?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Peptides are a promising alternative of conventional medical drugs for the treatment of different diseases because they have no or have very few side effects owing to the natural mechanisms for their elimination. There are a lot of examples of drugs on the pharmaceutical market based on modified amino acids and peptides. Herein, we report the synthesis and studies on the antimicrobial peptide (KLAKLAK)2-NH2 where Leu is replaced by the unnatural amino acid nor-Leu. In addition, a second pharmacophore with well proven anticancer properties is introduced to the peptide moiety. All structures were synthesized by conventional solid phase peptide synthesis. The antiproliferative and antimicrobial activities were studied using MTT-dye reduction assay and disk-diffusion test, respectively. Biological activity assays showed that the introduction of nor-Leu in the primary structure of the parent compound does not lead to an increase in the antiproliferative activity. However, the combination with the second pharmacophore 1,8-naphtalimide in a hybrid structure 1,8-NphtG-(KNleAKNleAK)2-NH2 leads to a significant increase in the antiproliferative properties. The antimicrobial tests showed that all tested compounds exhibit antimicrobial activity. The peptide and the second pharmacophore had a synergistic effect. In combination with complete hydrolytic stability for 72 h in model systems, the compound 1,8-NphtG-(KNleAKNleAK)2-NH2 is the best candidate for a medical drug in the treatment of mammary gland type A adenocarcinoma (MCF-7) in combination with antimicrobial properties.
Introduction
Many medical drugs on the pharmaceutical market are based on modified amino acids or peptides for the treatment of different impairments. Examples of such drugs are enalapril (name Vasotec) for the treatment of high blood pressure, diabetic kidney disease and heart failure [Citation1], acetylcysteine (chemical name N-acetyl-L-cysteine) for mucolytic therapy [Citation2, Citation3], peptide antibiotics, such as bacitracin [Citation4, Citation5] and gramicidin [Citation6], used against different microorganisms [Citation7]. The fight against cancer is not an exception, and several analogues of the peptide hormone somatostatin named octreotide, lanreotide and vapreotide are introduced in medicinal practice for the treatment of acromegaly, symptoms caused by neuroendocrine tumors [Citation8–11], pancreatic [Citation12], breast [Citation13], bone [Citation14, Citation15] and prostate cancers [Citation16]. Thus, peptides are a promising alternative for the treatment of different diseases because they have no or have very few side effects owing to the natural mechanisms for their elimination.
In addition, peptides have already proved their ability to transport different molecules to different targets in the organism [Citation17]. Thus, they are used as vectors for the delivery of some biologically active molecules in specific cells [Citation18, Citation19], tissues [Citation20], or other places in the organisms [Citation21, Citation22]. Cell-penetrating peptides (CPPs) have been experimentally validated for in vitro and in vivo delivery of small or large (up to 120 kDa) bioactive cargo inside cells [Citation23]. Habault and Poyet [Citation23] described the use of CPPs for transportation of pharmacophores in cancer therapy and especially for osteosarcoma.
Our previous study on the antiproliferative properties and antimicrobial activity of peptide (KLAKLAK)2-NH2 revealed that the introduction of β-Ala instead of Ala in the primary structure of the peptide and its single sequence analogue leads to higher biological activity and selectivity in the tested cell lines and microorganisms [Citation24, Citation25]. Taking into account all of the above as well as the fact that the introduction of unnatural amino acids in the peptide sequence often leads to better stability against proteinases and better pharmacokinetic properties, herein we report the synthesis and antiproliferative effect of new analogues of the antimicrobial peptide (KLAKLAK)2-NH2, where Leu is replaced with nor-Leu (Nle). In addition, bioconjugates of the target peptide (KNleAKNleAK)2-NH2 with second pharmacophores with proven anticancer properties, caffeic acid (Caf-) and 1,8-naphtalimide (Npht-), were synthesized and their activities were also studied.
