Abstract
The overuse of agrochemicals and development of pesticide resistance in plant pathogens is a main challenge in conventional agriculture. The use of biopesticides based on natural substances is recognized as an effective alternative of conventional pesticides. Besides the beneficial microorganisms, some plant species can be a source of microbiologically active compounds. Among the most promising are Origanum vulgare ssp. hirtum, Centaurea finazzeri, Achillea crithmifolia, Artemisia spp, Thymus pulegioides, Tanacetum parthenium, Clinopodium vulgare, Salvia sclarea, Lavandula officinalis and Mentha piperita. The filamentous fungi from genus Trichoderma are some of the most studied biocontrol agents because of their versatile mode of action. They employ several different strategies to combat plant pathogens, like direct mycoparasitism, production of enzymes and antibiotic substances, competition, ability to induce resistance in plants to a variety of stresses. Cost-effective cultivation and mass production of Trichoderma sp. biomass and formulations will allow utilization of locally available low-cost materials, such as different wastes and by-products that could be used as a growth substrate for production of adequate biomass containing effective propagules with or without minimal adjustments to the composition of cultivation media. This review outlines the two major mass production methods - solid and liquid state fermentation of Trichoderma spp. The possibilities for development of novel products for the bioindustry are highlighted.
Introduction
Conventional pesticides offer numerous benefits to agriculture like increased crop yield, improved food safety and human health, as well as reduction of labor and energy use. However, this comes at the price of a range of problems in agriculture, the environment and human health [Citation1]. Risks associated with pesticide use are multifaceted and well-known. The overuse of agrochemicals constitutes threats to both aquatic and terrestrial biodiversity and may lead to acute and chronic negative effects on human health. Another important challenge in conventional agriculture is the development of pesticide resistance in the pest populations [Citation2].
Since 1993, the policy in the European Union has been aimed at a declining trend in the use of pesticides in farming and low pesticide-input, giving increasing priority to non-chemical approaches. Additionally, the implementation of Integrated Pest Management (IPM) by farmers is recognized as one way to achieve these goals [Citation3]. Application of agroecological and other innovative approaches would benefit the competitiveness of the agricultural sector, creating conditions for the improvement of the input efficiency, increment of farm profitability and the social and economic development of rural regions [Citation4].
A study highlighted promising prospects for the development of biogas and bio-based fertilizers production in Bulgaria in 2017 [Citation5]. The authors point out that the investment in the production and use of such organic waste derived products would lead to a number of socio-economic benefits for society, the environment and farmers and would impact positively the quality of soil, groundwater and biodiversity as a whole [Citation5].
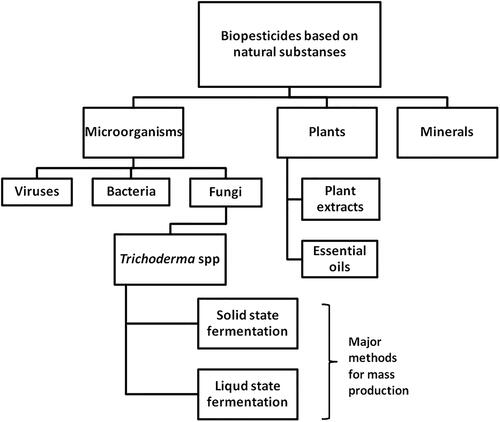
Biopesticides are effective tools in organic agriculture or in IPM programs. Biological pesticides are based on natural substances (derived from plants, different microorganisms or minerals, etc.) that can control invertebrate pests, plant pathogens and weeds. The microorganisms include bacteria, yeasts, fungi and viruses (). Among them, the fungal species are the most studied, due to their suitability and feasibility [Citation6].
Figure 1. Schematic representation of the different types biopesticides based on natural substanses addressed in this revew.
Many bacterial genera with activity against fungal and bacterial pathogens in plants have been described in the literature (reviewed by Bonaterra et al. [Citation7]). These species can use various mechanisms to limit the development of plant pathogens, like competition, antagonism and induction of systemic resistance in plants. There are several different methods to apply bacteria, for example, biopriming, encapsulation or fluid drilling of seeds, drenching, mixing or microbigation of soil, and foliar spraying or directly applying into the plant vascular system [Citation7]
The three most important genera of filamentous fungi used in biotechnology and bioengineering are Aspergillus, Penicillium and Trichoderma. Dedicated monographs are available in the literature for each one of them [Citation8]. The development of novel products for the bioindustry - chemicals, biofuels, biopharmaceuticals and others - is one of the paths to the maintenance of sustainable farming in Bulgaria [Citation9].
