were followed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability
All data that support this study are available from the corresponding author upon reasonable request.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Zhao N, Woodle MC, Mixson AJ. Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol. 2018;09(05):519.
- Thorn CF, Oshiro C, Marsh S, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–446.
- Radeva-Ilieva MP, Georgiev KD, Hvarchanova NR, et al. Protective effect of methylxanthine fractions isolated from bancha tea leaves against doxorubicin-induced cardio- and nephrotoxicities in rats. Biomed Res Int. 2020;2020:4018412.
- Cummings J, Willmott N, Hoey BM, et al. The consequences of doxorubicin quinone reduction in vivo in tumour tissue. Biochem Pharmacol. 1992;44(11):2165–2174.
- Jadhav VB, Thakare VN, Suralkar AA, et al. Ameliorative effect of luffa acutangula roxb. on doxorubicin induced cardiac and nephrotoxicity in mice. Indian J Exp Biol. 2013;51(2):149–156. PMID: 23923608
- Abushouk AI, Ismail A, Salem AMA, et al. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2017;90:935–946.
- Abdel-Daim MM, Kilany OE, Khalifa HA, et al. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother Pharmacol. 2017;80(4):745–753.
- Wang Y, Chao X, Ahmad FUD, et al. Phoenix dactylifera protects against doxorubicin-induced cardiotoxicity and nephrotoxicity. Cardiol Res Pract. 2019;2019:7395239.
- Khan G, Haque SE, Anwer T, et al. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol Pharm. 2014;71(5):861–868.
- Rafiee Z, Moaiedi MZ, Gorji AV, et al. p-Coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res. 2020;51(1):32–40.
- Pejin B, Iodice C, Tommonaro G, et al. Sugar composition of the moss Rhodobryum ontariense (Kindb.) Kindb. Nat Prod Res. 2012;26(3):209–215. 10.1080/14786419.2010.535163
- Pejin B, Kien-Thai Y, Stanimirovic B, et al. Heavy metal content of a medicinal moss tea for hypertension. Nat Prod Res. 2012;26(23):2239–2242. 10.1080/14786419.2011.648190
- Siddiqui AJ, Jahan S, Singh R, et al. Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. Biomed Res Int. 2022;2022:5425485. 10.1155/2022/5425485
- Magalhães V, Silva AR, Silv B, et al. Comparative studies on the anti-neuroinflammatory and antioxidant activities of black and red goji berries. J Funct Foods. 2022;92:105038.
- Ma ZF, Zhang H, Teh SS, et al. Goji berries as a potential natural antioxidant medicine: an insight into their molecular mechanisms of action. Oxid Med Cell Longev. 2019;2019:2437397.
- Bucheli P, Vidal K, Shen L, et al. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88(2):257–262.
- Liang B, Jin M, Liu H. Water-soluble polysaccharide from dried lycium barbarum fruits: isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011;83(4):1947–1951.
- Sun Q, Du M, Kang Y, et al. Prebiotic effects of goji berry in protection against inflammatory bowel disease. Crit Rev Food Sci Nutr. 2022;6:1–25.
- Georgiev KD, Slavov IJ, Iliev IA. Antioxidant activity and antiproliferative effects of Lycium barbarum’s (goji berry) fractions on breast cancer cell lines. Folia Med (Plovdiv). 2019;61(1):104–112.
- de Souza Zanchet MZ, Nardi GM, de Oliveira Souza Bratti L, et al. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxid Med Cell Longev. 2017;2017:9763210.
- Shah T, Bule M, Niaz K, et al. (Lycium barbarum) - A superfood. In Nabavi SM, Silva AS, editors. Nonvitamin and nonmineral nutritional supplements. Cambridge: Academic Press; 2019. p. 257–264.
- Zhou B, Xia H, Yang L, et al. The effect of lycium barbarum polysaccharide on the glucose and lipid metabolism: a systematic review and meta-analysis. J Am Coll Nutr. 2021;2:1–9.
- Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76(1):7–19.
- Skenderidis P, Lampakis D, Giavasis I, et al. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants. 2019;8(3):60.
- Benchennouf A, Grigorakis S, Loupassaki S, et al. Phytochemical analysis and antioxidant activity of lycium barbarum (goji) cultivated in Greece. Pharm Biol. 2017;55(1):596–602.
- Islam T, Yu X, Badwal TS, et al. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (lycium ruthenicum). Chem Cent J. 2017;11(1):59.
- Sun Y, Rukeya J, Tao W, et al. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci Rep. 2017;7:40605.
- European Parliament, Council of the European Union Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes (Council of Europe, Strasbourg, 2010), https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31986L0609&from=EN. Accessed 23 Nov 2022.
- Yu B, Wang W. Cardioprotective effects of morroniside in rats following acute myocardial infarction. Inflammation. 2018;41(2):432–436.
- Ifeanacho MO, Ikewuchi JC, Ikewuchi CC, et al. Prevention of doxorubicin-induced dyslipidaemia, plasma oxidative stress and electrolytes imbalance in wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. Sci Afr. 2021;11:e00636.
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267–3285.
- Octavia Y, Tocchetti CG, Gabrielson KL, et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–1225.
- Zhou S, Starkov A, Froberg MK, et al. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61(2):771–777. PMID11212281
- Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–1332.
- Gorini S, De Angelis A, Berrino L, et al. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev. 2018;2018:7582730.
- Varga ZV, Ferdinandy P, Liaudet L, et al. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309(9):H1453–H1467.
- Gao Y, Wei Y, Wang Y, et al. Lycium barbarum: a traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017;8(6):778–791.
- Ni J, Au M, Kong H, et al. Lycium barbarum polysaccharides in ageing and its potential use for prevention and treatment of osteoarthritis: a systematic review. BMC Complement Med Ther. 2021;21(1):212.
- Georgiev KD, Slavov IJ, Iliev IA. Synergistic growth inhibitory effects of Lycium barbarum (goji berry) extract with doxorubicin against human breast cancer cells. J Pharm Pharmacol Res. 2019;3:51–58.
- Yang T, Hu Y, Yan Y, et al. Characterization and evaluation of antioxidant and anti-inflammatory activities of flavonoids from the fruits of Lycium barbarum. Foods. 2022;11(3):306. 10.3390/foods11030306
- Xin YF, Zhou GL, Deng ZY, et al. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother Res. 2007;21(11):1020–1024. 10.1002/ptr.2186
- Xin YF, You ZQ, Gao HY, et al. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats. Phytother Res. 2012;26(5):716–721.
- Wu T, Xu J, Chen Y, et al. Oolong tea polysaccharide and polyphenols prevent obesity development in Sprague-Dawley rats. Food Nutr Res. 2018;62.
- Xu Y, Zhang M, Wu T, et al. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6(1):297–304.
- Abu Gazia M, El-Magd MA. Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs. 2018;206(1–2):62–72.
- Hijazi MA, Jambi HA, Aljehany BM, et al. Potential protective effect of Achillea fragrantissima against adriamycin-induced cardiotoxicity in rats via an antioxidant and anti-inflammatory pathway. Biomed Res Int. 2019;2019:5269074.