Materials and methods
Synthesis and analytical data
Specifically protected amino acids Nα-Fmoc-Lys(Boc)-OH, Fmoc-Nle-OH, Fmoc-Ala-OH as well as solid phase carrier Fmoc-Rink Amide MBHA Resin, activation agents HBTU (N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate), DIC (N,N′-diisopropylcarbodiimide) or PyBOP (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate), trifluoroacetic acid (TFA) and scavenger triisopropylsilane (TIS) were purchased from Iris Biotech (Germany). The solvents N,N’-dimethylformamide (DMF) and dichloromethane (DCM) were from Valerus (Bulgaria). Caffeic acid was from Alfa Aesar, and 1,8-naphthalanhydride from Sigma-Aldrich (Germany). All reagents and solvents were used without any preliminary treatment.
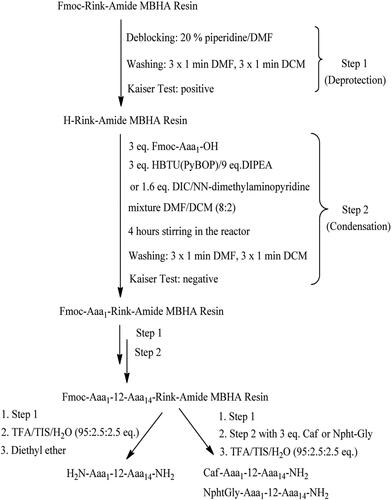
By analogy to our previous study [Citation24], Fmoc(9-fluorenylmethoxycarbonyl)/Ot-Bu solid-phase peptide synthesis (SPPS) on Rink-amide MBHA resin was used for the synthesis of target compounds. HBTU or DIC were used for amino acid activation. PyBOP reagent was applied to activate the second pharmacophore Caf- or NphtG- at the final stage of target molecule synthesis. The coupling reactions were performed using amino acid/HBTU(or PyBOP)/HOBt/DIEA/resin at a molar ratio of 3/3/3/9/1 or amino acid/DIC/resin at a molar ratio of 3/3/1. The protective Nα-Fmoc-group was removed from all amino acids during peptide chain elongation by treatment with 20% piperidine in DMF. As previously described [Citation25], the coupling and deprotection reactions were monitored by means of the standard Kaiser test. The final peptides were removed from the resin using a mixture of 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIS) and 2.5% dH2O. All peptides were obtained as a filtrate in TFA and precipitated with cold dry diethyl ether. The precipitate was filtered and subjected to subsequent high performance liquid chromatography coupled with mass spectrometry (HPLC/MS) analysis.
The peptide purity was monitored on an RP-HPLC Agilent Poroshell 120, 100 mm × 4.6 mm column using a Shimadzu LC MS/MS 8045 system, at a mobile phase flow rate of 0.30 mL/min, column temperature of 40 °C, and a linear binary gradient of two phases, Mobile phase А: H2O (10% AсСN; 0.1% HCOOH), and Mobile phase B: AсCN (5% H2O; 0,1% HCOOH) at the gradient of both phases in time presented in , as previously described [Citation25].
Table 1. Gradient system used for HPLC analysis of target compounds.
The compound structures were checked by electrospray ionization mass spectrometry in SCAN regime/ESI + mode of ionization with the parameters summarized in .
Table 2. Parameters of MS analysis of target compounds.
The optical rotation was measured with an automatic standard polarimeter Polamat A, Carl Zeis, Jena at c = 1 in water. Melting points were monitored on a standard Kofler hot-stage microscope. All analytical data are summarized in .
Table 3. Structure and analytical data for newly synthesized compounds.
The pharmacophore 1,8-naphtalimideglycine (NphtG-) was synthesized according to the procedure described by Marinov et al. [Citation26].
Biological activity assays
Cell cultures
Two human mammary carcinoma cell lines, MCF-7 (ER+, PR + and Her-2-) and MDA-MB-231 (ER-, PR- and Her-2-), were used as models for breast cancer. The cell line MCF-10A (human breast epithelial cell line) was used as a model for healthy tissue. Cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, USA). Cells were cultured in Dulbecco Modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Sigma-Aldrich, Schnelldorf, Germany) in an incubator at 37 °C, 5% CO2 and 95% humidity. Plastic flasks 75 cm2 (Biologix, Lenexa, Kansas, USA), were used to grow the cells.
In vitro antiproliferative activity
The procedure described in our previous study [Citation25] was applied with minor modifications. Briefly, the antiproliferative activity testing was performed on cell cultures from several human cell lines using the standard MTT-dye reduction assay described by Mosmann [Citation27]. The assay is based on the metabolism of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble formazan by mitochondrial reductases. The formazan concentration can be determined spectrophotometrically. The measured absorption is an indicator of cell viability and metabolic activity.