Plant extracts as biofungicides
Research on the potential of plant products as biofungicides has advanced since the last century, but this topic is being developed especially intensely at present [Citation10–13]. Usually, ethnobotanic studies and allelopathy data are used for the selection of plant species with antifungal properties. Among plant extracts, these of Aloe vera, Zingiber officinale, Datura stramonium, Allium cepa, Thea sinensis, Cynara cardunculus, Glycyrrhiza glabra, Nepeta sp. and others have been reported as promising for control of plant pathogens in some review studies [Citation12,Citation13]. In addition, some more examples of established inhibitory effect of plant extracts on the mycelium growth of phytopathogens reported in the last 10–15 years are summarized here. Wens and Geuens found that aqueous-ethanolic extract of Fallopia japonica is effective against Rhizoctonia solani, Botrytis cinerea and Sclerotinia minor [Citation14]. Mycelial growth inhibition of Cinnamomum cassia on B. cinerea on apple has been reported by Šerenaitĕ et al. [Citation15]. There are reports that aqueous extracts of Solanum indicum, Azadirachta indica, Oxalis latifolia [Citation16], Salvia guaranitica and Punica granatum [Citation17] inhibited the growth of Fusarium oxysporum f. sp. lycopersici. [Citation18]. Şesan et al. found that hydroalcoholic extracts of Hyssopus officinalis, Satureja hortensis, Allium sativum, Tagetes patula and Mentha sp. inhibited the growth of B. cinerea isolated from blackcurrant [Citation18].
Methanol extracts of Lavandula officinalis, Mentha piperita, Eucalyptus camaldulensis [Citation19] as well as aqueous extracts of Cymbopogon proximus, Zingiber officinale, Persea americana [Citation20] are effective in the control of growth of Alternaria alternata. Tapwal et al. have observed that aqueous extract of Cannabis sativa can be used for Curvularia lunata control [Citation21].
Essential oils as biofungicides
Much of the research on the potential of natural products such as biofungicides has focused on examination of the antifungal properties of essential oils [Citation22]. Accumulating evidence shows that these from Origanum and Thymus species, rich in carvacrol or thymol, exhibit the highest and broadest antifungal activity with very low active concentrations (0.05-5 µg/mL). The minimum inhibitory concentration (MIC) values of the pure compounds carvacrol and thymol are in the range of 0.02-1.5 µg/mL [Citation13]. Essential oils from Cinnamomum zeylanicu, Cananga odorata, Ocimum basilicum, Cymbopogon citratus, Boswellia thurifera, Majorana hortensis inhibit the growth of the common spoilage fungus at a concentration of 1 µL/mL [Citation13].
Recent studies on plant products as biofungicides
Significant antifungal activity of essential oils from three Micromeria species has been reported by Marinković et al. [Citation23]. Zatla et al. reported that essential oil and hydrosol from Marrubium vulgare exhibited inhibitory activity against B. cinerea, Penicillium expansum and A. alternata [Citation24].
Complete mycelial growth inhibition of Zataria multiflora, Thymus vulgaris and Thymus kotschyanus essential oils on Pythium aphanidermatum, Rhizoctonia solani, Fusarium graminearum, Sclerotinia sclerotiorum was found at 200 μL/L concentration by Amini et al. [Citation25]. Essential oils of Thymus vulgaris, T. tosevii, Mentha spicata and M. piperita showed strong antifungal activity against Alternaria alternata, Fusarium tricinctum and all Aspergillus species in the macrodilution assay [Citation26].
Various plant products, such as extracts, exudates and essential oils, can significantly inhibit the mycelium growth of phytopathogenic fungi. The essential oil of Origanum vulgare ssp. hirtum at concentrations of 0.2–0.8 μL/mL was reported to inhibit completely the growth of Fusarium solani, F. oxysporum, Alternaria solani, A. alternata and Botritys cinerea [Citation27]. Carvacrol has been identified as the main component in O. vulgare ssp. hirtum essential oil. The essential oil of Monarda didyma can be an effective inhibitor against Diaporthe nobilis, A. alternata and Phytophthora plurivora [Citation28].
Methanolic extract of Centaurea finazzeri as well as aqueous-methanolic extract of Achillea crithmifolia and Artemisia annua are effective in the control of A. alternata growth [Citation29,Citation30], while methanolic extract of Artemisia santhonicum displayed inhibitory activity against A. solani and B. cinerea [Citation31].