The cell lines used in the experiments were: mammary gland type A adenocarcinoma ER+, PR+, HER2- (MCF-7), triple negative breast cancer ER-, PR-, HER2- (MDA-MB-231) and breast, non-tumorigenic epithelial cell line (MCF-10A). The cell lines were routinely grown as monolayers in 75 cm2 tissue culture flasks in standard conditions. Cells were plated at a density of 1 × 103 cells in 100 µL in each well of 96-well flat-bottomed microplates and allowed to adhere for 24 h before treatment with test compounds. A concentration range from 7.5 to 2000 μmol/L was applied for 72 h. The formazan absorption was registered using a microplate reader at λ = 540 nm. Antiproliferative activities were expressed as IC50 values (concentration required for 50% inhibition of cell growth), calculated using nonlinear regression analysis (GraphPad Prizm4 Software).
The statistical analysis included one-way analysis of variance (ANOVA) followed by Bon-ferroni’s post hoc test. Differences were considered statistically significant at the level of p < 0.05. All results are presented as mean values with standard deviation (± SD).
Antimicrobial assay
The newly synthesized derivatives with general formula (X-KnLAKnLAK)2-NH2 where X is H, Caf- or NphtG- at concentrations 20 μmol/L in distilled water, were tested against model strains Esherichia coli NBIMCC K12 407, Bacillus subtilis NBIMCC 3562 and Candida albicans NBIMCC 74. The strains were obtained from the culture collection of the National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC, Bulgaria). Exponential cultures (1 × 107 cfu/mL) of strains E. coli K12 407 were obtained in Luria Bertani (LB, HiMedia, Mumbai, India). For B. subtilis 3562 an initial bacterial concentration of 1 × 106 cfu/mL was obtained in Nutrient broth (NB, HiMedia, Mumbai, India). For C. albicans 74 an initial bacterial concentration of 1 × 105 cfu/mL was obtained in Yeast Mold (YM) medium. Cell cultures were grown in an incubator shaker ES-20/60 (Biosan, Latvia) at 30 °C for B. subtilis 3562 and at 37 °C for E. coli K12 407 and C. albicans 74 with shaking at 220 rpm for 24 h. The microbiological tests were performed using the agar diffusion method. The agar plates containing LB/NB/YM media were inoculated by spreading 100 μL of each microbial exponential culture on their surface and incubated at 30/37 °C for penetration of suspension into the agar. After 30 min, sterile paper discs (6 mm in diameter) were soaked with the tested compounds in amounts of 6 µL and placed on the surface of the agar Petri dishes. The plates were incubated at 30/37 °C for 24 h. The antimicrobial activity was determined by measuring the diameter of the inhibition zones. Sterile paper discs soaked with water were used as a blank control. Mean values were calculated by performing the experiments in triplicates.
Hydrolytic stability
Three different pH values that mimic human pH in the stomach, blood plasma and small intestine were selected for the investigation of the hydrolytic stability of newly synthesized compounds. Model solutions used for the determination of hydrolytic stability were prepared according to the European Pharmacopoeia, 6th Edition as follows:
Buffer with pH 2.0 – 6.57 g of KCl were dissolved in water (CO2 free) and 119.0 mL of 0.1 mol/L HCl was added. The obtained solution was completed to 1000.0 mL with dH2O;
Buffer with pH 7.4 – 2.38 g of Na2HPO4, 0.19 g of KH2PO4 and 8.0 g of NaCl were dissolved in dH2O. The obtained solution was completed to 1000.0 mL with dH2O.
Buffer with pH 9.0 - 1000.0 mL of solution I were mixed with 420.0 mL of solution II. Solution I: 6.18 g H3BO3 was dissolved in 0.1 mol/L KCl and it was completed to 1000.0 mL with the same solvent; Solution II: 0.1 mol/L NaOH.
An HPLC MODEL 550 A (‘KONIK-TECH’ – Spain) with Photodiode Array Detectors and column Hypersil BDS C8 150 × 4.6 mm, 5 μm, was used for monitoring of the hydrolytic stability. The mobile phase was acetonitrile:water 50:50, elution rate: 0.9 mL/min, room temperature, scanning wavelength 190 nm, injected volume: 20 µL.
Results
Synthesis and characterization of compounds
Peptide mimetics of the antimicrobial peptide (KLAKLAK)2-NH2 with a general structure (X-KNleAKNleAK)2-NH2, where X is 1,8-naphthalimide-Gly (NphtG-) or caffeic acid (Caf-), were synthesized. They were obtained by the standard SPPS, Fmoc/Ot-Bu strategy. The target C-terminal amides were obtained using rink-amide MBHA resin as a solid-phase carrier. The activation of amino acids during peptide chain elongation was done with HBTU or DIC as condensation agents. The second pharmacophores Caf- or NphtG- were activated by means of PyBop. Analytical data for the newly synthesized peptides are summarized in Тable 3.
Antiproliferative activity
The MTT dye reduction assay was used to test the antiproliferative activity of the compounds. Cell cultures from lines MCF-10A, MCF-7 and MDA-MB-231 were incubated with the test substances at concentrations of 7.5 to 2000 μmol/L for 72 h. The antiproliferative activity was determined and expressed in % relative to the negative control. Dose-response dependence was observed for all substances (). The IC50 (half-manximal inhibitory concentration) values of the mean were calculated (). MCF-10A is a reliable model for normal human mammary epithelial cells, which served as a control.
Figure 1. Antiproliferative activity of newly synthesized compounds. Dose-response curves assessment in (A) breast non-tumorigenic epithelial cells (MCF-10A), (B) mammary gland type A adenocarcinoma (MCF-7) and (C) triple-negative breast cancer (MDA-MB-231).
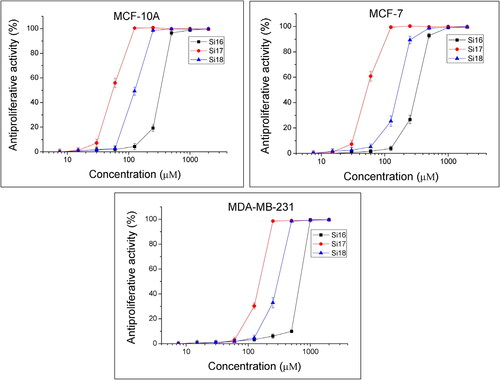
Table 4. Antiproliferative activity of the studied substances expressed by IC50 values of the mean (± SD).
The least potent antiproliferative effect in MCF-10A cells () was caused by Si16 (IC50 = 349.7 ± 6.11). In the cell line MCF-7, the strongest antiproliferative effect was for Si17, with IC50 = 54.1 ± 1.85 (). The tested compounds showed weak antiproliferative activity in the MDA-MB-231 cells ().
Antimicrobial activity
The antimicrobial experiments were conducted via the agar diffusion method. Disks soaked with 6 μL of 20 μmol/L solution of the tested compounds were placed onto the agar plates and the microbial growth was monitored for 24 h. Sterile zones, free of microbial growth, appeared around the paper disks confirming the antibacterial activity of the tested compounds. The obtained results are presented in .
Table 5. Antimicrobial activity against microorganisms.
Discussion
The introduction of peptides in medicinal practice is often limited by their short half-life in the plasma due to hydrolysis by human proteinases. Many modifications have proved their potential to increase peptide stability, such as replacement of a peptide bond with a pseudopeptide bond, introduction of unnatural amino acids, replacement of L- with D-amino acids, etc. Amino acids which closely mimic the structure of the natural amino acids are a promising alternative and their introduction in the primary structure of peptides mostly leads to improved pharmacokinetics and pharmacodynamic properties of new peptide mimetics [Citation28]. Moreover, the human organism has natural mechanisms to metabolize amino acids so they have no significant secondary side effects, but their introduction in some molecules could lead to increased biological activity [Citation29, Citation30]. Taking into account all mentioned above, as well as our promising preliminary results on the shortened analogues of KLAKLAK-NH2 containing the amino acid Nle, herein we synthesized the analogue containing the double sequence (KLAKLAK)2-NH2 and its conjugates with a second pharmacophore with proven anticancer properties to investigate their antiproliferative potential and antimicrobial properties.