In most cases, acetone exudates have been determined as more effective inhibitors on mycelium growth, compared to the methanolic extracts. Exudates are a mixture of mainly non-polar compounds with a protective role that are located on plant surface structures. Acetone exudates of Artemisia campestris, Artemisia santhonicum, Artemisia lerchiana, Thymus pulegioides, Tanacetum parthenium, Clinopodium vulgare, Salvia sclarea have been reported to be effective against Fusarium oxysporum [Citation29, Citation31]. Strong inhibition on mycelium growth of Phytophthora cambivora has been shown by acetone exudates of Clinopodium vulgare and Tanacetum vulgare [Citation29]. Exudates of Salvia officinalis leaves and flowers exhibit inhibitory activity against A. alternata and B. cinerea [Citation29].
The main active components of the exudates have been determined. For example, in Clinopodium vulgare and Thymus pulegioides exudates triterpenic acids - oleanolic, ursolic, betulinic; in Salvia sclarea exudate diterpene – sclareol. Acetone exudates of Salvia officinalis, Achillea critmifolia and Artemisia species are characterized with high content of methylated flavonoid aglycones [Citation29, Citation32,Citation33].
Species from genus Trichoderma as biocontrol agents
Genus Trichoderma has long been an object of major research efforts in the areas of biological control of plant disease and enzyme production [Citation34]. Representatives of this genus have attracted attention for producing a variety of interesting secondary metabolites with medicinal importance. Some Trichoderma species are used as biocontrol agents, while others are promising producers of enzymes for industrial purposes [Citation35]. Apparently, Trichoderma is identified as the genus with greatest potential comprising 25 biocontrol agents that have been used against a number of plant fungal diseases [Citation36].
Trichoderma ecology
Trichoderma species are opportunistic, avirulent plant symbionts, which are common in agricultural, prairie, forest, salt marsh and desert soils in all climatic zones, although individual species may have either cosmopolitan (e.g. T. harzianum) or limited (e.g. T. viride) geographic distribution [Citation37,Citation38]. The representatives of this genus can metabolize a remarkable diversity of substrates [Citation37]. Members of Trichoderma compete with other groups of fungi for ecological niches and nutrients, and produce various secondary metabolites, including antibiotics and mycotoxins. Some Trichoderma species have mycoparasitic ability, raising hopes as potential biocontrol agents against plant pathogenic fungi. Trichoderma species, such as T. harzianum, can colonize and degrade resistant structures of other plant pathogenic fungi [Citation39].
Trichoderma mechanisms of action
The genus includes model plant-growth–promoting species whose mode of action and regulation have been extensively studied [Citation40]. Trichoderma species have a pathogen suppressing arsenal that includes several mechanisms: direct antagonism (hyperparasitism), antibiosis, competition and induced resistance [Citation41]. Trichoderma spp. are widely used for suppression of soil-borne pathogens via competition and mycoparasitism [Citation42]. A recent study [Citation43] demonstrates the potential of Trichoderma spp. to inhibit both the colony growth and the aflatoxin B1 production of an aflatoxigenic Aspergillus flavus isolate. This indicates that the use of Trichoderma species as biocontrol agents against A. flavus and for prevention of aflatoxin accumulation could be an effective biocontrol strategy, especially when multiple isolates with different mechanisms of action are combined [Citation43].
The biosynthesis of silver nanoparticles, using fungi is a relatively new approach which finds application in agriculture. For the purpose, the nanoparticles are coated with biomolecules derived from different fungi, which confer their biological activity and improved stability [Citation44]. A recent study [Citation45] reported the successful synthesis of silver nanoparticles in filtrates from T. harzianum cultivated in the presence and absence of the cell wall of S. sclerotiorum. The resulting nanoparticles were able to control the growth of S. sclerotiorum with inhibition of mycelial growth and prevention of the formation of new sclerotia. These results open perspectives for further exploration of fungal biogenic nanoparticles against different agricultural pests [Citation45]. An innovative delivery approach of bio agents was proposed by Spasova et al. [Citation46]. The method is based on the use of specific nonionogenic, watersoluble, and nontoxic polymers. The electrospinning of plant sprouts with ecologically safe biohybrid nanofibrous mats containing chitosan and T. viride spores effectively inhibited the growth of Fusarium and Alternaria phytopathogenic strains [Citation46].