Plants have been studied since ancient times for their positive effects on human organisms. Some natural substances, such as curcumin produced by Curcuma longa [Citation31, Citation32], caffeine contained in the beans of Coffea arabica, and Coffea canephora [Citation33] as well as caffeic acid (Caf) [Citation34–38], became very popular last decade due to their ability to affect tumors. In addition, differently substituted derivatives of 1,8-naphtalimide (Npht) show good to excellent anticancer properties [Citation26, Citation39–42] and some of them are already used in medicinal practice [Citation43, Citation44]. The present study continues our previous works on (KLAKLAK)2-NH2 analogues [Citation24, Citation25]. We took into account the conclusion we made that the introduction of unnatural amino acids in the primary structure of a peptide influences its biological activity positively. The same structure-activity relationship was reported by Chayrov et al. and Pejin et al. [Citation29, Citation30]. So, herein we synthesized an analogue of doubled sequence (KLAKLAK)2-NH2, containing the unnatural amino acid Nle, to study the influence of this non-proteinogenic amino acid on both the antiproliferative ability and antimicrobial properties. In addition, we conjugated this new analogue with the second pharmacophore NphtG- or Caf-. All target compounds were synthesized using manual SPPS according to Scheme 1.
The antiproliferative activity assay was performed to examine the antiproliferative activity of these newly synthesized molecules. The parent compound Si1 (KLAKLAK)2-NH2 in non-tumorigenic cell line MCF-10A had IC50 = 154 ± 6.53 μmol/L [Citation24]. When Leu was replaced with Nle in the structure Si16, a significant decrease (p < 0.001) in antiproliferative activity (IC50 = 349.7 ± 6.11 μmol/L) occurred. The antiproliferative activity decreased also about 2.5 times in MCF-7 cells from IC50 = 124.1 ± 8.12 μmol/L for Si1 to IC50 = 321.5 ± 38.12 μmol/L for Si16. In contrast, no significant difference in IC50 values was observed in the MDA-MB-231 cell line. The addition of the second pharmacophore 1,8-NphtG- to (KnLAKnLAK)2-NH2 in compound Si17 led to a significant increase in the antiproliferative activity in the three cell lines. The introduction of a Caf- group in (KNleAKNleAK)2-NH2 (Si18) led to a low increase in antiproliferative activity compared to Si16. Both the obtained results in this study and our previous results show that the Nle analogues of (KLAKLAK)2-NH2 (Si16, Si17 and Si18) have significantly higher antiproliferative activity than the short analogues KNleAKNleAK-NH2, 1,8-NphtG-KNleAKNleAK-NH2 and Caf-KNleAKNleAK-NH2 (Si13, Si14 and Si15) [Citation24]. Moreover, the bioconjugates with 1,8-Npht-Gly (Si17) and Caf- (Si18) have better activity than the parent compound Si1 according to all tested cell lines. Based on all the obtained results, we suggest that there is a synergisitc effect between the two pharmacophores in the hybrid molecules.
The antimicrobial experiments were performed using a black sample disk soaked with 6 μL of distilled water. The obtained results revealed that the tested compounds Si16, Si17 and Si18 exhibited antimicrobial activity against all tested strains. All compounds were more effective against the bacterial strains E. coli K12 407 and B. subtilis 3562, where the inhibition zones were in the range of 12.00 mm to 14.66 mm. The compound Si17 also showed good antimicrobial activity against the yeast strain C. albicans 74, with a zone of inhibition of 18.33 mm, but the other compounds, Si16 and Si18, showed weak antifungal activity, 2.50 mm and 5.33 mm, respectively.
The hydrolytic stability of all newly synthesized molecules was studied for 72 h at three different pH values that mimic the pH in the human stomach, blood plasma and small intestine. All compounds were completely stable, and no hydrolysis occurred during the test period.