Antibiotic agents and enzymes produced by Trichoderma synergistically kill and/or degrade the target hyphae [Citation47]. These features allow them to control numerous plant pathogens. Another set of properties that may have great potential in agriculture is the ability of Trichoderma spp. to reprogram plant gene expression. Studies have demonstrated that some representatives are able to induce resistance to biotic stresses such as diseases and abiotic stresses, like drought and salinity, to enhance plant growth, improve the photosynthetic ability and nitrogen use efficiency [Citation47]. As reviewed elsewhere [Citation42], the species from genus Trichoderma are fast colonizers of the spermosphere (seed zone) and rhizosphere (root zone), which helps exclude invading pathogens when the biocontrol fungi are applied to seeds or roots. In direct antibiosis, secondary metabolites or secreted enzymes from Trichoderma inhibit pathogen growth or germination. Chitinases, β-glucanases, and proteases were established as key enzymes involved in mycoparasitism [Citation42]. The species benefits are wide and multifaceted, including commercial enzymes, plant growth-accelerating abilities, and biocontrol of plant diseases, indicating their promising industrial, agricultural and medicinal potential [Citation35].
Utilization of agricultural waste materials and by-products as a low-cost alternative for Trichoderma spp. growth and propagation
Cost-effective cultivation and mass production of Trichoderma sp. biomass and formulations requires utilization of locally adapted strains, as well as locally available low-cost materials such as different wastes and by-products. Such low-cost growth substrates could be used to obtain sufficient biomass yield containing effective propagules (e.g. chlamydospores) with or without minimal adjustments to the composition of cultivation media.
The mass production of Trichoderma biomass relies on two major methods: solid state fermentation (SSF) and liquid state fermentation (LSF). In SSF, the substrates for fungus growth are various cereal grains, agricultural wastes and by-products, and these products are mainly applied directly onto soil to suppress the soil-borne phytopathogens [Citation48]. The method is commonly used for mass production of fungal biopesticides, since it provides micropropagules with higher conidia content [Citation49,Citation50]. In LSF, the liquid media for growth of Trichoderma contain molasses and yeast extract. Growth takes place in deep tanks and the obtained biomass can be used for preparation of different forms of commercial products such as dusts, granules, pellets, wettable powders [Citation48]. The method finds application in the mass multiplication of fungal biocontrol agents. For such purpose the selected liquid medium should be readily available, inexpensive and with appropriate nutrient balance [Citation50].
Solid state fermentation of Trichoderma spp.
Fungal mass propagation through SSF should fit to farm needs, and thus requires appropriate low-cost and presumably locally available materials as alternative nutrient substrates. A number of studies have tested various agricultural products and wastes as a suitable media for potential large-scale SSF of Trichoderma sp.
Various grains like sorghum [Citation51], rice [Citation52–54], millet and ragi [Citation55] have been successfully used as inexpensive growth substrates for Trichoderma spp. In general, grains are first moistened and sterilized and then inoculated with Trichoderma for production of spores, which occurs within 10–15 days [Citation50].
Although grains have been shown to be suitable solid-state media for growing Trichoderma spp., the most promising and cost-effective approach would be the utilization of different agricultural by-products and wastes. In 1999, Gopal et al. compared different solid-state media for growing T. harzianum and T. virens for a period of 21 days and found tea waste to be the best for mass multiplication when compared with other materials such as neem cake, farm yard manure and coffee husk [Citation56]. Another study showed that out of five tested materials (rice bran, rice chaffy grain, farmyard manure, banana pseudostem and dried banana leaf), dried banana leaves proved to be the best carrier material for production of high-density propagules from T. harzianum. Addition of jaggery to the leaves increased the fungal propagation and vitality [Citation54]. Wastes and peels of brinjal (eggplant), banana, papaya, guava, potato, carrot, spinach, sugarcane and tea leaves proved to be suitable for production and growth of T. harzianum and T. viride following wetting with molasses. The materials were used as solid and liquid substrate and incubated for spore production for 20 days [Citation57]. Other wastes like pea, maize and rice husks, wheat bran and saw dust also support spore production and growth of T. harzianum and T. viride following incubation for 15 days for sporulation [Citation58]. The authors of two studies have proposed the sugarcane bagasse as a cost-effective substrate for mass cultivation of T. harzianum and T. lixii following comparison with materials like vegetable wastes, spent tea leaves, cow-dung, vermicompost and paddy straw [Citation51, Citation53]. In a recent study, Citation59 suggested an innovative use of whole digestate, obtained after biomethane production mixed with agro-food waste as a valuable substrate for growing T. asperellum, T. atroviride, T. harzianum, T. reesei [Citation59].
The fungal biomass obtained after SSF is usually air-dried, ground or powdered and further used for treatment of seeds or soil application. Some commercial formulations are prepared by diluting the fungal powder with talcum powder containing 1% carboxymethyl-cellulose to adjust the desired concentration of biocontrol agent [Citation48].
Liquid state fermentation of Trichoderma spp.
LSF of Trichoderma spp. in laboratory conditions is usually performed using potato dextrose broth. However, as in the case of SSF, a number of studies have been targeted towards finding optimal cultivation conditions as well as the utilization of locally available low-cost liquid materials suitable for LSF of Trichoderma spp.
Investigating the effects of the LSF parameters, Al-Taweil et al. found that the optimal cultivation conditions for maximizing the biomass production in batch cultures of T. viride included: medium with carbon and nitrogen content of 45 g/L and 0.35 g/L, respectively, incubation at 30 °C, agitation at 175 rpm and pH 6. This composition yielded the optimum mycelium biomass following 5 days of cultivation. The authors found that nitrogen content was the limiting factor for fungal growth in their study, suggesting that liquid wastes lacking sufficient carbon and nitrogen sources should be additionally supplemented to support the fungal growth [Citation60].
In a screening of locally available household and industrial liquid wastes, Emerson and Mikuntan tested various products for T. viride cultivation, including black gram-soaked water, coconut water, rice mill effluent, 1% jaggery solution, and 1% extract of palmyra fruit pulp. After an incubation period of 14 days, the highest T. viride growth and sporulation was achieved using black gram-soaked water [Citation61].
Utilization of locally available liquid wastes was also in the focus of a recent study by Rusanova et al. who assessed the capacity of T. asperellum to grow in rose oil distillation wastewater (RODW) rich in natural phenolics and sugars in Bulgaria. The results showed that T. asperellum successfully grows and sporulates in RODW (pH ≈ 4) due to assimilation of naturally occurring sugars and does not require addition of external carbon sources or pH correction. Furthermore, the observation that T. asperellum fermentation does not significantly change the composition of the biologically active RODW phenolics, makes this type of cultivation appropriate for further utilization of bioactive phenolic compounds contained in RODW and promising a low-cost alternative for fungal propagation [Citation62].
Trichoderma-based formulations
Successful biological control depends not only on the discovery of a proper easily propagated microorganism that is antagonistic to pathogens, but also on development of a product which enables viability of the biocontrol agent and facilitates its action in plant disease control. Various Trichoderma-based formulations have been developed for mass production and field application. As summarized by Kumar et al. [Citation50], formulation is a process of blending of active ingredients such as fungal spores with an inert material such as diluents and surfactants in order to alter the physical characteristics of the biocontrol agent to a more desirable form [Citation50]. The well-developed formulation should be easy to handle, stable over a range of −5 °C to +35 °C and to have a minimum shelf-life of two years at room temperature [Citation50].
There are different methods for treatment with Trichoderma formulations in practice: dry seed treatment, seed biopriming, dipping the roots in Trichoderma suspensions before planting or using of pellets preparations directly in soil [Citation63]. In their review article, Ramanujam et al. present the most widely used Trichoderma formulations utilizing inert materials, such as talcum, vermiculite, kaolin, mineral and vegetable oils, as well as by-products, such as wheat bran, press mud and coffee husk [Citation63].
Microencapsulation as a method to prolong the shelf life of liquid and solid Trichoderma formulations is reviewed by Cumagun [Citation64]. In order to avoid loss of viability during the process of drying, conidia are incapsulated with sugars (sucrose, molasses, maltodextrin) or glycerol, which results in higher conidia survival [Citation64].
In another paper [Citation65], Fraceto et al. reviewed in details the most used physical (spray drying), chemical (polymerization) and physico-chemical (coacervation, ionic gelation) microencapsulation methods and discussed their perspective for industrial application and sustaining activity of the Trichoderma-based products and prolonging their shelf life [Citation65].
In their research [Citation66], Herrera et al. developed two formulations which were able to improve T. asperellum antagonism against Rhizoctonia solani on corn seedlings. Compared to the control, the liquid formulations prepared by emulsified vegetable and mineral oils, improved the viability of spores because of the UV-protecting effect of oils. A granular formulation prepared with degreased corn germ and T. asperellum spores protected the corn seedlings from R. solani under greenhouse conditions, with 73% reduction of infected plants and 93% reduction of necrotic spot size [Citation66].
Various Trichoderma-based formulations are currently available on the global market aiming effective biocontrol of phytopathogens and facilitation of crops growth. However, there still remains the need of development of cost-effective products with prolonged shelf life and stability when applied on fields.