Conclusions
The obtained data from this investigation show that the introduction of the unnatural amino acid Nle in the primary structure of the parent compound (KLAKLAK)2-NH2 does not lead to an increase in the antiproliferative activity. However, the combination with a second pharmacophore, 1,8-naphtalimide, in the hybrid structure Si17 leads to a significant increase in the antiproliferative properties. Thus, a synergistic effect is observed between the two parts of the hybrid molecule. According to the antimicrobial activity assays, all tested compounds exhibit antimicrobial activity against all used model strains, E. coli K12 407, B. subtilis 3562 and C. albicans 74, but compound Si17 has the highest antimicrobial activity. In a combination with complete hydrolytic stability for 72 h in the model systems used in this study, the compound 1,8-NphtG-(KNleAKNleAK)2-NH2 (Si17) is the best candidate for a potential medical drug in the treatment of cancers in combination with antimicrobial properties.
Author contributions
Sirine Jaber: synthesis of target compounds, Veronica Nemska: antimicrobial activity study, Ivan Iliev: anticancer activity study and results interpretation, manuscript preparation, Elena Ivanova: anticancer activity study, Tsvetelina Foteva: antimicrobial activity study, Nelly Georgieva: antimicrobial studies conceptualization and interpretation, manuscript preparation, Ivan Givechev: HPLC/MS analysis and interpretation, Dimiter Tanev: HPLC/MS analysis and interpretation, Emilia Naydenova: conceptualization and manuscript preparation, Dancho Danalev: synthesis of target compounds, work conceptualization and manuscript preparation.
Ethical approval
This study does not contain any studies with animals or humans performed by any of the authors.
Acknowledgements
The authors would like to thank Testing center Global Test Ltd. for HPLC/MS/MS analysis.
Disclosure statement
All authors declare that they have no conflict of interest. They are entitled to the authorship and have approved the final version of the manuscript.
Additional information
Funding
References
- He YM, Feng L, Huo DM, et al. Enalapril versus losartan for adults with chronic kidney disease: a systematic review and meta-analysis. Nephrology (Carlton). 2013;18(9):605–614.
- Grandjean EM, Berthet P, Ruffmann R, et al. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209–221.
- Sadowska AM, Verbraecken J, Darquennes K, et al. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(4):425–434.
- Zintel HA, Ma RA, Nichols AC, et al. The absorption, distribution, excretion and toxicity of bacitracin in man. Am J Med Sci. 1949;218(4):439–445.
- Johnson BA, Anker H, Meleney FL. Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science. 1945;102(2650):376–377.
- Wang F, Qin L, Pace CJ, et al. Solubilized gramicidin A as potential systemic antibiotics. ChemBioChem. 2012;13(1):51–55.
- Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20(1):122–128.
- Pyronnet S, Bousquet C, Najib S, et al. Antitumor effect of somatostatin. Mol Cell Endocrinol. 2008;286(1-2):230–237.
- Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29(1):1–19.
- Pollak M. The potential role of somatostatin analogues in breast cancer treatment. Yale J Biol Med. 1997;70(5-6):535–539.
- Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16(24):2963–2970.
- Paz-Bouza JI, Redding TW, Schally AV. Treatment of nitrosamine-induced pancreatic tumors in hamsters with analogs of somatostatin and luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA. 1987;84(4):1112–1116.
- Rose DP, Gottardis M, Noonan JJ. Rat mammary carcinoma regressions during suppression of serum growth hormone and prolactin. Anticancer Res. 1983;3(5):323–325.
- Schally AV, Comaru-Schally AM, Redding TW. Antitumor effects of analogs of hypothalamic hormones in endocrine-dependent cancers. Proc Soc Exp Biol Med. 1984;175(3):259–281.
- Schally AV, Redding TW, Comaru-Schally AM. Potential use of analogs of luteinizing hormone-releasing hormones in the treatment of hormone-sensitive neoplasms. Cancer Treat Rep. 1984;68(1):281–289.
- Zalatnai A, Paz-Bouza JI, Redding TW, et al. Histologic changes in the rat prostate cancer model after treatment with somatostatin analogs and D-Trp-6-LH-RH. Prostate. 1988;12(1):85–98.
- Farkhani SM, Valizadeh A, Karami H, et al. Cell penetrating peptides: efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides. 2014;57:78–94.
- Bian J, Popović ZB, Benejam C, et al. Effect of cell-based intercellular delivery of transcription factor GATA4 on ischemic cardiomyopathy. Circ Res. 2007;100(11):1626–1633.
- Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91(2):664–668.