Conclusions
There is а positive trend of increased interest in biological farming in recent years. There is growing evidence that biopesticides and biocontrol agents are effective, species-specific and environmentally friendly. In the European Union, biopesticides are evaluated according to the regulations that apply to synthetic active substances, and this is considered to be one of the reasons for the comparatively smaller number of scientific studies in this area. However, it is recognized that there is a need to expand the range of approved active substances that are non-chemical, low-risk or basic to increase pest control options, and at the same time are available to farmers, thereby reducing their dependence on the most hazardous active substances. Investment in the production and use of organic waste derived products like bio-based fertilizers will bring a variety of socio-economic, environmental and biodiversity benefits. Bulgaria has all prerequisites and potential for advancing research and development in this area, like favorable climatic conditions, established traditions in organic production, as well as the capacity to produce innovative products.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Additional information
Funding
References
- Lamichhane JR, Dachbrodt-Saaydeh S, Kudsk P, et al. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Disease. 2016;100(1):10–24.
- Mahmood I, Imadi SR, Shazadi K, et al. Effects of pesticides on environment. In: Plant, soil and microbes. Cham: Springer; 2016. p. 253–269.
- Hillocks RJ. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot. 2012;31(1):85–93.
- Bencheva N. Transition of Bulgarian agriculture: present situation, problems and perspectives for development. J Central Eur Agri. 2005;6(4):473–480.
- Bencheva N, Tepavicharova M. Opportunities and attitudes of farmers to the production of biogas and bio-based fertilisers in Bulgaria. Problems Agri Econ. 2017;3(352):92–102.
- Fountain E, Wratten S. Conservation biological control and biopesticides in agricultural. In: Fath BD, editor. Encyclopedia of ecology. 2nd ed., Vol. 1. Amsterdam, Netherlands: Elsevier; 2013. p. 377–381.
- Bonaterra A, Badosa E, Daranas N, et al. Bacteria as biological control agents of plant diseases. Microorganisms. 2022;10(9):1759.
- Gomes EN, Elsherbiny EA, Aleem B, et al. Beyond classical biocontrol: new perspectives on Trichoderma. In Fungal biotechnology and bioengineering. Cham: Springer; 2020. p. 437–455.
- Atanassov A, Shishinyova M, Rakleova G, et al. Biologichno zemedelie - problemi i perspektivi [biological agriculture - problems and perspectives]. Biological crop, livestock and food. Sofia, Bulgarian: Agricultural Academy; 2014.
- Arraiza MP, González-Coloma A, Andres MF, et al. Antifungal effect of essential oils. In: El-Shemy HA, editor. Potential of essential oils. London, UK: IntechOpen; 2018. pp. 145–164.
- Baraka M, Radwan FM, Shaban W, et al. Efficiency of some plant extracts, natural oils, biofungicides and fungicides against root rot disease of date palm. J Biol Chem Environ Sci. 2011;6(2):405–429.
- Choudhury D, Dobhal P, Srivastava S, et al. Role of botanical plant extracts to control plant pathogens-A review. Ind J Agri Res. 2018;52(4):341–346.
- Soković MD, Glamočlija JM, Ćirić AD. Natural products from plants and fungi as fungicides. In: Nita M, editor. Fungicides-showcases of integrated plant disease management from around the world. London, UK: IntechOpen; 2013. pp. 185–232.
- Wens A, Geuens J. In vitro and in vivo antifungal activity of plant extracts against common phytopathogenic fungi. J BioSci Biotechnol. 2022;11(1):15–21.
- Šernaitė L, Rasiukevičiūtė N, Valiuškaitė A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods. 2020;9(10):1430.
- Ramaiah AK, Garampalli RKH. In vitro antifungal activity of some plant extracts against Fusarium oxysporum f. sp. lycopersici. Asian J Plant Sci Res. 2015;5(1):22–27.
- Rongai D, Pulcini P, Pesce B, et al. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015;10(1):409–416.
- Şesan TE, Enache E, Iacomi BM, et al. Antifungal activity of some plant extracts against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L). Acta Sci Pol Hortorum Cultus. 2015;14(1):29–43.
- Zaker M, Mosallanejad H. Antifungal activity of some plant extracts on Alternaria alternata, the causal agent of alternaria leaf spot of potato. Pak J Biol Sci. 2010;13(21):1023–1029.
- Fawzi E, Khalil A, Afifi A. Antifungal effect of some plant extracts on Alternaria alternata and Fusarium oxysporum. Afr J Biotechnol. 2009;8(11):2590–2597.
- Tapwal A, Garg S, Gautam N, et al. In vitro antifungal potency of plant extracts against five phytopathogens. Braz Arch Biol Technol. 2011;54(6):1093–1098.
- Soković M, Liaras K. Chapter 4 - Natural products as antifungals. In: Soković M, Liaras K, editors. Antifungal compounds discovery. Amsterdam, Netherlands: Elsevier; 2021. pp. 67–165.
- Marinković B, Marin PD, Knezević-Vukcević J, et al. Activity of essential oils of three micromeria species (Lamiaceae) against micromycetes and bacteria. Phytother Res. 2002;16(4):336–339.
- Zatla AT, Mami I, Dib ME, et al. Efficacy of essential oil and hydrosol extract of marrubium vulgare on fungi responsible for apples rot. AIA. 2020;18(3):285–293.
- Amini M, Safaie N, Salmani M, et al. Antifungal activity of three medicinal plant essential oils against some phytopathogenic fungi. Trakia J Sci. 2012;10(1):1–8.
- Soković MD, Vukojević J, Marin PD, et al. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14(1):238–249.
- Krumova E, Nikolova M, Miteva-Staleva J, et al. Bio-efficacy of the essential oil isolated from Origanum vulgare subsp. hirtum against fungal pathogens of potato. Comptes Rendus De L’académie Bulgare Des Sciences. 2021;74(10):1571–1578.
- Christova PK, Dincheva IN, Slavov SB, et al. Evaluation of growth response of phytopathogens Alternaria alternata, Diaporthe nobilis and Phytophthora plurivora to inhibitory potential of three essential oils of Monarda didyma genotypes. J Plant Dis Prot. 2021;128(6):1531–1545.
- Nikolova MT, Yordanov P, Slavov S, et al. Antifungal activity of plant extracts against phytopathogenic fungi. J BioSci Biotechnol. 2017;6(2):155–161.
- Slavov S, Nikolova M. 2021. Effect of two Asteraceae species on plant pathogenic fungi. Annual of Sofia University “St. KlimentOhridski” Faculty of Biology, 106(Book 4 - Scientific Sessions of the Faculty of Biology), Scientific Conference "Kliment’s Days". Sofia 4–11.
- Krumova E, Kostadinova N, Miteva-Staleva J, et al. 2020. Biocidal activity of extracts from Bulgarian plants-screening and potato crop protection. FEMS online Conference on Microbiology. Online
- Nikolova M, Aneva I, Dimitrova M, et al. Metabolic profiling of Artemisia santhonicum and Artemisia lerchiana by GC/MS. Voprosi biologicheskoi, meditsinskoi i varmatsevticheskoi himii. 2019;22(7):26–26.
- Nikolova M, Gussev C, Nguyen T. Evaluation of the antioxidant action and flavonoid composition of Artemisia species extracts. Biotechnol Biotechnol Equip. 2010;24(sup1):101–103.
- Samuels GJ. Trichoderma: a review of biology and systematics of the genus. Mycol Res. 1996;100(8):923–935.
- Zhang J-L, Tang W-L, Huang Q-R, et al. Trichoderma: a treasure house of structurally diverse secondary metabolites with medicinal importance. Front Microbiol. 2021;12:723828.
- Thambugala KM, Daranagama DA, Phillips AJ, et al. Fungi vs. fungi in biocontrol: an overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol. 2020;10:604923.
- Klein D, Eveleigh D. Ecology of Trichoderma. In: Kubicek CP, Harman GE, editors. Trichoderma and Gliocladium. Basic biology, taxonomy and genetics. 1st ed., Vol. 1. Boca Raton, Florida, United States: Taylor and Francis Ltd; 1998. pp. 57–74.
- Samuels GJ. Trichoderma: systematics, the sexual state, and ecology. Phytopathology. 2006;96(2):195–206.
- del Carmen H, Rodríguez M, Evans HC, et al. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci Rep. 2021;11(1):1–30.
- Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84(1):11–18.
- Sharma S, Kour D, Rana KL, et al. Trichoderma: biodiversity, ecological significances, and industrial applications. In: Recent advancement in white biotechnology through fungi. Cham: Springer; 2019. pp. 85–120.
- Mukherjee PK, Mendoza-Mendoza A, Zeilinger S, et al. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol Rev. 2022;39:15–33.
- Ren X, Branà MT, Haidukowski M, et al. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins. 2022;14(2):86.
- Abdelghany T, Al-Rajhi AM, Al Abboud MA, et al. Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. BioNanoSci. 2018;8(1):5–16.