- Rousselle C, Clair P, Lefauconnier JM, et al. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57(4):679–686.
- Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558(1-3):63–68.
- Sebbage V. Cell-penetrating peptides and their therapeutic applications. Biosci Horiz. 2009;2(1):64–72.
- Habault J, Poyet J-L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules. 2019;24(5):927.
- Jaber S, Iliev I, Angelova T, et al. Synthesis, antitumor and antibacterial studies of new shortened analogues of (KLAKLAK)2-NH2 and their conjugates containing unnatural amino acids. Molecules. 2021;26(4):898.
- Jaber S, Nemska V, Iliev I, et al. Synthesis and biological studies on (KLAKLAK)2-NH2 analog containing unnatural amino acid β-Ala and conjugates with second pharmacophore. Molecules. 2021;26(23):7321.
- Marinov MN, Naydenova ED, Momekov GT, et al. Synthesis, characterization, quantum-chemical calculations and cytotoxic activity of 1,8-naphthalimide derivatives with non-protein amino acids. Anticancer Agents Med Chem. 2019;19(10):1276–1284.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63.
- Cabrele C, Martinek TA, Reiser O, et al. Peptides containing β‑amino acid patterns: challenges and successes in medicinal chemistry. J Med Chem. 2014;57(23):9718–−9739. 10.1021/jm5010896.
- Chayrov R, Parisis N, Chatziathanasiadou M, et al. Synthetic analogues of aminoadamantane as influenza viral inhibitors-in vitro, in silico and QSAR studies. Molecules. 2020;25(17):3989.
- Pejin B, Iodice C, Tommonaro G, et al. Further in vitro evaluation of cytotoxicity of the marine natural product derivative 4′-leucine-avarone. Nat Prod Res. 2014;28(5):347–350.
- Sathuvan M, Thangam R, Gajendiran M, et al. κ-Carrageenan: an effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr Polym. 2017;160:184–193.
- Tsekova P, Spasova M, Manolova N, et al. Еlectrospun сellulose acetate membranes decorated with curcumin-PVP particles: preparation, antibacterial and antitumor activities. J Mater Sci Mater Med. 2017;29(1):9.
- Rosendahl AH, Perks CM, Zeng L, et al. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin Cancer Res. 2015;21(8):1877–1887.
- Chung T-W, Moon S-K, Chang Y-C, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dualmechanism. FASEB J. 2004;18(14):1670–1681.
- Chang W-C, Hsieh C-H, Hsiao M-W, et al. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J Obstet Gynecol. 2010;49(4):419–424.
- Prasad NR, Karthikeyan A, Karthikeyan S, et al. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol Cell Biochem. 2011;349(1-2):11–19.
- Murad LD, Soares NCP, Brand C, et al. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr Cancer. 2015;67(3):532–542.
- Ignatova MG, Manolova NE, Rashkov IB, et al. Poly(3-hydroxybutyrate)/caffeic acid electrospun fibrous materials coated with polyelectrolyte complex and their antibacterial activity and in vitro antitumor effect against HeLa cells. Mater Sci Eng C Mater Biol Appl. 2016;65:379–392.
- Braña MF, Ramos A. Naphthalimides as anticancer agents: synthesis and biological activity. Curr Med Chem Anticancer Agents. 2001;1(3):237–255.
- Banerjee S, Veale EB, Phelan CM, et al. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem Soc Rev. 2013;42(4):1601–1618.
- Kamal A, Bolla NR, Srikanth PS, et al. Naphthalimide derivatives with therapeutic characteristics: a patent review. Expert Opin Ther Pat. 2013;23(3):299–317.
- Wang K-R, Qian F, Wang X-M, et al. Cytotoxic activity and DNA binding of naphthalimide derivatives with amino acid and dichloroacetamide functionalizations. Chin Chem Lett. 2014;25(7):1087–1093.
- Braña MF, Castellano JM, Jiménez A, et al. Synthesis, cytostatic activity and mode of action of a new series of imide derivatives of 3-nitro-11α naphtalic acid. Curr Chemother. 1978;2:1216–1217.
- Braña MF, Castellano JM, Roldán CM, et al. Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitro-1,8-naphthalic acid. Cancer Chemother Pharmacol. 1980;4(1):61–66.