- Guilger-Casagrande M, Germano-Costa T, Pasquoto-Stigliani T, et al. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci Rep. 2019;9(1):1–9.
- Spasova M, Manolova N, Naydenov M, et al. Electrospun biohybrid materials for plant biocontrol containing chitosan and Trichoderma viride spores. J Bioactive and Compatible Polymers. 2011;26(1):48–55.
- Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48(1):21–43.
- Bahadur A, Dutta P. Trchoderma spp.: their impact in crops diseases management. In: Trichoderma - Technology and uses. London, UK: IntechOpen; 2022.
- Boblina B, Beura S, Mishra M, et al. Growth of Trichoderma spp. on different solid substrates. IntJCurrMicrobiolAppSci. 2019;8(09):2519–2529.
- Kumar S, Thakur M, Rani A. Trichoderma: mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr J Agri Res. 2014;9(53):3838–3852.
- Subash N, Meenakshisundaram M, Sasikumar C, et al. Mass cultivation of Trichoderma harzianum using agricultural waste as a substrate for the management of damping off disease and growth promotion in chilli plants (Capsicum annuum L.). Int J Pharm Pharm Sci. 2014;6(5):188–192.
- Kumhar KC, Babu A, Bordoloi M, et al. Evaluation of culture media for biomass production of trichoderma viride (KBN 24) and their production economics. Am J Agri Forestr. 2014;2(6):317–320.
- Sachdev S, Singh A, Singh RP. Optimization of culture conditions for mass production and bio-formulation of Trichoderma using response surface methodology. 3 Biotech. 2018;8(8):1–8.
- Thangavelu R, Palaniswami A, Velazhahan R. Mass production of trichoderma harzianum for managing fusarium wilt of banana. Agriculture, Ecosyst Environ. 2004;103(1):259–263.
- Jeyarajan R. Prospects of indigenous mass production and formulation of Trichoderma. Current status of biological control of plant diseases using antagonistic organisms in India. Proceedings of the group meeting on antagonistic organisms in plant disease management held at Project Directorate of Biological Control, Bangalore, India, 10–11th July 2003; 2006.
- Gopal KV, Anandaraj M, Sarma Y. Evaluation of substrates for mass multiplication of fungal biocontrol agents Trichoderma harzianum and T. virens. J Spices and Aromatic Crops. 1999;8(2):207–210.
- Simon S. Agro-based waste products as a substrate for mass production of Trichoderma spp. J Agric Sci. 2011;3(4):168.
- Yadav LS. Antagonistic activity of Trichoderma sp. and evaluation of various agro wastes for mass production. Ind J Plant Sci. 2012;1(1):109–112.
- Alias C, Bulgari D, Gobbi E. It works! organic-waste-assisted Trichoderma spp. solid-state fermentation on agricultural digestate. Microorganisms. 2022;10(1):164.
- Al-Taweil HI, Osman MB, Aidil A, et al. Optimizing of Trichoderma viride cultivation in submerged state fermentation. Am J Appl Sci. 2009;6(7):1284.
- Emerson F, Mikunthan G. Small scale production of Trichoderma viride on locally available liquid waste and other substrates. Am-Eur J Agri Environ Sci. 2015;15:1666–1671.
- Rusanova M, Rusanov K, Momchilova S, et al. Assessment of the fermentation of rose oil distillation wastewater (RODW) by Trichoderma asperellum SL-45 as additional step for fungal biomass production, to the RODW phenolics extraction. Annual of Sofia University “St. Kliment Ohridski” Faculty of Biology, Book. 2019;4, volume 104:52–61.
- Ramanujam B, Prasad R, Sriram S, et al. Mass production, formulation, quality control and delivery of Trichoderma for plant disease management. J Plant Protect Sci. 2010;2(2):1–8.
- Cumagun CJR. Advances in formulation of Trichoderma for biocontrol. In: Biotechnology and biology of Trichoderma. Amsterdam, The Netherlands: Elsevier; 2014. p. 527–531.
- Fraceto LF, Maruyama CR, Guilger M, et al. Trichoderma harzianum∼based novel formulations: potential applications for management of next∼gen agricultural challenges. J Chem Technol Biotechnol. 2018;93(8):2056–2063.
- Herrera W, Valbuena O, Pavone-Maniscalco D. Formulation of Trichoderma asperellum TV190 for biological control of Rhizoctonia solani on corn seedlings. Egypt J Biol Pest Control. 2020;30(1):1–8.
